A Comparison of Lymphoid and Myeloid Cells Derived from Human Hematopoietic Stem Cells Xenografted into NOD-Derived Mouse Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Mice and Xenotransplantation
2.3. Cytometric Bead Array (CBA) Analysis
2.4. Flow Cytometry Assays
2.5. Detection of Platelets
2.6. Bone Marrow Dissection
2.7. Liver and Spleen Dissection
2.8. Statistical Analysis
3. Results
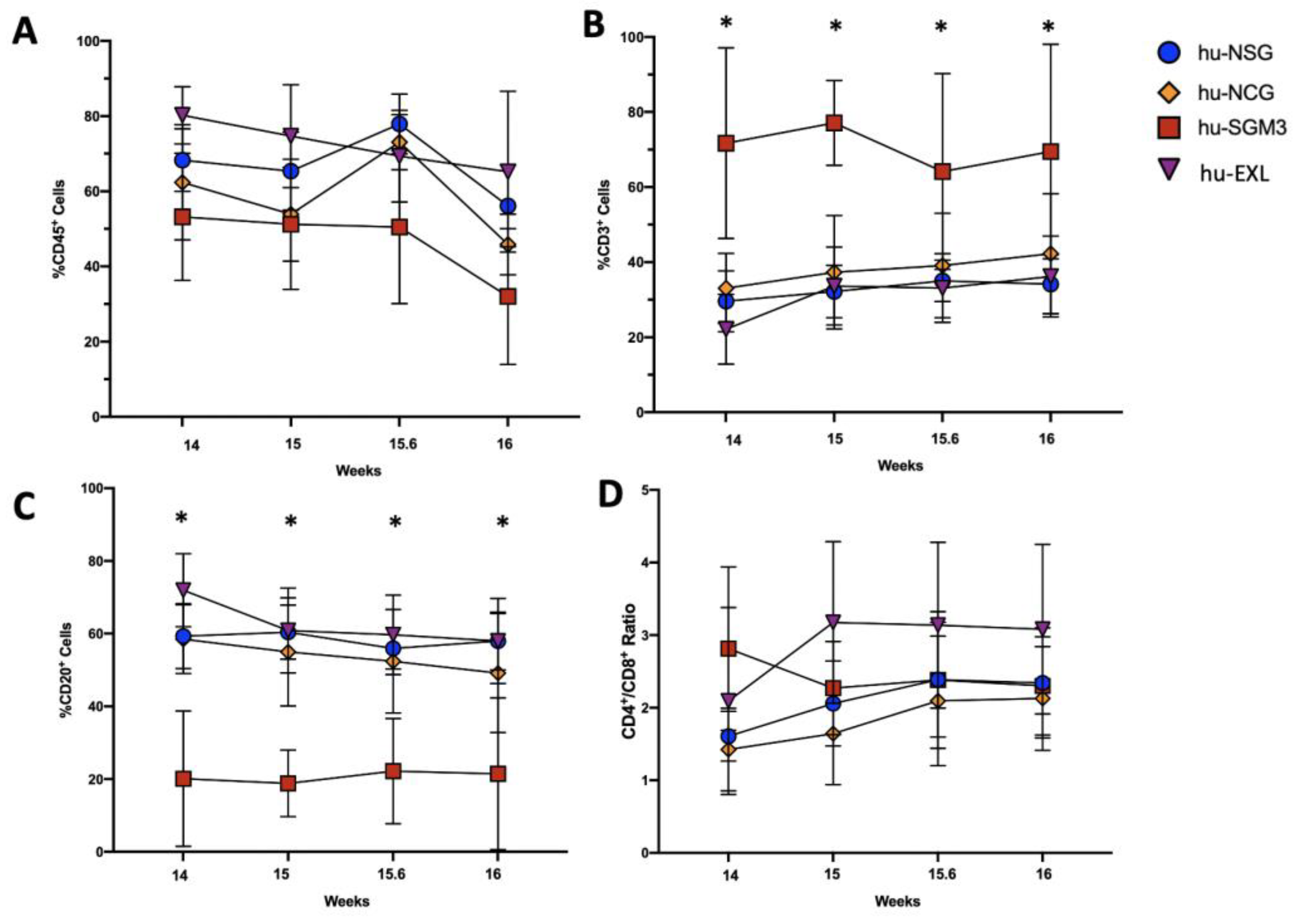
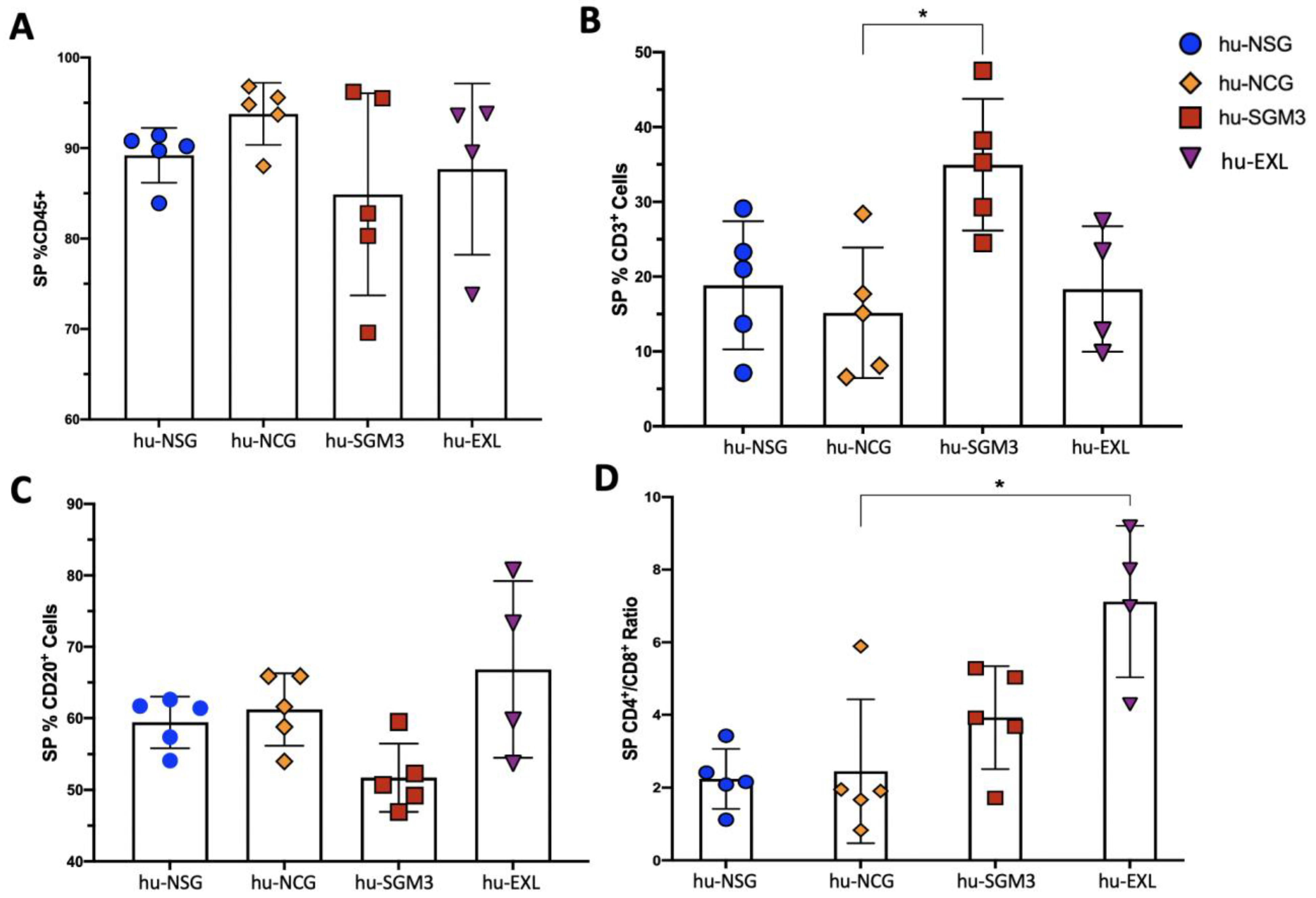
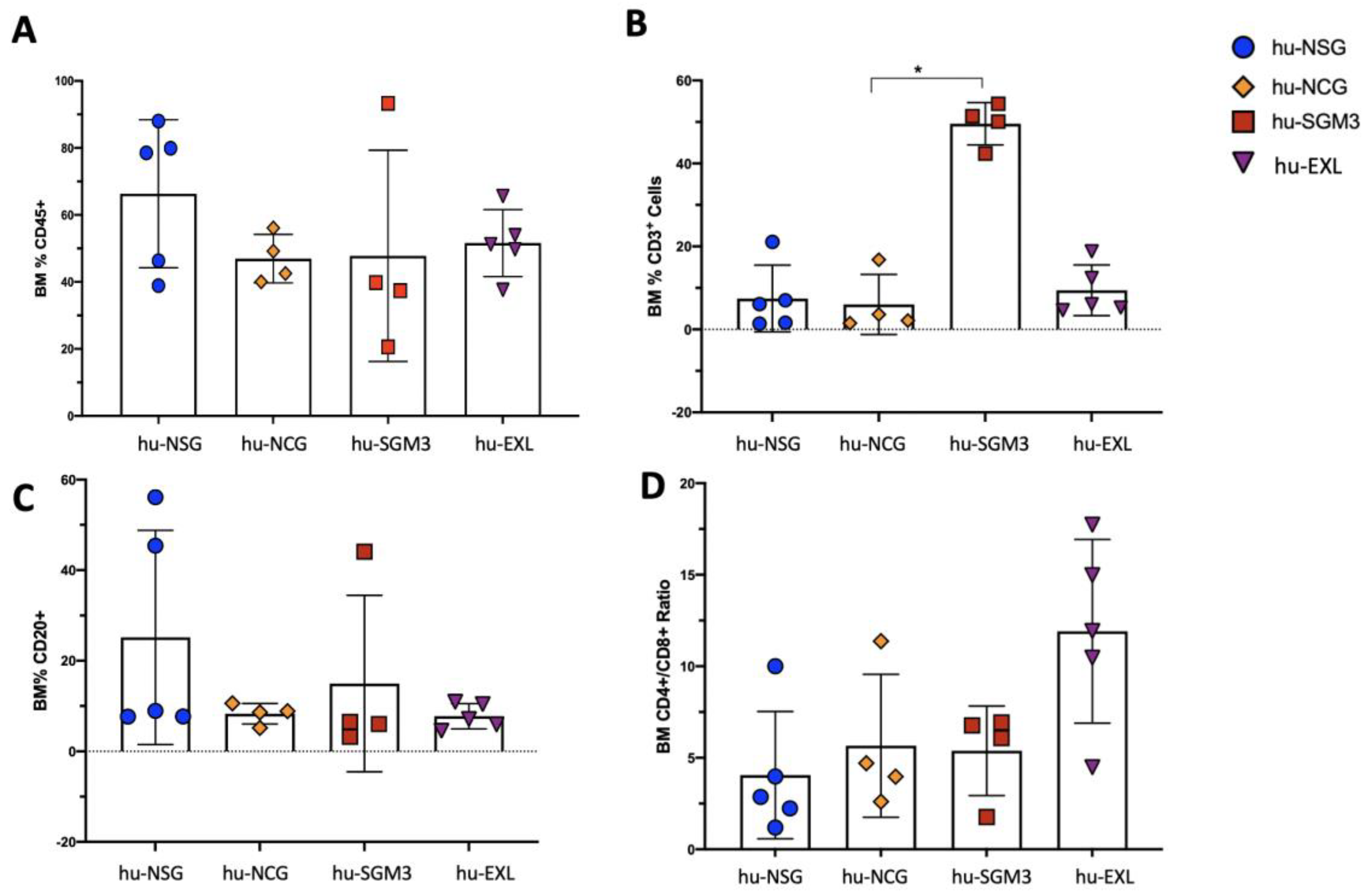
3.1. A Higher Frequency of Human CD3+ Cells in the Peripheral Blood and Tissues in hu-SGM3 Mice
3.2. Increased Frequency of Peripheral CD14+ Monocytes in the hu-SGM3 Model in the Blood and Tissues
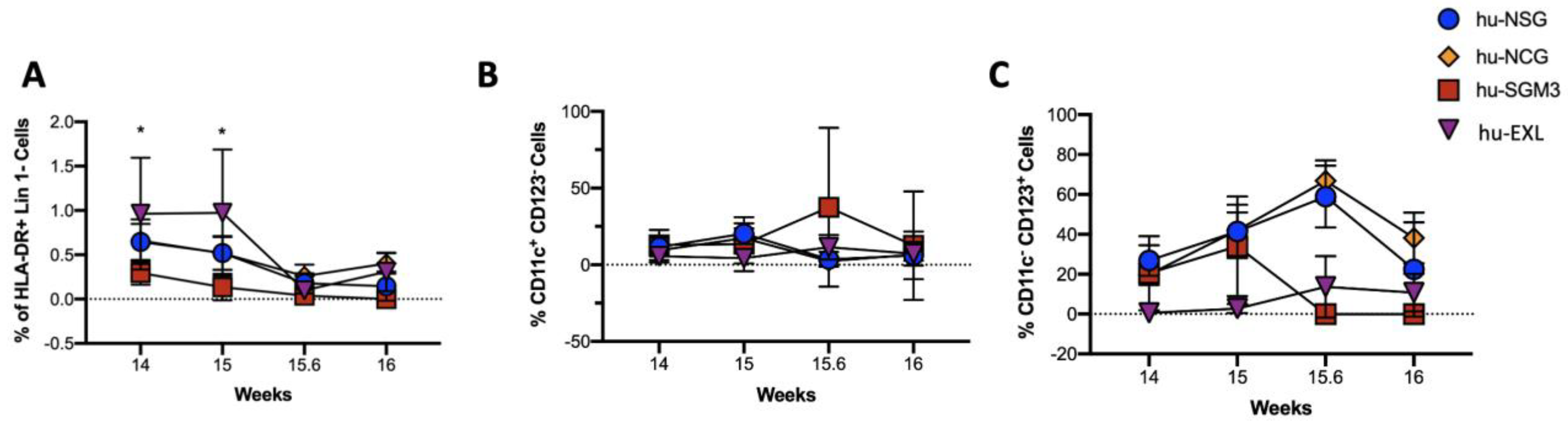
3.3. Enriched Frequency of Myeloid Dendricitc Cells in the Bone Marrow and Spleen in the hu-SGM3 Model
3.4. Enhancement of Human Megakaryocyte Cell Development in the hu-SGM3 and hu-EXL Models
3.5. Enhancement of Human Platelet Development in the hu-EXL Model
3.6. Enhancement of Human Mast Cell Development in the hu-SGM3 and hu-EXL Models
3.7. Human Cytokine Expression during Xenotransplantation in the hu-NSG, hu-NCG, hu-SGM3, and hu-EXL Humanized Mouse Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Claiborne, D.T.; Maldini, C.R.; Phelps, M.; Vrbanac, V.; Karpel, M.E.; Krupp, K.L.; Power, K.A.; Boutwell, C.L.; Balazs, A.B.; et al. Innate Immune Reconstitution in Humanized Bone Marrow-Liver-Thymus (HuBLT) Mice Governs Adaptive Cellular Immune Function and Responses to HIV-1 Infection. Front. Immunol. 2021, 12, 667393. [Google Scholar] [CrossRef]
- Harvey, B.S.; Baker, C.J.; Edwards, M.S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect. Immun. 1992, 60, 3635–3640. [Google Scholar] [CrossRef] [PubMed]
- Serreze, D.V.; Chapman, H.D.; Varnum, D.S.; Gerling, I.; Leiter, E.H.; Shultz, L.D. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J. Immunol. 1997, 158, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Serreze, D.V.; Leiter, E.H. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr. Opin. Immunol. 1994, 6, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Blunt, T.; Finnie, N.J.; Taccioli, G.E.; Smith, G.C.; Demengeot, J.; Gottlieb, T.M.; Mizuta, R.; Varghese, A.J.; Alt, F.W.; Jeggo, P.A.; et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 1995, 80, 813–823. [Google Scholar] [CrossRef]
- Bosma, M.; Schuler, W.; Bosma, G. The scid mouse mutant. Curr. Top. Microbiol. Immunol. 1988, 137, 197–202. [Google Scholar] [CrossRef]
- Charles River. NOD CRISPR Prkdc IL2r Gamma (NCG) Triple-Immunodeficient Mouse Model. Available online: https://www.criver.com/products-services/find-model/ncg-mouse?region=3611 (accessed on 18 March 2023).
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef]
- Laboratory, T.J. NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ. Available online: https://www.jax.org/strain/013062 (accessed on 18 March 2023).
- Coughlan, A.M.; Harmon, C.; Whelan, S.; O’Brien, E.C.; O’Reilly, V.P.; Crotty, P.; Kelly, P.; Ryan, M.; Hickey, F.B.; O’Farrelly, C.; et al. Myeloid Engraftment in Humanized Mice: Impact of Granulocyte-Colony Stimulating Factor Treatment and Transgenic Mouse Strain. Stem Cells Dev. 2016, 25, 530–541. [Google Scholar] [CrossRef]
- Watanabe, Y.; Takahashi, T.; Okajima, A.; Shiokawa, M.; Ishii, N.; Katano, I.; Ito, R.; Ito, M.; Minegishi, M.; Minegishi, N.; et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice). Int. Immunol. 2009, 21, 843–858. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Medina-Moreno, S.; Davis, H.; Bryant, J.; Taborda, N.A.; Rugeles, M.T.; Kottilil, S.; Zapata, J.C. Characterization of CXCR5(+) CD8(+) T-cells in humanized NSG mice. Immunobiology 2020, 225, 151885. [Google Scholar] [CrossRef]
- Hess, N.J.; Lindner, P.N.; Vazquez, J.; Grindel, S.; Hudson, A.W.; Stanic, A.K.; Ikeda, A.; Hematti, P.; Gumperz, J.E. Different Human Immune Lineage Compositions Are Generated in Non-Conditioned NBSGW Mice Depending on HSPC Source. Front. Immunol. 2020, 11, 573406. [Google Scholar] [CrossRef]
- Ballen, K.K.; Valinski, H.; Greiner, D.; Shultz, L.D.; Becker, P.S.; Hsieh, C.C.; Stewart, F.M.; Quesenberry, P.J. Variables to predict engraftment of umbilical cord blood into immunodeficient mice: Usefulness of the non-obese diabetic--severe combined immunodeficient assay. Br. J. Haematol. 2001, 114, 211–218. [Google Scholar] [CrossRef]
- Verstegen, M.M.; van Hennik, P.B.; Terpstra, W.; van den Bos, C.; Wielenga, J.J.; van Rooijen, N.; Ploemacher, R.E.; Wagemaker, G.; Wognum, A.W. Transplantation of human umbilical cord blood cells in macrophage-depleted SCID mice: Evidence for accessory cell involvement in expansion of immature CD34+CD38- cells. Blood 1998, 91, 1966–1976. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Spencer Clinton, J.L.; Rico-Hesse, R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl. Trop. Dis. 2019, 13, e0007837. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Avellaneda, D.; Mosso-Pani, M.A.; Sánchez-Torres, L.E.; Castro-Mussot, M.E.; Corona-de la Peña, N.A.; Salazar, M.I. Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Bush, L.M.; Healy, C.P.; Marvin, J.E.; Deans, T.L. High-throughput enrichment and isolation of megakaryocyte progenitor cells from the mouse bone marrow. Sci. Rep. 2021, 11, 8268. [Google Scholar] [CrossRef]
- Ishikawa, F.; Yasukawa, M.; Lyons, B.; Yoshida, S.; Miyamoto, T.; Yoshimoto, G.; Watanabe, T.; Akashi, K.; Shultz, L.D.; Harada, M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005, 106, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Knibbe-Hollinger, J.S.; Fields, N.R.; Chaudoin, T.R.; Epstein, A.A.; Makarov, E.; Akhter, S.P.; Gorantla, S.; Bonasera, S.J.; Gendelman, H.E.; Poluektova, L.Y. Influence of age, irradiation and humanization on NSG mouse phenotypes. Biol. Open 2015, 4, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.J.; Hudson, A.W.; Hematti, P.; Gumperz, J.E. Early T Cell Activation Metrics Predict Graft-versus-Host Disease in a Humanized Mouse Model of Hematopoietic Stem Cell Transplantation. J. Immunol. 2020, 205, 272–281. [Google Scholar] [CrossRef]
- Prochazka, M.; Gaskins, H.R.; Shultz, L.D.; Leiter, E.H. The nonobese diabetic scid mouse: Model for spontaneous thymomagenesis associated with immunodeficiency. Proc. Natl. Acad. Sci. USA 1992, 89, 3290–3294. [Google Scholar] [CrossRef]
- King, M.A.; Covassin, L.; Brehm, M.A.; Racki, W.; Pearson, T.; Leif, J.; Laning, J.; Fodor, W.; Foreman, O.; Burzenski, L.; et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin. Exp. Immunol. 2009, 157, 104–118. [Google Scholar] [CrossRef]
- Abeynaike, S.A.; Huynh, T.R.; Mehmood, A.; Kim, T.; Frank, K.; Gao, K.; Zalfa, C.; Gandarilla, A.; Shultz, L.; Paust, S. Human Hematopoietic Stem Cell Engrafted IL-15 Transgenic NSG Mice Support Robust NK Cell Responses and Sustained HIV-1 Infection. Viruses 2023, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Rettenmier, C.W.; Roussel, M.F. Macrophage colony-stimulating factor, CSF-1, and its proto-oncogene-encoded receptor. Cold Spring Harb. Symp. Quant. Biol. 1988, 53 Pt 1, 521–530. [Google Scholar] [CrossRef]
- Rathinam, C.; Poueymirou, W.T.; Rojas, J.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Rongvaux, A.; Eynon, E.E.; Manz, M.G.; Flavell, R.A. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood 2011, 118, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Traver, D.; Akashi, K.; Manz, M.; Merad, M.; Miyamoto, T.; Engleman, E.G.; Weissman, I.L. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science 2000, 290, 2152–2154. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Esterházy, D.; Loschko, J.; London, M.; Jove, V.; Oliveira, T.Y.; Mucida, D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol. 2016, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen-Kerkhoff, N.; Lundberg, K.; Westers, T.M.; Kordasti, S.; Bontkes, H.J.; Lindstedt, M.; de Gruijl, T.D.; van de Loosdrecht, A.A. Human Bone Marrow-Derived Myeloid Dendritic Cells Show an Immature Transcriptional and Functional Profile Compared to Their Peripheral Blood Counterparts and Separate from Slan+ Non-Classical Monocytes. Front. Immunol. 2018, 9, 1619. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Medina-Moreno, S.; Davis, H.; Bryant, J.; Zapata, J.C. HIV Replication in Humanized IL-3/GM-CSF-Transgenic NOG Mice. Pathogens 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- McKenna, H.J.; Stocking, K.L.; Miller, R.E.; Brasel, K.; De Smedt, T.; Maraskovsky, E.; Maliszewski, C.R.; Lynch, D.H.; Smith, J.; Pulendran, B.; et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000, 95, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; von Muenchow, L.; Capoferri, G.; Heiler, S.; Alberti-Servera, L.; Rolink, H.; Engdahl, C.; Rolink, M.; Mitrovic, M.; Cvijetic, G.; et al. Accumulation of Multipotent Hematopoietic Progenitors in Peripheral Lymphoid Organs of Mice Over-expressing Interleukin-7 and Flt3-Ligand. Front. Immunol. 2018, 9, 2258. [Google Scholar] [CrossRef]
- Thordardottir, S.; Hangalapura, B.N.; Hutten, T.; Cossu, M.; Spanholtz, J.; Schaap, N.; Radstake, T.R.; van der Voort, R.; Dolstra, H. The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cells Dev. 2014, 23, 955–967. [Google Scholar] [CrossRef]
- Liu, H.; Ramachandran, I.; Gabrilovich, D.I. Regulation of plasmacytoid dendritic cell development in mice by aryl hydrocarbon receptor. Immunol. Cell Biol. 2014, 92, 200–203. [Google Scholar] [CrossRef]
- Coelho-Dos-Reis, J.G.A.; Funakoshi, R.; Huang, J.; Pereira, F.V.; Iketani, S.; Tsuji, M. Functional Human CD141+ Dendritic Cells in Human Immune System Mice. J. Infect. Dis. 2020, 221, 201–213. [Google Scholar] [CrossRef]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef]
- Egeland, T.; Steen, R.; Quarsten, H.; Gaudernack, G.; Yang, Y.C.; Thorsby, E. Myeloid differentiation of purified CD34+ cells after stimulation with recombinant human granulocyte-monocyte colony-stimulating factor (CSF), granulocyte-CSF, monocyte-CSF, and interleukin-3. Blood 1991, 78, 3192–3199. [Google Scholar] [CrossRef]
- Brugger, W.; Möcklin, W.; Heimfeld, S.; Berenson, R.J.; Mertelsmann, R.; Kanz, L. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood 1993, 81, 2579–2584. [Google Scholar] [CrossRef]
- Petzer, A.L.; Zandstra, P.W.; Piret, J.M.; Eaves, C.J. Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: Novel responses to Flt3-ligand and thrombopoietin. J. Exp. Med. 1996, 183, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Ohmizono, Y.; Sakabe, H.; Kimura, T.; Tanimukai, S.; Matsumura, T.; Miyazaki, H.; Lyman, S.D.; Sonoda, Y. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia 1997, 11, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Piacibello, W.; Fubini, L.; Sanavio, F.; Brizzi, M.F.; Severino, A.; Garetto, L.; Stacchini, A.; Pegoraro, L.; Aglietta, M. Effects of human FLT3 ligand on myeloid leukemia cell growth: Heterogeneity in response and synergy with other hematopoietic growth factors. Blood 1995, 86, 4105–4114. [Google Scholar] [CrossRef]
- Latinovic, O.S.; Neal, L.M.; Tagaya, Y.; Heredia, A.; Medina-Moreno, S.; Zapata, J.C.; Reitz, M.; Bryant, J.; Redfield, R.R. Suppression of Active HIV-1 Infection in CD34(+) Hematopoietic Humanized NSG Mice by a Combination of Combined Antiretroviral Therapy and CCR5 Targeting Drugs. AIDS Res. Hum. Retrovir. 2019, 35, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Kondas, A.V.; Ritter, J.M.; Reed, Z.; Ostergaard, S.D.; Morgan, C.N.; Gallardo-Romero, N.; Tansey, C.; Mauldin, M.R.; Salzer, J.S.; et al. Teaching a new mouse old tricks: Humanized mice as an infection model for Variola virus. PLoS Pathog. 2021, 17, e1009633. [Google Scholar] [CrossRef] [PubMed]
- Bility, M.T.; Cheng, L.; Zhang, Z.; Luan, Y.; Li, F.; Chi, L.; Zhang, L.; Tu, Z.; Gao, Y.; Fu, Y.; et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: Induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014, 10, e1004032. [Google Scholar] [CrossRef]
- Zheng, Z.; Sze, C.W.; Keng, C.T.; Al-Haddawi, M.; Liu, M.; Tan, S.Y.; Kwek, H.L.; Her, Z.; Chan, X.Y.; Barnwal, B.; et al. Hepatitis C virus mediated chronic inflammation and tumorigenesis in the humanised immune system and liver mouse model. PLoS ONE 2017, 12, e0184127. [Google Scholar] [CrossRef]
- Tomić, A.; Varanasi, P.R.; Golemac, M.; Malić, S.; Riese, P.; Borst, E.M.; Mischak-Weissinger, E.; Guzmán, C.A.; Krmpotić, A.; Jonjić, S.; et al. Activation of Innate and Adaptive Immunity by a Recombinant Human Cytomegalovirus Strain Expressing an NKG2D Ligand. PLoS Pathog. 2016, 12, e1006015. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, J.L.; Ji, M.X.; Tian, D.; Wang, L.Y.; Chen, C.; Tian, M. Attenuated P. falciparum Parasite Shows Cytokine Variations in Humanized Mice. Front. Immunol. 2020, 11, 1801. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Medina-Moreno, S.; Davis, H.; Bryant, J.; Taborda, N.A.; Rugeles, M.T.; Kottilil, S.; Zapata, J.C. High activation and skewed T cell differentiation are associated with low IL-17A levels in a hu-PBL-NSG-SGM3 mouse model of HIV infection. Clin. Exp. Immunol. 2020, 200, 185–198. [Google Scholar] [CrossRef]
- Spengler, J.R.; Saturday, G.; Lavender, K.J.; Martellaro, C.; Keck, J.G.; Nichol, S.T.; Spiropoulou, C.F.; Feldmann, H.; Prescott, J. Severity of Disease in Humanized Mice Infected With Ebola Virus or Reston Virus Is Associated With Magnitude of Early Viral Replication in Liver. J. Infect. Dis. 2017, 217, 58–63. [Google Scholar] [CrossRef]
- Jangalwe, S.; Shultz, L.D.; Mathew, A.; Brehm, M.A. Improved B cell development in humanized NOD-scid IL2Rγ(null) mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016, 4, 427–440. [Google Scholar] [CrossRef]
- Hung, S.; Kasperkowitz, A.; Kurz, F.; Dreher, L.; Diessner, J.; Ibrahim, E.S.; Schwarz, S.; Ohlsen, K.; Hertlein, T. Next-generation humanized NSG-SGM3 mice are highly susceptible to Staphylococcus aureus infection. Front. Immunol. 2023, 14, 1127709. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Wanek, A.; Eddens, T.; Volden, P.; Kolls, J.K. Toward a humanized mouse model of Pneumocystis pneumonia. JCI Insight 2021, 6, e139573. [Google Scholar] [CrossRef]
- Yamauchi, T.; Takenaka, K.; Urata, S.; Shima, T.; Kikushige, Y.; Tokuyama, T.; Iwamoto, C.; Nishihara, M.; Iwasaki, H.; Miyamoto, T.; et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 2013, 121, 1316–1325. [Google Scholar] [CrossRef]
- Jinnouchi, F.; Yamauchi, T.; Yurino, A.; Nunomura, T.; Nakano, M.; Iwamoto, C.; Obara, T.; Miyawaki, K.; Kikushige, Y.; Kato, K.; et al. A human SIRPA knock-in xenograft mouse model to study human hematopoietic and cancer stem cells. Blood 2020, 135, 1661–1672. [Google Scholar] [CrossRef]
- Legrand, N.; Ploss, A.; Balling, R.; Becker, P.D.; Borsotti, C.; Brezillon, N.; Debarry, J.; de Jong, Y.; Deng, H.; Di Santo, J.P.; et al. Humanized mice for modeling human infectious disease: Challenges, progress, and outlook. Cell Host Microbe 2009, 6, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Jacquelin, S.; Licata, F.; Dorgham, K.; Hermand, P.; Poupel, L.; Guyon, E.; Deterre, P.; Hume, D.A.; Combadière, C.; Boissonnas, A. CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice. Blood 2013, 122, 674–683. [Google Scholar] [CrossRef]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The role of platelets in the pathogenesis of viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, Y.G. Full reconstitution of human platelets in humanized mice after macrophage depletion. Blood 2012, 120, 1713–1716. [Google Scholar] [CrossRef]
- Iemura, A.; Tsai, M.; Ando, A.; Wershil, B.K.; Galli, S.J. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am. J. Pathol. 1994, 144, 321–328. [Google Scholar] [PubMed]
- Dastych, J.; Metcalfe, D.D. Stem cell factor induces mast cell adhesion to fibronectin. J. Immunol. 1994, 152, 213–219. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, A.J.; Junkins, R.D.; Wu, Z.; Lin, T.J. Stem cell factor induces AP-1-dependent mast cell IL-6 production via MAPK kinase 3 activity. J. Leukoc. Biol. 2014, 95, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Meininger, C.J.; Yano, H.; Rottapel, R.; Bernstein, A.; Zsebo, K.M.; Zetter, B.R. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood 1992, 79, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsumoto, K.; Okayama, Y.; Sakai, K.; Maeno, T.; Suga, T.; Miura, T.; Takai, S.; Kurabayashi, M.; Saito, H. Interleukin-3 does not affect the differentiation of mast cells derived from human bone marrow progenitors. Immunol. Investig. 2008, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.S.; Ekoff, M.; Grootens, J.; Löf, L.; Amini, R.M.; Hagberg, H.; Ungerstedt, J.S.; Olsson-Strömberg, U.; Nilsson, G. KIT signaling is dispensable for human mast cell progenitor development. Blood 2017, 130, 1785–1794. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Pérez-Rodríguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef]
- Mencarelli, A.; Gunawan, M.; Yong, K.S.M.; Bist, P.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Huang, E.K.; Fan, Y.; Chan, J.K.Y.; et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J. Leukoc. Biol. 2020, 107, 797–807. [Google Scholar] [CrossRef]
- Ito, R.; Takahashi, T.; Katano, I.; Kawai, K.; Kamisako, T.; Ogura, T.; Ida-Tanaka, M.; Suemizu, H.; Nunomura, S.; Ra, C.; et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J. Immunol. 2013, 191, 2890–2899. [Google Scholar] [CrossRef]
- Weigmann, B.; Schughart, N.; Wiebe, C.; Sudowe, S.; Lehr, H.A.; Jonuleit, H.; Vogel, L.; Becker, C.; Neurath, M.F.; Grabbe, S.; et al. Allergen-induced IgE-dependent gut inflammation in a human PBMC-engrafted murine model of allergy. J. Allergy Clin. Immunol. 2012, 129, 1126–1135. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lepus, C.M.; Raghu, H.; Reber, L.L.; Tsai, M.M.; Wong, H.H.; von Kaeppler, E.; Lingampalli, N.; Bloom, M.S.; Hu, N.; et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. Elife 2019, 8, e39905. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, T.; Nakatani, T.; Uda, K.; Ogura, H.; Shin, W.; Kurokawa, N.; Saito, K.; Fujikawa, N.; Date, T.; Takasaki, M.; et al. A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations. Blood 2018, 131, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Kappel, L.W.; Goldberg, G.L.; King, C.G.; Suh, D.Y.; Smith, O.M.; Ligh, C.; Holland, A.M.; Grubin, J.; Mark, N.M.; Liu, C.; et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood 2009, 113, 945–952. [Google Scholar] [CrossRef]
- Radojcic, V.; Pletneva, M.A.; Yen, H.R.; Ivcevic, S.; Panoskaltsis-Mortari, A.; Gilliam, A.C.; Drake, C.G.; Blazar, B.R.; Luznik, L. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J. Immunol. 2010, 184, 764–774. [Google Scholar] [CrossRef]
- Haring, E.; Zeiser, R.; Apostolova, P. Interfering With Inflammation: Heterogeneous Effects of Interferons in Graft-Versus-Host Disease of the Gastrointestinal Tract and Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 705342. [Google Scholar] [CrossRef]
- Saito, A.; Ichimura, Y.; Kubota, N.; Tanaka, R.; Nakamura, Y.; Fujisawa, Y.; Watanabe, R.; Ishitsuka, Y.; Fujimoto, M.; Okiyama, N. IFN-γ-Stimulated Apoptotic Keratinocytes Promote Sclerodermatous Changes in Chronic Graft-Versus-Host Disease. J. Investig. Dermatol. 2021, 141, 1473–1481.e1474. [Google Scholar] [CrossRef]
- Kennedy, G.A.; Varelias, A.; Vuckovic, S.; Le Texier, L.; Gartlan, K.H.; Zhang, P.; Thomas, G.; Anderson, L.; Boyle, G.; Cloonan, N.; et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014, 15, 1451–1459. [Google Scholar] [CrossRef]
- Burkett, P.R.; Meyer zu Horste, G.; Kuchroo, V.K. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J. Clin. Investig. 2015, 125, 2211–2219. [Google Scholar] [CrossRef]
- Gagari, E.; Tsai, M.; Lantz, C.S.; Fox, L.G.; Galli, S.J. Differential release of mast cell interleukin-6 via c-kit. Blood 1997, 89, 2654–2663. [Google Scholar] [CrossRef]
- Ustun, C.; DeFor, T.E.; Karadag, F.K.; Don Yun, H.; Nathan, S.; Brunstein, C.G.; Blazar, B.R.; Weisdorf, D.J.; Holtan, S.G.; Amin, K. Tissue mast cell counts may be associated with decreased severity of gastrointestinal acute GVHD and nonrelapse mortality. Blood Adv. 2020, 4, 2317–2324. [Google Scholar] [CrossRef] [PubMed]


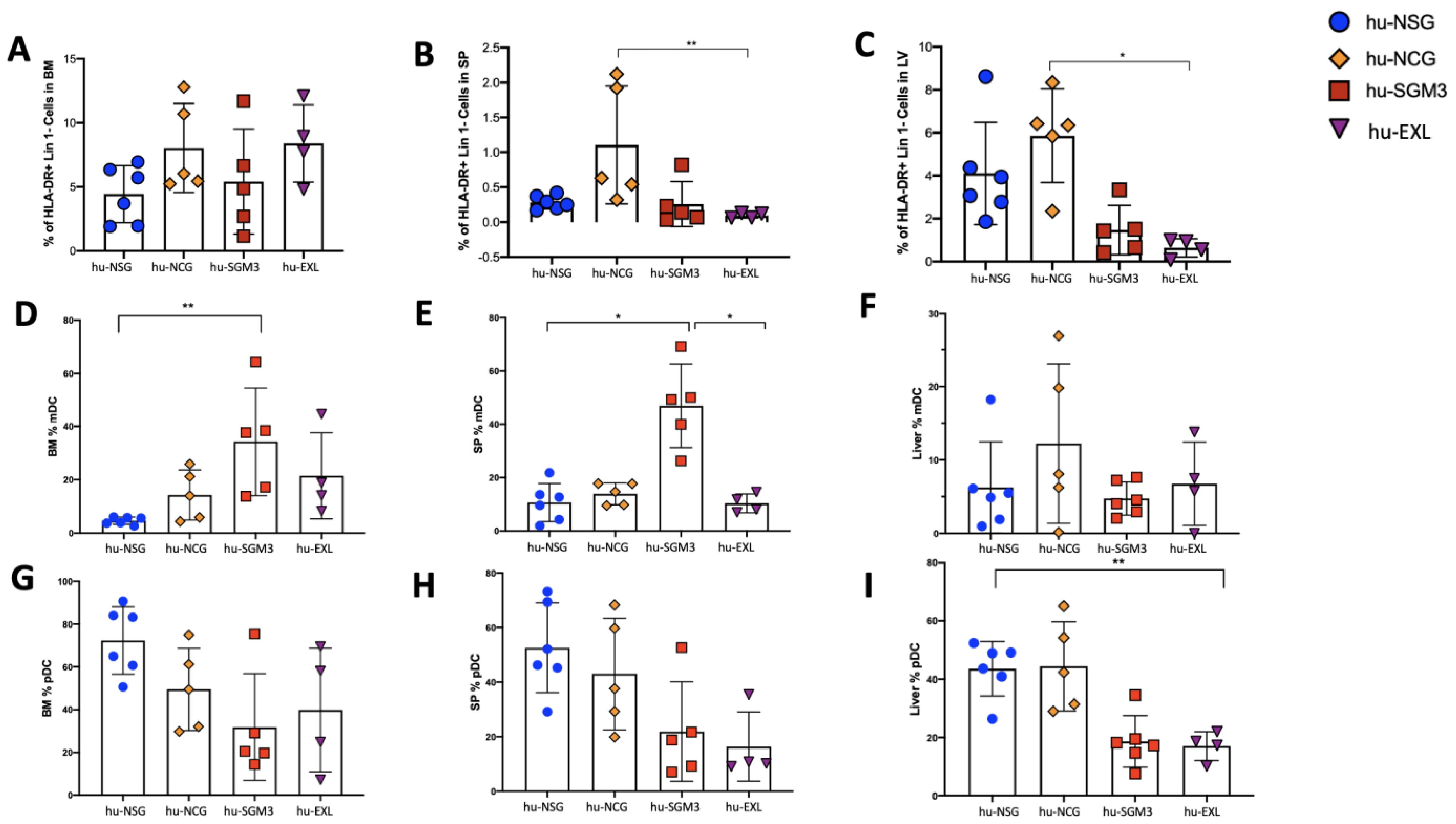



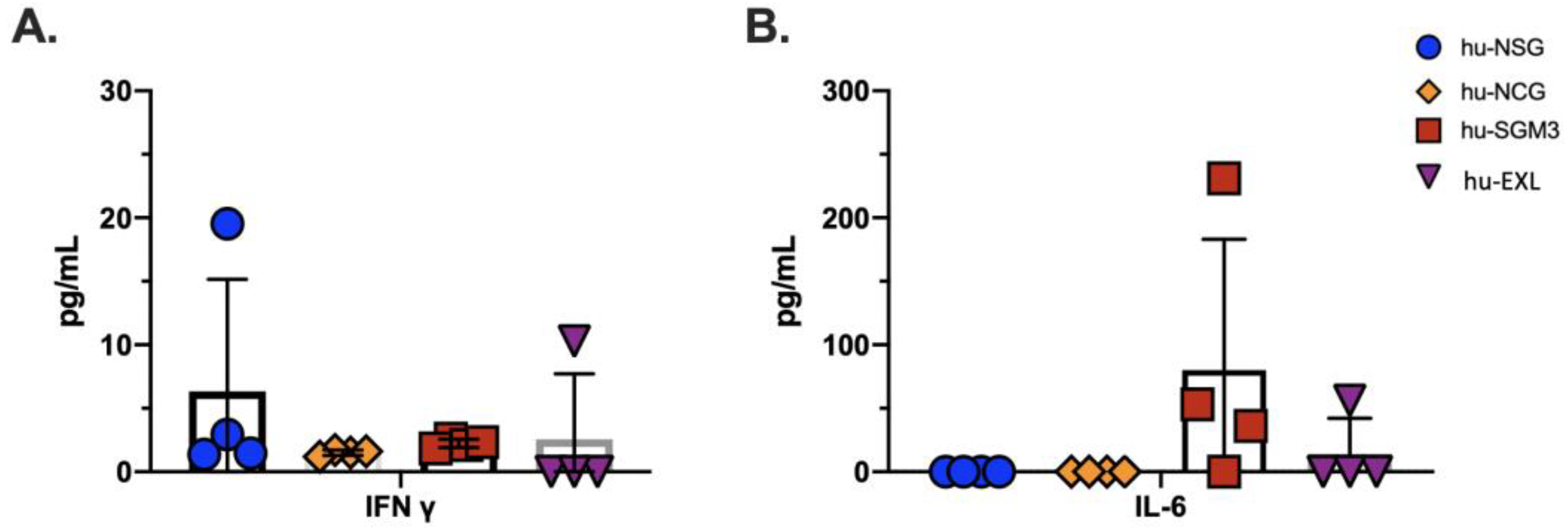
| Cytokine | hu-NSG | hu-NCG | hu-SGM3 | hu-EXL |
|---|---|---|---|---|
| TNF-α | − | − | − | − |
| IFN-α | − | − | − | − |
| IFN-γ | + | + | + | + |
| IL-6 | − | − | + | + |
| IL12p70 | − | − | − | − |
| IL10 | − | − | − | − |
| IP10 | − | − | − | − |
| Model | Advantages | Limitations | Infections Models |
|---|---|---|---|
| hu-NSG |
BM: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes SP: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes |
| DENV [18], HIV [14,45], Variola virus [46], HBV [47], HCV [48], CMV [49], P. falciparum [50] |
| hu-NCG |
BM: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes SP: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes |
| NA |
| hu-SGM3 |
BM: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes, mast cells SP: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes, mast cells |
| HIV [51], Ebola [52], DENV [53], Staphylococcus aureus [54] |
| hu-EXL |
BM: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes, mast cells SP: CD45 lymphocytes, CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD41+ megakaryocytes, mast cells |
| HIV [33], Pneumocystis [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Barbosa, H.; Medina-Moreno, S.; Perdomo-Celis, F.; Davis, H.; Coronel-Ruiz, C.; Zapata, J.C.; Chua, J.V. A Comparison of Lymphoid and Myeloid Cells Derived from Human Hematopoietic Stem Cells Xenografted into NOD-Derived Mouse Strains. Microorganisms 2023, 11, 1548. https://doi.org/10.3390/microorganisms11061548
Gutierrez-Barbosa H, Medina-Moreno S, Perdomo-Celis F, Davis H, Coronel-Ruiz C, Zapata JC, Chua JV. A Comparison of Lymphoid and Myeloid Cells Derived from Human Hematopoietic Stem Cells Xenografted into NOD-Derived Mouse Strains. Microorganisms. 2023; 11(6):1548. https://doi.org/10.3390/microorganisms11061548
Chicago/Turabian StyleGutierrez-Barbosa, Hernando, Sandra Medina-Moreno, Federico Perdomo-Celis, Harry Davis, Carolina Coronel-Ruiz, Juan C. Zapata, and Joel V. Chua. 2023. "A Comparison of Lymphoid and Myeloid Cells Derived from Human Hematopoietic Stem Cells Xenografted into NOD-Derived Mouse Strains" Microorganisms 11, no. 6: 1548. https://doi.org/10.3390/microorganisms11061548
APA StyleGutierrez-Barbosa, H., Medina-Moreno, S., Perdomo-Celis, F., Davis, H., Coronel-Ruiz, C., Zapata, J. C., & Chua, J. V. (2023). A Comparison of Lymphoid and Myeloid Cells Derived from Human Hematopoietic Stem Cells Xenografted into NOD-Derived Mouse Strains. Microorganisms, 11(6), 1548. https://doi.org/10.3390/microorganisms11061548






