Characterization of the Bacterial Communities in Cichorium intybus According to Cultivation and Storage Conditions
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Metagenomic DNA Extraction from Chicory Leaves
2.3. Bacterial 16S rRNA Gene Amplification and MiSeq Sequencing
2.4. Microbiota Profiling and Diversity Analysis
2.5. Total Bacterial and Potential Pathogenic Bacterial Quantification Using Quantitative Real-Time PCR (qRT-PCR)
2.6. Artificial Contamination of Enterohemorrhagic E. coli (EHEC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Bacterial Loads and Diversity Indices on Chicory Leaves among Sampling Times and Sites
3.2. Microbiota Composition of Chicory Leaves by Principal Coordinate Analysis (PCoA)
3.3. Comparison of Microbiota at the Phylum, Order, Family, and Genus Levels
3.4. Quantification of Potential Pathogenic Species on Chicory Leaves
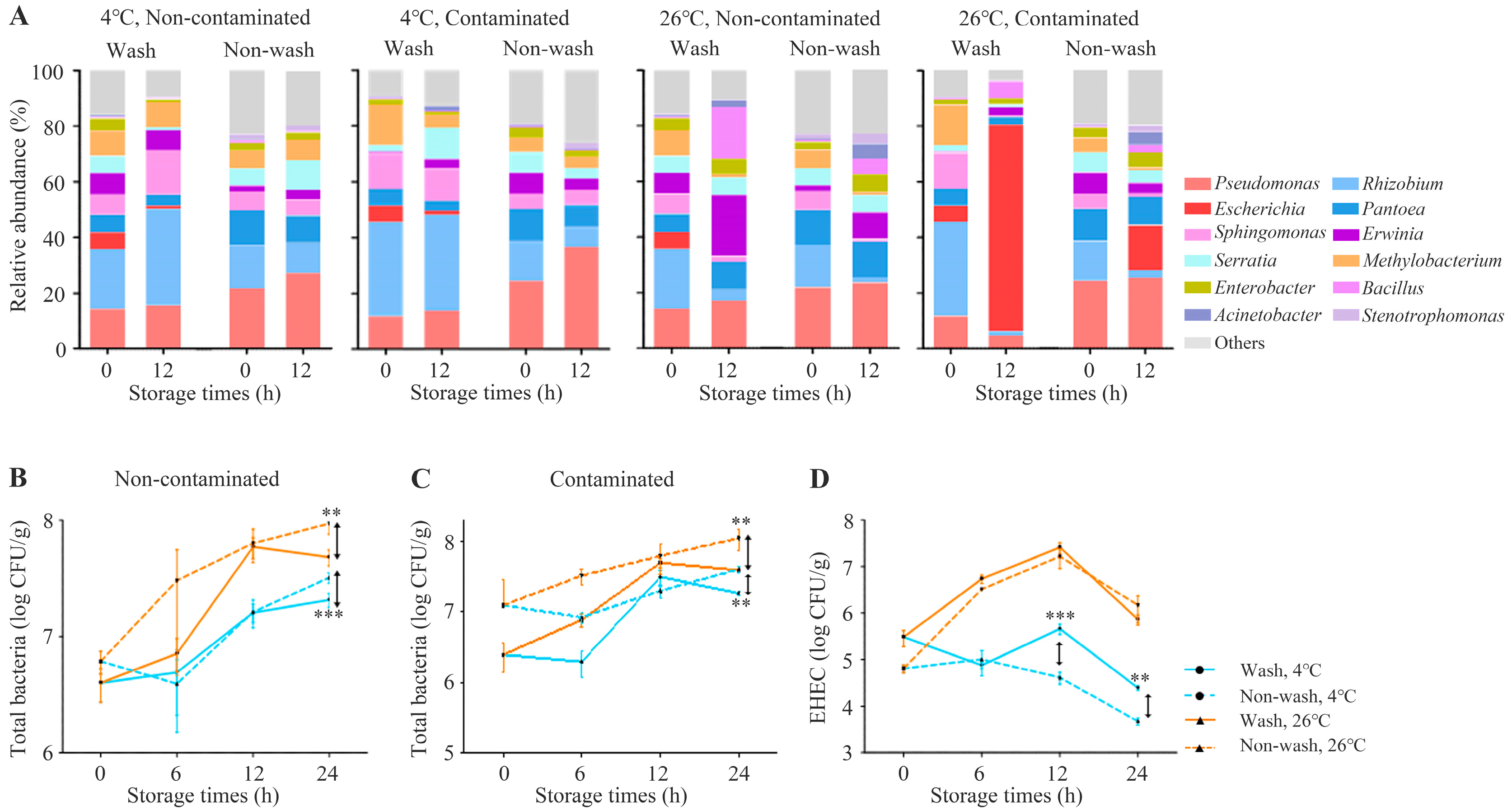
3.5. A Shift in Chicory Leaf Microbiota following Artificial Infection at Various Storage Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeon, D.-Y.; Yum, S.-J.; Seo, D.W.; Kim, S.M.; Jeong, H.G. Leaf-associated microbiota on perilla (Perilla frutescens var. frutescens) cultivated in South Korea to detect the potential risk of food poisoning. Food Res. Int. 2019, 126, 108664. [Google Scholar] [CrossRef]
- Seo, D.W.; Yum, S.-j.; Lee, H.R.; Kim, S.M.; Jeong, H.G. Microbiota Analysis and Microbiological Hazard Assessment in Chinese Chive (Allium tuberosum Rottler) Depending on Retail Types. J. Microbiol. Biotechnol. 2022, 32, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today’s world. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Okoh, A.I. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taban, B.M.; Halkman, A.K. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe 2011, 17, 286–287. [Google Scholar] [CrossRef]
- Kim, B.-R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.-H.; Lee, J.-H.; Kim, H.B.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [Green Version]
- Escobar-Zepeda, A.; Sanchez-Flores, A.; Baruch, M.Q. Metagenomic analysis of a Mexican ripened cheese reveals a unique complex microbiota. Food Microbiol. 2016, 57, 116–127. [Google Scholar] [CrossRef]
- Cichota, R.; McAuliffe, R.; Lee, J.; Minnee, E.; Martin, K.; Brown, H.E.; Moot, D.J.; Snow, V.O. Forage chicory model: Development and evaluation. Field Crops Res. 2020, 246, 107633. [Google Scholar] [CrossRef]
- Park, C.-K.; Jeon, B.-S.; Shim, K.-H. Biological Activities of Roasted Chicory Root. Korean J. Med. Crop Sci. 2003, 11, 329–335. [Google Scholar]
- Abbas, Z.K.; Saggu, S.; Sakeran, M.I.; Zidan, N.; Rehman, H.; Ansari, A.A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2015, 22, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushparaj, P.; Low, H.; Manikandan, J.; Tan, B.; Tan, C. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007, 111, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Dolinski, R.; Olek, A. Micro propagation of wild chicory (Cichorium intybus L. Var. Silvestre Bisch.) from leaf explants. Acta Sci. Pol. Hortorum Cult. 2013, 12, 33–44. [Google Scholar]
- MacDonald, E.; Einöder-Moreno, M.; Borgen, K.; Brandal, L.T.; Diab, L.; Fossli, Ø.; Herrador, B.G.; Hassan, A.A.; Johannessen, G.S.; Johansen, E.J. National outbreak of Yersinia enterocolitica infections in military and civilian populations associated with consumption of mixed salad, Norway, 2014. Euro Surveill. 2016, 21, 30321. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, E.; Heier, B.T.; Nygård, K.; Stalheim, T.; Cudjoe, K.S.; Skjerdal, T.; Wester, A.L.; Lindstedt, B.-A.; Stavnes, T.-L.; Vold, L. Yersinia enterocolitica outbreak associated with ready-to-eat salad mix, Norway, 2011. Emerg. Infect. Dis. 2012, 18, 1496. [Google Scholar] [CrossRef]
- Mikesell, P.; Ivins, B.E.; Ristroph, J.D.; Dreier, T.M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun. 1983, 39, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Felske, A.; Engelen, B.; Nübel, U.; Backhaus, H. Direct ribosome isolation from soil to extract bacterial rRNA for community analysis. App. Environ. Microbiol. 1996, 62, 4162–4167. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Brooker, M.R.; Dowd, S.E.; Camerlengo, T. Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS ONE 2011, 6, e20956. [Google Scholar] [CrossRef]
- Hanshew, A.S.; Mason, C.J.; Raffa, K.F.; Currie, C.R. Minimization of chloroplast contamination in 16S rRNA gene pyrosequencing of insect herbivore bacterial communities. J. Microbiol. Methods 2013, 95, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-H.; Song, K.B. Antimicrobial activity of honeybush (Cyclopia intermedia) ethanol extract against foodborne pathogens and its application in washing fresh-cut Swiss chard. Food Control 2021, 121, 107674. [Google Scholar] [CrossRef]
- Ingraham, J. Growth of psychrophilic bacteria. J. Bacteriol. 1958, 76, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-C.; Yum, S.-J.; Jeon, D.-Y.; Jeong, H.-G. Analysis of the microbiota on lettuce (Lactuca sativa L.) cultivated in South Korea to identify foodborne pathogens. J. Microbiol. Biotechnol. 2018, 28, 1318–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazali, N.S.H.; Rashid, N. Molecular identification of bacterial communities from vegetables samples as revealed by DNA sequencing of universal primer 16S rRNA gene. Int. J. Med. Sci. 2019, 4, 19–26. [Google Scholar]
- Li, C.-h.; Tang, L.-s.; Jia, Z.-j.; Li, Y. Profile changes in the soil microbial community when desert becomes oasis. PLoS ONE 2015, 10, e0139626. [Google Scholar] [CrossRef] [Green Version]
- Sylla, J.; Alsanius, B.W.; Krüger, E.; Reineke, A.; Bischoff-Schaefer, M.; Wohanka, W. Introduction of Aureobasidium pullulans to the phyllosphere of organically grown strawberries with focus on its establishment and interactions with the resident microbiome. Agronomy 2013, 3, 704–731. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Presence and survival of Escherichia coli O157: H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int. J. Food Microbiol. 2012, 156, 133–140. [Google Scholar] [CrossRef]
- Becker, B.; Weiss, C.; Holzapfel, W.H. An evaluation of the use of three phenotypic test-systems for biochemical identification of Enterobacteriaceae and Pseudomonadaceae. Food Control 2009, 20, 815–821. [Google Scholar] [CrossRef]
- Miteva, V.; Sheridan, P.; Brenchley, J. Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl. Environ. Microbiol. 2004, 70, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Zhang, S.; Gao, H.; Hu, N. Characterization of a cold-active esterase from Serratia sp. and improvement of thermostability by directed evolution. BMC Biotechnol. 2016, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, P.; Moreno, R.; Rojo, F. Growth of Pseudomonas putida at low temperature: Global transcriptomic and proteomic analyses. Environ. Microbiol. Rep. 2011, 3, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, E.; Wang, L.; Arakawa, M.; Yano, I. Survival of Pseudomonas pseudomallei strains at 5 °C. Kansenshogaku Zasshi. J. Jpn. Assoc. Infect. Dis. 1993, 67, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Moxley, R.A. Veterinary Microbiology, 40th ed.; McVey, D.S., Kennedy, M., Eds.; Elservier: Amsterdam, The Netherlands, 2022; Chapter 5; pp. 41–55. [Google Scholar]
- Stewart, G.C. Veterinary Microbiology; McVey, D.S., Kennedy, M., Eds.; Elservier: Amsterdam, The Netherlands, 2022; Chapter 28; pp. 257–264. [Google Scholar]
- Edelstein, M.; Sundborger, C.; Hergens, M.-P.; Ivarsson, S.; Dryselius, R.; Insulander, M.; Jernberg, C.; Hutin, Y.; Wallensten, A. Barriers to trace-back in a salad-associated EHEC outbreak, Sweden, June 2013. PLoS Curr. 2014, 6, 1–20. [Google Scholar] [CrossRef]
- Mellmann, A.; Bielaszewska, M.; Köck, R.; Friedrich, A.W.; Fruth, A.; Middendorf, B.; Harmsen, D.; Schmidt, M.A.; Karch, H. Analysis of collection of hemolytic uremic syndrome–associated enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 2008, 14, 1287. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef]
- Iguchi, A.; Nagaya, Y.; Pradel, E.; Ooka, T.; Ogura, Y.; Katsura, K.; Kurokawa, K.; Oshima, K.; Hattori, M.; Parkhill, J. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol. Evol. 2014, 6, 2096–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhao, Y.; Liu, C.; Chen, Z.; Zhou, D. Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2014, 9, 1071–1081. [Google Scholar] [CrossRef]
- Davies, Y.M.; Cunha, M.P.V.; Oliveira, M.; Oliveira, M.; Philadelpho, N.; Romero, D.; Milanelo, L.; Guimarães, M.B.; Ferreira, A.J.P.; Moreno, A.M. Virulence and antimicrobial resistance of Klebsiella pneumoniae isolated from passerine and psittacine birds. Avian Pathol. 2016, 45, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crippen, C.S.; Jr, M.J.R.; Sanchez, S.; Szymanski, C.M. Multidrug resistant Acinetobacter isolates release resistance determinants through contact-dependent killing and bacteriophage lysis. Front. Microbiol. 2020, 11, 1918. [Google Scholar] [CrossRef]
- Robinson, R.K. Encyclopedia of Food Microbiology; Academic Press: Illinois, IL, USA, 2014; pp. 3–76. [Google Scholar]
- Abdelfattah, A.; Whitehead, S.R.; Macarisin, D.; Liu, J.; Burchard, E.; Freilich, S.; Dardick, C.; Droby, S.; Wisniewski, M. Effect of washing, waxing and low-temperature storage on the postharvest microbiome of apple. Microorganisms 2020, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Itoh, K. Prediction of microbial growth in fresh-cut vegetables treated with acidic electrolyzed water during storage under various temperature conditions. J. Food Prot. 2001, 64, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Liu, H. Nutrient-imbalanced conditions shift the interplay between zooplankton and gut microbiota. BMC Genom. 2021, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [Green Version]
- Macieira, A.; Barbosa, J.; Teixeira, P. Food safety in local farming of fruits and vegetables. Int. J. Environ. Res. Public Health 2021, 18, 9733. [Google Scholar] [CrossRef]




| Sampling Information | Normalized Reads | Total Read/Sample | Total Bacteria (Average log CFU/g) | α-Diversity Indices | ||
|---|---|---|---|---|---|---|
| Chao1 | Observed OTUs | Shannon | ||||
| Spring | 18,840 | 41,494 | 6.78 A | 610.09 A | 379.48 B | 4.51 B |
| Summer | 34,907 | 6.11 B | 600.3 A | 431.37 A | 5.17 A | |
| Gongju | 39,105 | 6.76 a | 635.13 a | 418.72 a | 5.18 a | |
| Busan | 37,296 | 6.19 b | 575.26 a | 392.12 b | 4.51 b | |
| Sampling Times | Bacterial Loads of Pathogenic Bacteria (CFU/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| EHEC | EPEC | ETEC | K. pneumoniae | B. cereus | S. marcescens | S. aureus | A. lwoffii | |
| Spring | 6.59 × 102 (5.00% *) | 3.28 × 102 (7.50%) | N.D. ** | N.D. | 5.12 × 103 (2.50%) | N.D. | N.D. | N.D. |
| Summer | N.D. | N.D. | N.D. | 7.36 × 102 (8.75%) | N.D. | 2.57 × 103 (1.25%) | N.D. | 1.09 × 102 (2.50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yum, S.-J.; Lee, H.-R.; Yu, S.Y.; Seo, D.W.; Kwon, J.H.; Kim, S.M.; Kim, J.H.; Jeong, H.-G. Characterization of the Bacterial Communities in Cichorium intybus According to Cultivation and Storage Conditions. Microorganisms 2023, 11, 1560. https://doi.org/10.3390/microorganisms11061560
Yum S-J, Lee H-R, Yu SY, Seo DW, Kwon JH, Kim SM, Kim JH, Jeong H-G. Characterization of the Bacterial Communities in Cichorium intybus According to Cultivation and Storage Conditions. Microorganisms. 2023; 11(6):1560. https://doi.org/10.3390/microorganisms11061560
Chicago/Turabian StyleYum, Su-Jin, Heoun-Reoul Lee, Seon Yeong Yu, Dong Woo Seo, Jun Hyeok Kwon, Seung Min Kim, Jong Hun Kim, and Hee-Gon Jeong. 2023. "Characterization of the Bacterial Communities in Cichorium intybus According to Cultivation and Storage Conditions" Microorganisms 11, no. 6: 1560. https://doi.org/10.3390/microorganisms11061560
APA StyleYum, S.-J., Lee, H.-R., Yu, S. Y., Seo, D. W., Kwon, J. H., Kim, S. M., Kim, J. H., & Jeong, H.-G. (2023). Characterization of the Bacterial Communities in Cichorium intybus According to Cultivation and Storage Conditions. Microorganisms, 11(6), 1560. https://doi.org/10.3390/microorganisms11061560






