Molecular Detection and Phylogenetic Analyses of Babesia spp. and Theileria spp. in Livestock in Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
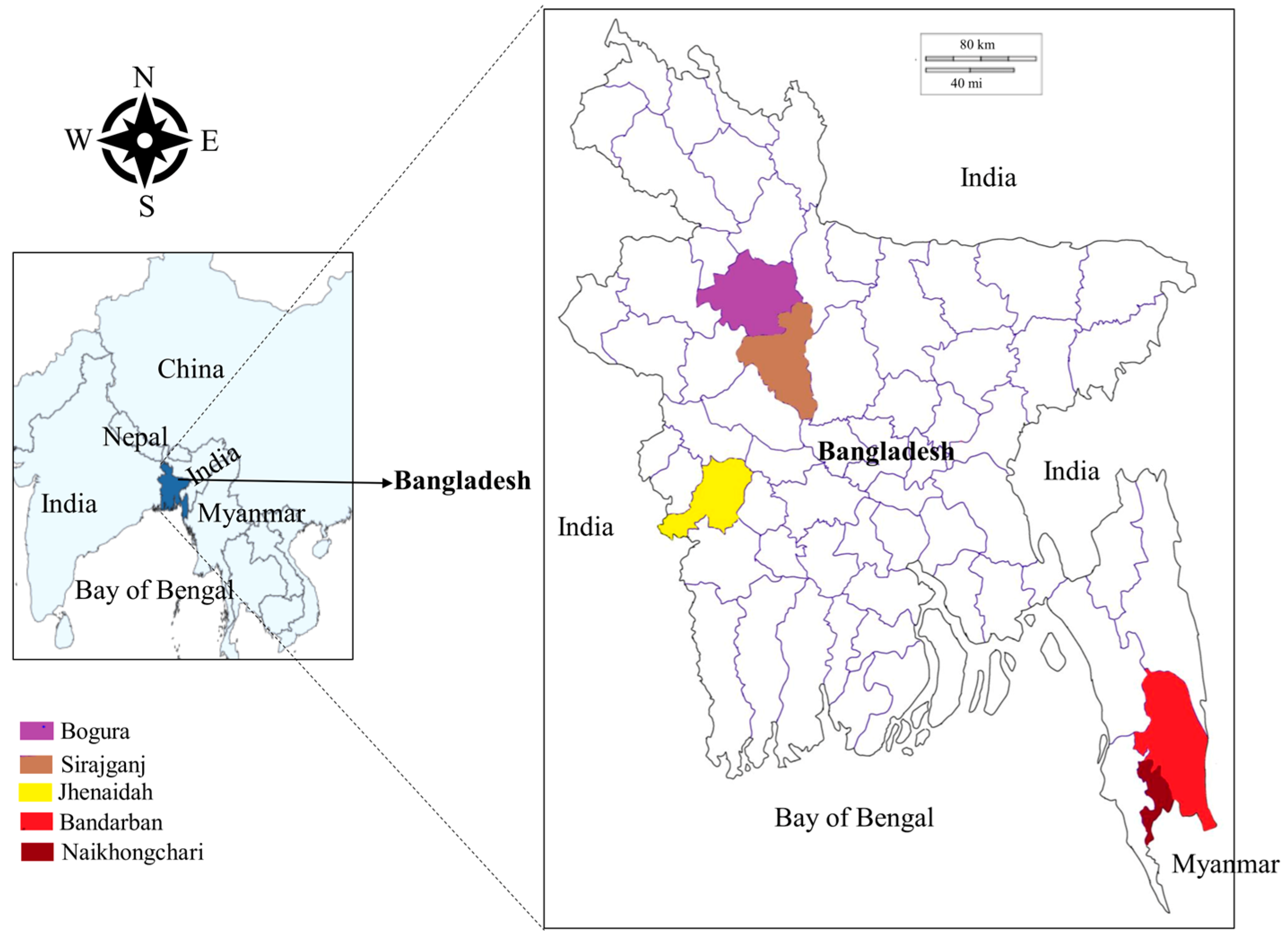
2.2. Study Sites and Sample Collection
2.3. Dried Blood Spot Preparation on FTATM Elute Micro Card and DNA Elution
2.4. Molecular Detection of Piroplasms
2.5. Sequencing of the PCR-Positive Samples
2.6. Phylogeny Construction
2.7. GenBank Accession Numbers
2.8. Statistical Analyses
3. Results
3.1. Overall Prevalence
3.2. Co-Infections with Different Piroplasms
3.3. Gene Sequence Analyses
3.4. Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uilenberg, G. International collaborative research: Significance of Tick-Borne Hemoparasitic Diseases to World Animal Health. Vet. Parasitol. 1995, 57, 19–41. [Google Scholar] [CrossRef]
- Uilenberg, G. Babesia-A Historical Overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef]
- Minjauw, B.; McLeod, A. Tick-Borne Diseases and Poverty: The Impact of Ticks and Tick-Borne Diseases on the Livelihoods of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa; DFID Animal Health Programme, Centre for Tropical Veterinary Medicine: Edinburgh, UK, 2003. [Google Scholar]
- McLeod, R.; Kristjanson, P. Economic Impact of Ticks and Tick-Borne Diseases to Livestock in Africa, Asia and Australia; International Livestock Research Institute: Nairobi, Kenya, 1999. [Google Scholar]
- Hunfeld, K.; Hildebrandt, A.; Gray, J. Babesiosis: Recent Insights into an Ancient Disease. Int. J. Parasitol. 2008, 38, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of Cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef]
- Aktaş, M.; Altay, K.; Dumanli, N. Development of a Polymerase Chain Reaction Method for Diagnosis of Babesia ovis Infection in Sheep and Goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Zhao, H.; Li, Y.; Xie, J.; Liu, A.; Hassan, M.A.; Yin, H.; Guan, G.; Luo, J. Evaluating an Indirect RMPSP Enzyme-Linked Immunosorbent Assay for the Detection of Bovine Theileria Infection in China. Parasitol. Res. 2017, 116, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-Borne Pathogens of Zoonotic and Veterinary Importance in Nigerian Cattle. Parasit. Vectors. 2016, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ringo, A.E.; Nonga, H.E.; Galon, E.M.; Ji, S.; Rizk, M.A.; El-Sayed, S.A.E.S.; Mohanta, U.K.; Ma, Z.; Chikufenji, B.; Do, T.T.; et al. Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania. Animals 2022, 12, 3171. [Google Scholar] [CrossRef] [PubMed]
- D’Oliveira, C.; van der Weide, M.; Habela, M.A.; Jacquiet, P.; Jongejan, F. Detection of Theileria annulata in Blood Samples of Carrier Cattle by PCR. J. Clin. Microbiol. 1995, 33, 2665–2669. [Google Scholar] [CrossRef] [Green Version]
- Ziam, H.; Kernif, T.; Saidani, K.; Kelanemer, R.; Hammaz, Z.; Geysen, D. Bovine Piroplasmosis-Anaplasmosis and Clinical Signs of Tropical Theileriosis in the Plains of Djurdjura (North Algeria). Vet. Med. Sci. 2020, 6, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Neitz, W. Theileriosis, Gonderioses and Cytauxzoonoses: A Review. Onderstepoort J. Vet. Res. 1957, 27, 275–430. [Google Scholar]
- Minami, T.; Fujinaga, T.; Furuya, K.; Ishihara, T. Clinico-Hematologic and Serological Comparison of Japanese and Russian Strains of Theileria sergenti. Natl. Inst. Anim. Health. Q. 1980, 20, 44–52. [Google Scholar]
- Sugimoto, C.; Fujisaki, K. Non-transforming Theileria Parasites of Ruminants. In Theileria. World Class Parasites; Dobbelaere, D.A.E., McKeever, D.J., Eds.; Springer: Boston, MA, USA, 2002; Volume 3, pp. 93–106. [Google Scholar]
- Perera, P.K.; Gasser, R.B.; Firestone, S.M.; Anderson, G.A.; Malmo, J.; Davis, G.; Beggs, D.S.; Jabbar, A. Oriental Theileriosis in Dairy Cows Causes a Significant Milk Production Loss. Parasit. Vectors. 2014, 7, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparna, M.; Ravindran, R.; Vimalkumar, M.B.; Lakshmanan, B.; Rameshkumar, P.; Kumar, K.G.A.; Promod, K.; Ajithkumar, S.; Ravishankar, C.; Devada, K.; et al. Molecular Characterization of Theileria orientalis Causing Fatal Infection in Crossbred Adult Bovines of South India. Parasitol. Int. 2011, 60, 524–529. [Google Scholar] [CrossRef]
- Patial, V.; Gupta, T.; Angaria, S.; Bali, D.; Katoch, A.; Gautam, M.; Singh, N.K.; Sharma, M.; Chahota, R. Theileria orientalis Outbreak in an Organized Cattle Breeding Farm. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100572. [Google Scholar] [CrossRef] [PubMed]
- McFadden, A.; Rawdon, T.; Meyer, J.; Makin, J.; Morley, C.; Clough, R.; Tham, K.; Müllner, P.; Geysen, D. An Outbreak of Haemolytic Anaemia Associated with Infection of Theileria orientalis in Naïve Cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef]
- Eamens, G.J.; Gonsalves, J.R.; Jenkins, C.; Collins, D.; Bailey, G. Theileria orientalis MPSP Types in Australian Cattle Herds Associated with Outbreaks of Clinical Disease and Their Association with Clinical Pathology Findings. Vet. Parasitol. 2013, 191, 209–217. [Google Scholar] [CrossRef]
- BBS (Bangladesh Bureau of Statistics). Statistical Yearbook of Bangladesh 2020, 40th ed.; Statistics & Informatics Division (SID), Ministry of Planning, Government of The People’s Republic of Bangladesh: Dhaka, Bangladesh, 2021. [Google Scholar]
- Ghosh, S.; Bansal, G.C.; Gupta, S.C.; Ray, D.; Khan, M.Q.; Irshad, H.; Shahiduzzaman, M.; Seitzer, U.; Ahmed, J.S. Status of Tick Distribution in Bangladesh, India and Pakistan. Parasitol. Res. 2007, 101 (Suppl. S2), S207–S216. [Google Scholar] [CrossRef]
- Islam, M.K.; Alim, M.A.; Tsuji, N.; Mondal, M.M.H. An Investigation into the Distribution, Host-Preference and Population Density of Ixodid Ticks Affecting Domestic Animals in Bangladesh. Trop. Anim. Health. Prod. 2006, 38, 485–490. [Google Scholar] [CrossRef]
- Kabir, M.H.B.; Mondal, M.M.H.; Eliyas, M.; Mannan, M.A.; Hashem, M.A.; Debnath, N.C.; Miazi, O.F.; Mohiuddin, C.; Kashem, M.A.; Islam, M.R.; et al. An Epidemiological Survey on Investigation of Tick Infestation in Cattle at Chittagong District, Bangladesh. Afr. J. Microbiol. Res. 2011, 5, 346–352. [Google Scholar]
- Al-Mahmud, M.A.; Belal, S.S.H.; Hossain, M.A. Prevalence of Theileriosis and Babesiosis in Cattle in Sirajganj District of Bangladesh. Res. Agric. Livest. Fish. 2015, 2, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Alim, M.A.; Das, S.; Roy, K.; Masuduzzaman, M.; Sikder, S.; Hassan, M.M.; Siddiki, A.Z.; Hossain, M.A. Prevalence of Hemoprotozoan Diseases in Cattle Population of Chittagong Division, Bangladesh. Pak. Vet. J. 2012, 32, 221–224. [Google Scholar]
- Chowdhury, S.; Hossain, M.; Barua, S.; Islam, S. Occurrence of Common Blood Parasites of Cattle in Sirajgonj Sadar Area of Bangladesh. Bang. J. Vet. Med. 2006, 4, 143–145. [Google Scholar] [CrossRef] [Green Version]
- Belal, S.S.H.; Al Mahmud, A.; Ferdous, M.J. Prevalence of Anaplasmosis in Cattle in Sirajganj District of Bangladesh. Res. Agric. Livest. Fish. 2015, 1, 97–103. [Google Scholar] [CrossRef]
- Mohanta, U.K.; Anisuzzaman; Mondal, M.M.H. Tick and Tick Borne Protozoan Diseases of Livestock in the Selected Hilly Areas of Bangladesh. Int. J. Agril. Res. Innov. Technol. 2011, 1, 60–63. [Google Scholar] [CrossRef]
- Roy, B.C.; Krücken, J.; Ahmed, J.S.; Majumder, S.; Baumann, M.P.; Clausen, P.H.; Nijhof, A.M. Molecular Identification of Tick-Borne Pathogens Infecting Cattle in Mymensingh District of Bangladesh Reveals Emerging Species of Anaplasma and Babesia. Transbound. Emerg. Dis. 2018, 65, e231–e242. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Raut, S.; Singh, R.P.; Mishra, P.; Hossain, M.S.; Dey, A.R.; Kabir, A.; Anisuzzaman; Talukder, M.H.; Shahiduzzaman, M. Molecular Detection of Babesia and Theileria from Crossbred Cattle in Sirajganj and Rangpur Districts of Bangladesh. Vet. Med. Sci. 2023, 9, 899–906. [Google Scholar] [CrossRef]
- Sivakumar, T.; Tuvshintulga, B.; Zhyldyz, A.; Kothalawala, H.; Yapa, P.R.; Kanagaratnam, R.; Vimalakumar, S.C.; Abeysekera, T.S.; Weerasingha, A.S.; Yamagishi, J.; et al. Genetic Analysis of Babesia Isolates from Cattle with Clinical Babesiosis in Sri Lanka. J. Clin. Microbiol. 2018, 56, e00895-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, N.; Mizuno, D.; Kuboki, N.; Igarashi, I.; Nakamura, Y.; Yamashina, H.; Hanzaike, T.; Fujii, K.; Onoe, S.; Hata, H.; et al. Epidemiological Survey of Theileria orientalis Infection in Grazing Cattle in the Eastern Part of Hokkaido, Japan. J. Vet. Med. Sci. 2009, 71, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Jittapalapong, S.; et al. Molecular and Serological Prevalence of Babesia bovis and Babesia bigemina in Water Buffaloes in the Northeast Region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Quddus, M.A. Crop Production Growth in Different Agro-Ecological Zones of Bangladesh. J. Bang. Agril. Univ. 2009, 7, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Faruque, M.; Rahaman, M.; Hoque, M.; Ikeya, K.; Amano, T.; Han, J.; Dorji, T.; Omar, A. Present Status of Gayal (Bos frontalis) in the Home Tract of Bangladesh. Bang. J. Anim. Sci. 2015, 44, 75–84. [Google Scholar] [CrossRef]
- Rizk, M.A.; Salama, A.; El-Sayed, S.A.E.S.; Elsify, A.; El-Ashkar, M.; Ibrahim, H.; Youssef, M.; El-Khodery, S. Animal Level Risk Factors Associated with Babesia and Theileria Infections in Cattle in Egypt. Acta Parasitol. 2017, 62, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Fitzgerald, E.; Strickland, K.L. Prevalence of Clinical Babesiosis in an Area in North Co Meath, Ireland. Vet. Rec. 1983, 113, 537–539. [Google Scholar] [PubMed]
- Christensson, D.A.; Thorburn, M.A. Age Distribution of Naturally Occurring Acute Babesiosis in Cattle in Sweden. Acta Vet. Scand. 1987, 28, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a Bovine Blood Parasite of Veterinary and Zoonotic Importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [Green Version]
- Fuehrer, H.P.; Igel, P.; Treiber, M.; Baumann, T.A.; Riedl, J.; Swoboda, P.; Joachim, A.; Noedl, H. Ectoparasites of Livestock, Dogs, and Wild Rodents in the Chittagong Hill Tracts in Southeastern Bangladesh. Parasitol. Res. 2012, 111, 1867–1870. [Google Scholar] [CrossRef]




| Target Gene | Assays | Primer Sequences Forward 5′ → 3′ Reverse | Annealing Temp. (°C) | References | |
|---|---|---|---|---|---|
| B. bigemina (BbigRAP-1a) | PCR | GAGTCTGCCAAATCCTTAC | TCCTCTACAGCTGCTTCG | 55 | [34] |
| nPCR | AGCTTGCTTTCACAACTCGCC | TTGGTGCTTTGACCGACGACAT | 50 | ||
| B. bovis (BboSBP-4) | PCR | AGTTGTTGGAGGAGGCTAAT | TCCTTCTCGGCGTCCTTTTC | 55 | [34] |
| nPCR | GAAATCCCTGTTCCAGAG | TCGTTGATAACACTGCAA | 50 | ||
| B. naoakii (AMA-1) | PCR | TGGCGCCGACTTCCTGGAGCCCATCTCCAA | AGCTGGGGCCCTCCTTCGATGAACCGTCGG | 64 | [32] |
| B. ovis (ssu rRNA) | PCR | TGGGCAGGACCTTGGTTCTTCT | CCGCGTAGCGCCGGCTAAATA | 62 | [7] |
| T. annulata (Tams-1) | PCR | GTAACCTTTAAAAACGT | GTTACGAACATGGGTTT | 54 | [11] |
| nPCR | CACCTCAACATACCCC | TGACCCACTTATCGTCC | 54 | ||
| T. orientalis (MPSP) | PCR | CTTTGCCTAGGATACTTCCT | ACGGCAAGTGGTGAGAACT | 58 | [33] |
| Pathogens | Locations | Total n = 276 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Jhenaidah n = 29 | Bogura n = 14 | Sirajganj n = 107 | Bandarban n = 105 | Naikhongchari n = 21 | |||
| B. bigemina | 9 (31.03%) | 8 (57.14%) | 66 (61.68%) | 51 (48.57%) | 2 (9.52%) | 136 (49.28%) | <0.001 |
| B. bovis | n.d. | n.d. | 1 (0.93%) | 1 (0.95%) | n.d. | 2 (0.72%) | NA |
| B. naoakii | n.d. | n.d. | 3 (2.80%) | n.d. | n.d. | 3 (1.09%) | NA |
| B. ovis * | 6 (27.27%) | n.d. | 23 (37.09%) | 1 (14.29%) | n.d. | 30 (32.26%) | NA |
| T. annulata | n.d. | n.d. | n.d. | 4 (3.81%) | 14 (66.67%) | 18 (6.52%) | NA |
| T. orientalis | 7 (24.13%) | 7 (50%) | 32 (29.90%) | 72 (68.57%) | 9 (42.86%) | 127 (46.01%) | <0.001 |
| Pathogens | Animal Species | Total (n = 276) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cattle (n = 174) | Gayals (n = 9) | Goats (n = 93) | ||||||||
| <2 yrs (n = 64) | ≥2 yrs (n = 110) | Sub Total | <2 yrs (n = 5) | ≥2 yrs (n = 4) | Sub Total | <2 yrs (n = 63) | ≥2 yrs (n = 30) | Sub Total | ||
| B. bigemina | 40 (22.99%) | 55 (50.00%) | 95 (54.60%) * | 1 (20.00%) | n.d. | 1 (11.11%) * | 25 (39.68%) | 15 (50.00%) | 40 (43.01%) * | 136 (49.28%) |
| B. bovis | 1 (0.57%) | n.d. | 1 (0.57%) | n.d. | n.d. | n.d. | 1 (1.59%) | n.d. | 1 (1.08%) | 2 (0.72%) |
| B. naoakii | 1 (0.57%) | n.d. | 1 (0.57%) | n.d. | n.d. | n.d. | n.d. | 2 (6.67%) | 2 (2.15%) | 3 (1.09%) |
| B. ovis † | n.s. | n.s. | n.s. | n.d. | n.s. | n.s. | 24 (38.10%) | 6 (20.00%) | 30 (32.26%) | 30 (32.26%) |
| T. annulata | 4 (2.30) | 5 (4.55%) | 9 (5.17%) | 5 (100.00%) | 2 (50.00%) | 7 (77.78%) | 1 (1.59%) | 1 (3.33%) | 2 (2.15%) | 18 (6.52%) |
| T. orientalis | 46 (26.44%) | 71 (64.55%) | 117 (67.24%) ** | 3 (60.00%) | 1 (25.00%) | 4 (44.44%) ** | 2 (3.17%) * | 5 (16.67%) * | 7 (7.53%) ** | 128 (46.01%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanta, U.K.; Chikufenji, B.; Galon, E.M.; Ji, S.; Ma, Z.; El-Sayed, S.A.E.-S.; Ringo, A.E.; Do, T.T.; Xuan, X. Molecular Detection and Phylogenetic Analyses of Babesia spp. and Theileria spp. in Livestock in Bangladesh. Microorganisms 2023, 11, 1563. https://doi.org/10.3390/microorganisms11061563
Mohanta UK, Chikufenji B, Galon EM, Ji S, Ma Z, El-Sayed SAE-S, Ringo AE, Do TT, Xuan X. Molecular Detection and Phylogenetic Analyses of Babesia spp. and Theileria spp. in Livestock in Bangladesh. Microorganisms. 2023; 11(6):1563. https://doi.org/10.3390/microorganisms11061563
Chicago/Turabian StyleMohanta, Uday Kumar, Boniface Chikufenji, Eloiza May Galon, Shengwei Ji, Zhuowei Ma, Shimaa Abd El-Salam El-Sayed, Aaron Edmond Ringo, Thanh Thom Do, and Xuenan Xuan. 2023. "Molecular Detection and Phylogenetic Analyses of Babesia spp. and Theileria spp. in Livestock in Bangladesh" Microorganisms 11, no. 6: 1563. https://doi.org/10.3390/microorganisms11061563
APA StyleMohanta, U. K., Chikufenji, B., Galon, E. M., Ji, S., Ma, Z., El-Sayed, S. A. E.-S., Ringo, A. E., Do, T. T., & Xuan, X. (2023). Molecular Detection and Phylogenetic Analyses of Babesia spp. and Theileria spp. in Livestock in Bangladesh. Microorganisms, 11(6), 1563. https://doi.org/10.3390/microorganisms11061563








