Abstract
Body tissues are subjected to various oxygenic gradients and fluctuations and hence can become transiently hypoxic. Hypoxia-inducible factor (HIF) is the master transcriptional regulator of the cellular hypoxic response and is capable of modulating cellular metabolism, immune responses, epithelial barrier integrity, and local microbiota. Recent reports have characterized the hypoxic response to various infections. However, little is known about the role of HIF activation in the context of protozoan parasitic infections. Growing evidence suggests that tissue and blood protozoa can activate HIF and subsequent HIF target genes in the host, helping or hindering their pathogenicity. In the gut, enteric protozoa are adapted to steep longitudinal and radial oxygen gradients to complete their life cycle, yet the role of HIF during these protozoan infections remains unclear. This review focuses on the hypoxic response to protozoa and its role in the pathophysiology of parasitic infections. We also discuss how hypoxia modulates host immune responses in the context of protozoan infections.
Keywords:
Giardia duodenalis; protozoa; hypoxia; HIF; Entamoeba; Leishmania; Plasmodium; Cryptosporidium; parasite 1. Introduction
Normoxia describes a state in which a tissue is adequately oxygenized and able to carry out aerobic metabolism. This state is typically achieved with an external atmosphere consisting of ~21% oxygen, which translates to ~145 mmHg pO2 at sea level [1,2]. A coordinated network of oxygen gradients exist in the lungs, extending to all organ systems. In homeostasis, organs including the brain, lungs, and liver generally have an oxygen concentration ranging from 3.4% to 6.8% [3]. The gastrointestinal (GI) system exhibits one of the steepest oxygen gradients in the body, ranging from >33 mmHg in the proximal small intestine to 3 mmHg in the distal colon, subjecting the mucosa and local microbiota to fluctuating oxygen concentrations [4,5]. In vertebrates, intense exercise and insufficient environmental oxygen availability can cause hypoxia, a state that in turn engages anaerobic metabolism. Cell injury during hypoxia is generally reversible, in stark contrast with the irreversible damage incurred in anoxic conditions [6,7]. Hypoxia has been widely characterized in various cancers, organ pathologies, and metabolic diseases. Beyond the realm of cancer and chronic diseases, hypoxia plays an important role in infectious diseases, leading to changes in cellular metabolism that may contribute to or protect against pathophysiology. This article provides a state-of-the-art review on the role of tissue hypoxia and HIF activation in the context of parasitic infections, with a focus on tissue, blood, and enteric protozoa.
2. Hypoxia and Hypoxia-Inducible Factor
Mechanisms of Tissue Hypoxia: HIF, Prolyl-Hydroxylases (PHDs), and Metabolism
Tissues can detect and respond to hypoxic conditions, wherein cells attempt to mitigate harm and promote survival during this undesirable state. Hypoxia-inducible factor (HIF) is one of the main players in the cellular detection of hypoxia. HIF is composed of two subunits: the constitutively expressed alpha subunit and nuclear beta subunit which dimerize to form the HIF complex [8]. The HIF complex needs to be readily available to rapidly modulate subsequent gene expression. There are three known alpha subunit isoforms (HIF-1α, HIF-2α, and HIF-3α), each of which dimerize with the HIF-1β, also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) [9,10]. HIF-1α is generally expressed in all nucleated cells and contributes to many transcriptional regulatory processes in response to hypoxia. Although HIF-2α shares many similar target genes to HIF-1α, HIF-2α exhibits more restrictive expression [9,11]. A shift from predominantly acting HIF-1α to HIF-2α occurs during prolonged hypoxia [12,13]. HIF-3α, the most recently identified HIF-α, is primarily expressed in kidneys and lung epithelial cells, contributing to more specialized responses to hypoxia [14,15,16]. Under normoxic conditions, prolyl-hydroxylases (PHDs) and factor-inhibiting HIF (FIH) carry out their primary function, using oxygen as a substrate. PHDs specifically hydroxylate HIF-α isoforms on (one or two) conserved proline residue(s) in the oxygen-dependent degradation domain (ODDD), which allows HIF-α isoforms to be recognized and ubiquitinated by the von Hippel–Lindau protein (VHL), an E3 ubiquitin, and subsequently targeted for proteasomal degradation [9,10,17,18]. FIH-induced hydroxylation of HIF-α isoforms prevents the binding of p300/cAMP response element-binding protein (CREB) cofactors to the HIF complex [19,20,21]. Thus, under normoxic conditions, the dimerization of the HIF complex is inhibited as PHDs and FIH have sufficient oxygen substrates.
Under hypoxic conditions, HIF-α isoforms are stabilized and further translocate to the nucleus where they dimerize with HIF-1β, acting as a transcription factor for numerous genes. Dimerized and nuclear HIF will bind to hypoxia response elements (HREs) present in the promoter of target genes whilst bound to p300/CREB cofactors [19,20,21]. Families of genes that are regulated by HIF include genes involved in cellular metabolism, angiogenesis, proliferation, survival, combatting oxidative stress, redox homeostasis, epithelial barrier function maintenance, and erythropoiesis [9,10,11,22]. HRE consensus sequences (5′-(A/G)CGTG-3′) have been identified in the promoter of hundreds of genes, suggesting dramatic and widespread effects of HIF-1 stabilization [23]. However, other HRE sequences have been elucidated, including the HIF ancillary sequence (HAS) (5′-CAGGT-3′) [22]. The main targets of HIF are genes aimed at combatting cell oxidative stress and promoting angiogenesis. Importantly, HIF-1 is the immediate activator of proangiogenic genes such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO), while HIF-2 is responsible for activating genes promoting vascular maturation, including VEGF receptors (VEGFR1–3), angiopoietin growth factors (ANG1, ANG2, ANG3/4), and their associated receptors (TIE1/2) [23,24,25,26]. The activation of hundreds of genes by HIF-1 is a complex process that may further rely on the activation of other transcription factors. For instance, heat shock transcription factor 1 (HSF1) can subsequently regulate HIF-1 signaling [27]. However, this transcription factor responds to heat shock, suggesting that activation of genes such as heat shock protein 70 (HSP70), a known HIF target gene, may also be alternatively regulated by stressors independent of oxygen.
Metabolic reprogramming is another biological target of HIF, helping cells sustain themselves in the absence of oxidative phosphorylation (OXPHOS). To do so, HIF-1 activates genes encoding the enzymes phosphoglycerate kinase-1 (PGK1), enolase (ENOL), aldolase (ALDA), lactate dehydrogenase-A (LDHA), and pyruvate dehydrogenase kinase (PDK1), effectively increasing glycolytic flux and inhibiting OXPHOS [28]. Other classes of HIF target genes include those promoting self-renewal (i.e., ADN, EDN1, PGM, GPI), proliferation (i.e., CD73, ITF, MET, CTGF), apoptosis (i.e., BNIP3/3L, NRDG, NOXA), and redox homeostasis (GPX3, HMOX1, SOD2) genes [22].
3. Protozoan Parasites and Hypoxia
Protozoa are known to engage in symbiotic or parasitic relations with their mammalian hosts. Due to specific tissue tropisms, many parasites exhibit a certain oxygenic affinity. Considering the steep, dual oxygenic gradients that exist throughout the gut, enteric parasites must be well adapted to the environment to establish an infection and proceed through their life cycle. As a result, numerous protozoans that colonize the GI tract are accustomed to low oxygen concentrations and may require microaerophilic conditions to survive and thrive. In this section we discuss the role of oxygen and hypoxia in the parasitism of enteric as well as tissue and blood protozoa.
3.1. Enteric Protozoan Parasites: Oxygen Metabolism and Host Hypoxic Response
3.1.1. Hypoxia in the Gastrointestinal Tract
In the GI tract, hypoxia occurs upon low blood oxygen concentration or as a result of poor blood flow. Blood flow is important when it comes to gastrointestinal hypoxia as the amount of blood is highly dependent on meal consumption [1,29,30]. Several structural and biological factors can futher determine the oxygen levels in the GI tract, such as the region of the intestine, the mucosal layer, and the gut microbiota [1,4,9,29,30,31,32,33,34]. There are two oxygen gradients in the gut: (i) a longitudinal oxygen gradient, decreasing from the small intestine to the colon, and (ii) a radial gradient, with oxygen levels decreasing from the submucosa to the lumen [1,4,31]. The small intestinal radial gradient ranges from <10 mmHg in the lumen up to 59 mmHg, while the colonic radial gradient ranges from as low as 3 mmHg and 11 mmHg in the lumen (sigmoid and ascending colon, respectively) to as high as 42–71 mmHg in the muscularis externa [4,31]. Intestinal epithelial cells (IECs) are therefore reliant on HIF to promote cellular survival and adaptation when oxygen fluctuations subject them to a state of hypoxia [35]. Furthermore, inflammation in the GI tract leads to an influx of immune cells, resulting in a heightened hypoxic state due to depleted oxygen availability and altered cellular metabolism [36]. Colorectal cancer severity and metastasis have both been linked to increased tissue hypoxia. HIF activates numerous genes involved in tumor proliferation and angiogenesis [37,38,39,40]. For example, HIF-1α can also promote activation of ataxia-telangiectasia mutated kinase to protect colorectal cancer cells against apoptosis, an important mechanism by which tumors utilize hypoxia for enhanced survival [41]. Antibodies against HIF target gene products such as VEGF can further increase the duration of survival in patients when used in combination with other chemotherapeutic drugs [42].
IECs can also utilize the hypoxic response for protection against various system perturbations. For instance, Clostridium difficile toxins increase transcription and subsequent translation of HIF-1α in a dose-dependent manner, suggesting that IEC HIF-1α is protective against C. difficile-induced injury and inflammation [43]. Clinical symptoms of murine TNBS-colitis are exasperated when HIF-1α is decreased and, conversely, overexpression of HIF-1α can be protective [44]. Furthermore, hypoxia is implicated in various inflammatory conditions wherein cellular responses to hypoxia can promote the resolution of inflammation [36,45].
3.1.2. Entamoeba histolytica
Entamoeba histolytica, the causative agent of amebic dysentery or amebiasis, is a leading cause of diarrheal illness in humans, with 50 million symptomatic infections per year [46]. After ingestion, cysts undergo excystation into trophozoites, which travel to the colon where they colonize and invade the epithelial tissue [46]. During this process, trophozoites traverse a steep gradient from the oxygen-poor colonic lumen to the oxygenated lamina propria [47]. E. histolytica has developed a complex machinery against nitric oxide (NO) and reactive oxygen species (ROS) released by neutrophils and macrophages to prevent macromolecular damage during the tissue invasion process, which subsequently maintains a strict state of intracellular hypoxia [48,49,50,51]. Oxygen is one of the main host stressors that influences E. histolytica’s survival and pathogenicity. E. histolytica can upregulate its antioxidant enzyme machinery, which includes iron superoxide dismutase, peroxiredoxin, and thioredoxin to evade host immune defenses [49,52,53,54].
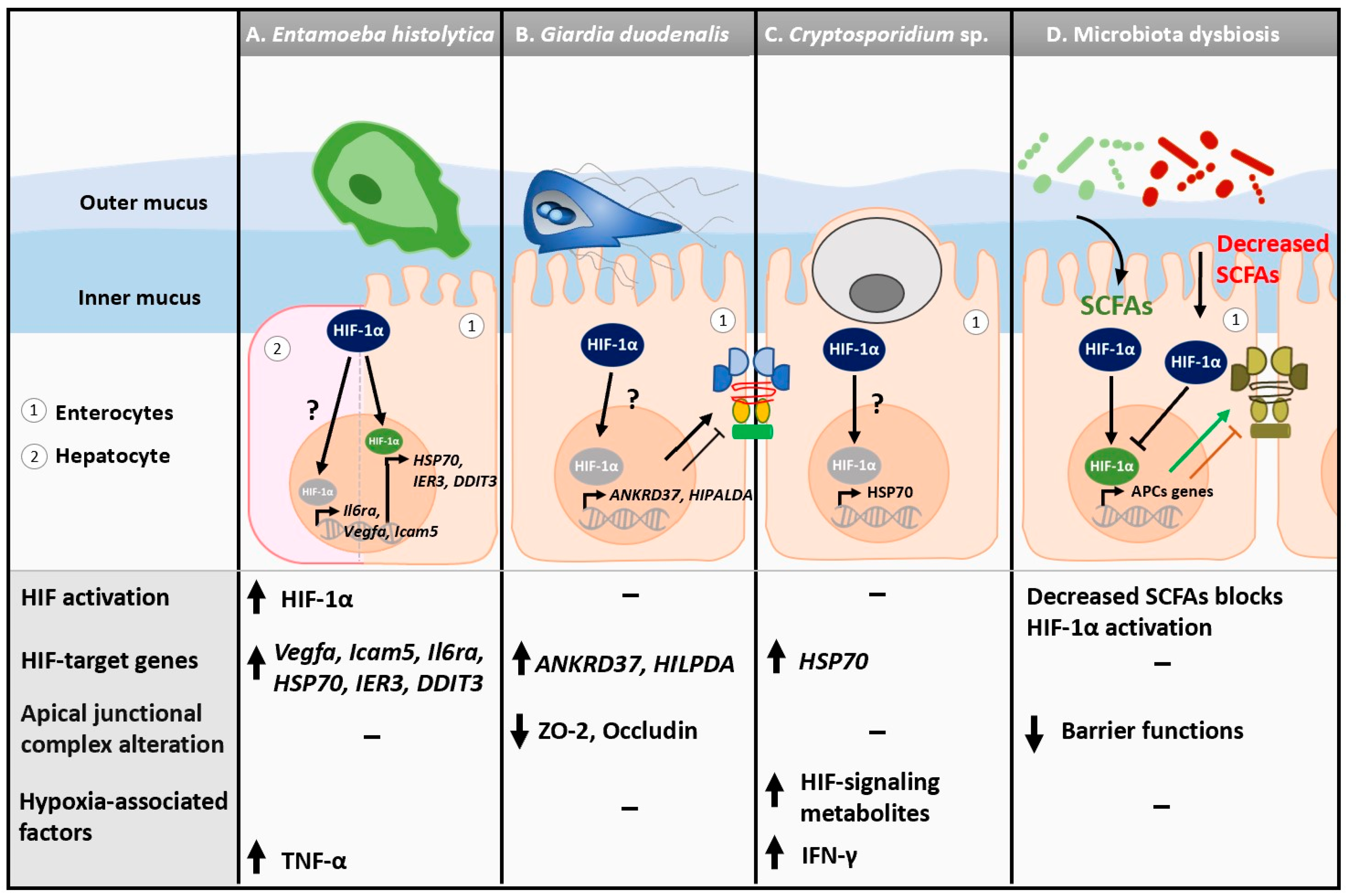
Alterations to HIF-1α expression and hypoxic-related signaling have been described during amebiasis (Figure 1A). E. histolytica is associated with hepatic hypoxic responses as HIF-1α and HIF-dependent genes Vegfa, Icam1, and Il6ra are upregulated in a murine model of E. histolytica infection [55] (Table 1). Interestingly, HIF-1α is required for adequate secretion of IL-6, indirectly modulating the anti-E. histolytica Th17 immune response [55]. Human intestinal xenografts transplanted into SCID mice exhibit upregulated expression of numerous genes associated with hypoxic responses upon E. histolytica infection, including those coding for HIF1α, metallothioneins (MT1G, MT1H, MT1P, MT2A, MT3), members of the c-Jun and c-Fos family (JUN, JUNB, JUND, FOS), growth arrest and DNA damage inducible 3 (DDIT3), alpha crystallin (CRYAB), immediate early response 3 (IER3), and heat shock protein 70 (HSP70) [56] (Table 1). Furthermore, intestinal biopsies from humans infected with E. histolytica show elevated HIF activation, as well as enrichment for the response to hypoxia as a biological process [57]. Considering E. histolytica is an anaerobe, this finding suggests that the parasite may modulate its microenvironment to make the conditions more favorable, triggering an HIF-dependent cellular response.
Figure 1.
Alterations to mucosal HIF-1α expression and hypoxia-related signaling during enteric protozoan infections and microbiota dysbiosis. (A) Murine hepatocytes exhibit elevated levels of HIF-associated genes Icam1 and Il6ra in response to Entamoeba histolytica infection. Human intestinal xenografts transplanted into SCID mice show elevated expression of several hypoxia-related genes, including HSP70, IER3, DDIT3, and HIF-1α, upon E. histolytica infection. (B) Giardia duodenalis (WB) is associated with increased expression of HIF target genes ANKRD37, HILPDA, NOS2, GADD45A, HIG2, ITF, MIR210HG, and SLC2A3. Expression of tight junctional proteins ZO-2 and Occludin are decreased upon Giardia infection in anaerobic conditions. (C) Cryptosporidium muris infection is associated with elevated fecal HIF-1-associated signaling metabolites. Hypoxia-induced heat shock protein 70 (HSP70) is elevated in response to Cryptosporidium parvum. (D) Microbiota dysbiosis can lead to decreased secretion of short-chain fatty acids (SCFAs), lessening HIF activation and barrier function. Although enteric parasites are associated with microbiota dysbiosis, no direct link between HIF signaling and microbial-secreted metabolites has been established in the context of enteric protozoan infections.

Table 1.
Murine and human HIF target genes upregulated upon infection with protozoan parasites.
3.1.3. Giardia Duodenalis
The intestinal protozoan parasite Giardia duodenalis (syn. G. intestinalis, G. lamblia) causes giardiasis, one of the leading causes of diarrheal disease worldwide with over 200 million symptomatic cases annually in humans [73,74]. Clinical manifestations of giardiasis include acute presentations of diarrhea and abdominal pain, as well as post-infectious disorders such as post-infectious irritable bowel syndrome (PI-IBS), chronic fatigue, and failure to thrive in children [75]. After ingestion via the fecal–oral route, cysts undergo excystation and trophozoites colonize the upper small intestine by attaching to the surface of enterocytes [76,77]. Giardia is classified as a microaerophilic parasite, lacking mitochondria and enzymes for the electron transport chain (ETC) and tricarboxylic acid (TCA) cycle [9,78,79]. Instead, Giardia has a fermentative style of metabolism, utilizing glycolysis and substrate-level phosphorylation to generate energy while also possessing oxygen-scavenging enzymes such as flavodiiron proteins (FDPs), peroxiredoxin, flavohemoglobin, superoxide reductase, NADH peroxidase, and NADH oxidase to combat luminal ROS [80,81,82,83,84]. The ability to process oxygen also depends on Giardia’s life stage, wherein Giardia trophozoite uptake of oxygen is stimulated via exogenous glucose while cyst oxygen uptake is triggered by ethanol [85].
G. duodenalis has demonstrated the ability to activate a hypoxic-like response in cells (Figure 1B). Transcriptomic analysis on human duodenal organoid-derived monolayers infected with G. duodenalis strain WB clone 6 exhibit an enrichment of hypoxia response-related genes (i.e., Molecular Signatures Database) after 24 h of infection [86]. Similar observations have been made using Caco-2 epithelial cells, where HIF-induced genes including NOS2, ANKRD37, GADD45A, ITF, MIR210HG, and SLC2A3 are also upregulated in Giardia-exposed cells, suggesting that IECs respond to increased oxidative stress upon infection [22,58,59,87] (Table 1). Furthermore, hypoxia-inducible gene 2 (HIG2), a peptide inhibitor of adipose triglyceride lipase encoded by a target gene of HIF-1 named HILPDA (Hypoxia-Inducible Lipid Droplet-Associated), is upregulated early on in infection, indicating that HIF activation may occur quickly upon cellular interaction with Giardia [58,88] (Table 1). However, little is known about the mechanisms and roles of HIF activation upon exposure to Giardia.
3.1.4. Cryptosporidium spp.
Cryptosporidium species such as Cryptosporidium hominis and Cryptosporidium parvum are leading causes of diarrheal illness [89]. Cryptosporidium oocysts excyst upon ingestion into four sporozoites, which subsequently invade IECs and progress through their life cycle intracellularly [90]. As Cryptosporidium migrates from the gut lumen to the IECs, sporozoites are exposed to fluctuating but predominantly oxygen-poor environments [90]. Cryptosporidium can carry out both aerobic and anaerobic metabolic pathways, encoding a pyruvate:NADP+ oxidoreductase and an alternative oxidase, respectively [91,92]. This suggests that Cryptosporidium can not only tolerate but also make use of the variable oxygenic conditions of the gut [92]. Interestingly, the oocysts are present in higher abundances in water samples with lower dissolved oxygen, implying the oocyst may be more sensitive to oxygen than the invasive merozoites [93].
In a neonatal rat model of cryptosporidiosis, cardiomyocytes have hyperexpression of HIF-1α; however, this finding is more suggestive of a link between gastroenteritis and cardiovascular disease than a direct induction of hypoxia by C. parvum [60]. In the GI tract, the fecal HIF-1 signaling-associated metabolite oxoglutaric acid is increased in C. muris-infected mice, suggesting that mice can enact a hypoxic response upon Cryptosporidium infection [94] (Figure 1C). Finally, in HCT-08 colonic monolayers infected with C. parvum, heat shock protein 70 (HSP70), which possesses a hypoxia response element, is also upregulated 24 h post infection [61] (Table 1). The full biological significance of these observations requires further investigation.
3.1.5. Hypoxia and Gut Epithelial Barrier Functions during Protozoan Infections
During states of inflammation and hypoxia, epithelial barrier function can be reduced as a result of the dysregulation of various tight junction proteins, including claudin-1 [95,96,97]. To combat this, the hypoxic response activates genes such as ATG9A and CLDN1 to promote tight junction biogenesis and barrier integrity [98,99]. Importantly, enteric protozoa can also directly disrupt tight junctions and increase barrier permeability via the adherence of the active form of the parasite to the epithelial layer. For example, E. histolytica can both alter expression of and degrade zonulin-1, claudin-2, and occludin, a result of secreted cysteine protease A5 [100,101]. Similarly, G. duodenalis secretes cysteine proteases that can disrupt the claudin-1, claudin-4, and occludin arrangement in IECs [102,103]. Under normoxic or anaerobic conditions, G. duodenalis infection is associated with the disruption of epithelial junctional complexes (EJCs), which results in epithelial barrier dysfunction [104,105] (Figure 1B). Cryptosporidium spp. can also modulate the organization and expression of various tight junction proteins, including occludin, E-cadherin, and claudin-4; however, these changes are suggested to be mediated via the induction of protein degradation pathways rather than by the secretion of cysteine proteases by the protozoa [65]. Given the evidence of the modulation of both barrier function and HIF activation associated enteric protozoa, the role of hypoxia in modulation of IEC tight junctional complexes requires further investigation.
In the gut, protozoan infections have also been associated with microbiota dysbiosis and biofilm disruption, both of which may lead to the liberation of invasive pathobionts [106,107,108,109,110,111]. Interestingly, microbiota-derived products, including short-chain fatty acids (SCFAs) such as butyrate, play a role in the stabilization of HIF, which in turn improves epithelial barrier functions [112] (Figure 1D). In an enteroid model of hypoxia, pre-treatment or concurrent treatment with different ratios of SCFA cocktails such as acetate, propionate, and butyrate led to increased transepithelial electrical resistance, as well as increased expression of key gut barrier and metabolism genes [113]. Thus, more research is warranted to characterize changes in EJCs during protozoan infections under hypoxic conditions, as well as the role of protozoa-associated dysbiotic microbiota and derived products in the loss of barrier functions.
3.2. Tissue and Blood Parasites: Oxygen Metabolism and Host Hypoxic Responses
3.2.1. Tissue Hypoxia
The optimal concentration of dissolved oxygen varies depending on the localization, function, and vascularization of the tissue. Although the state of physiologic hypoxia is pertinent to the gastrointestinal tract, many tissues can become hypoxic and must respond accordingly. For example, the brain must be sufficiently oxygenized (~35 mmHg) to carry out proper functions [114]. Under conditions of cerebral hypoxia due to ischemia or injury, the brain increases metabolic consumption of oxygen [114,115,116]. In the lungs, human influenza A virus infection can lead to localized hypoxia and alveolar cell death, resulting in oxygen depletion below the preferred range of 100–160 mmHg [115,117]. Alternatively, the skin functions optimally with a much lower concentration of oxygen that increases from ~8 to 35 mmHg across the superficial to the subpapillary plexus, respectively, while perturbations to these concentrations can impair keratinocyte proliferation and junction integrity [115,118]. Hence, given the various oxygenic environments sustained by each tissue, fluctuations in oxygen concentration can subject virtually any bodily tissue to hypoxia and incur damage in the absence of an appropriate cellular response.
Tissue and blood protozoan parasites are responsible for some of the most prevalent human parasitic infections, including malaria, Chagas disease, leishmaniasis, and toxoplasmosis [119]. Unlike enteric protozoa, which must be adapted to the fluctuating low levels of oxygen in the gut, tissue and blood protozoa exhibit a more diverse array of oxygenic preferences depending on where in the body they reside, and numerous investigations have aimed to characterize the hypoxic signatures of the host. Indeed, numerous tissue and blood protozoa either require or upregulate expression of HIF to enact a successful infection.
3.2.2. Leishmania spp.
Leishmania spp. are a group of flagellated protozoa that are responsible for three different forms of leishmaniasis: visceral, cutaneous, and mucocutaneous [120]. The promastigote stage of the parasite replicates extracellularly within a sandfly (approximately 90 sandfly species can transmit Leishmania), which can then deliver the protozoan to the mammalian host during a blood meal [120,121]. In the mammalian host, obligate intracellular amastigotes will be phagocytosed by macrophages or dendritic cells, allowing them to travel to distant sites in the body [122]. A study assessing parasitic growth under anoxic conditions noted a loss of motility and increased secretion of lactate in promastigotes, illustrating their poor adaptability to anoxic conditions; hence, they are classified as aerobes [123]. Promastigotes require a high concentration of both extracellular and intracellular oxygen to produce superoxide, enabling the parasite to carry out aerobic metabolism and utilize oxygen as the final electron acceptor in the electron transport chain [124]. Additionally, all Leishmania spp. possess trypanothione reductase to combat oxidative stress, allowing them to survive within a macrophage [125].
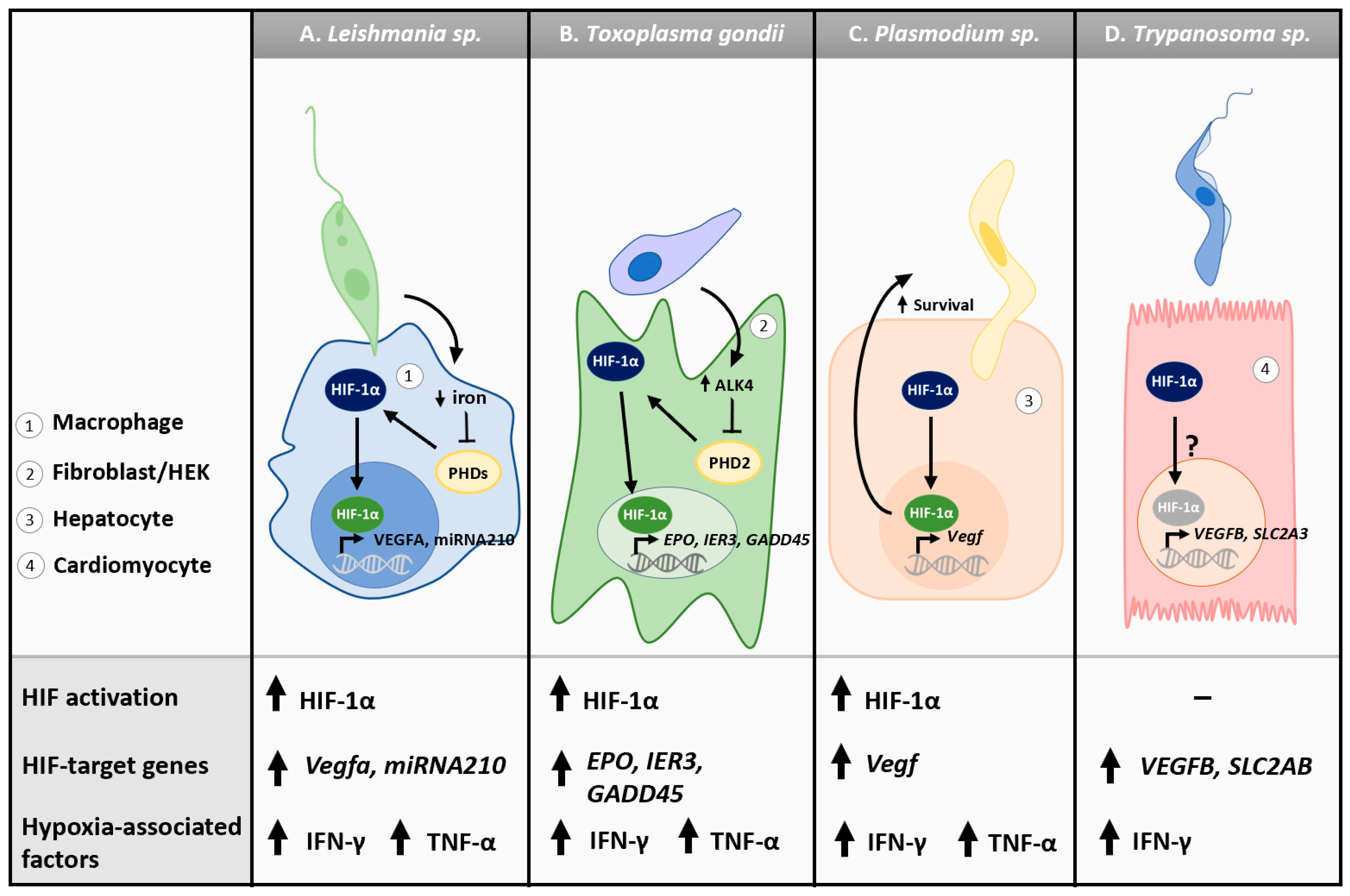
Although Leishmania spp. are aerobic, they induce a hypoxic environment within the macrophage, increasing the expression of macrophage HIF-1α and subsequent expression of HIF-target gene micro-RNA-210 (miRNA210) [62,63,64,65] (Table 1). Increases in miRNA210 enhance L. donovani survival as silencing of both HIF-1α and miRNA210 in macrophages reduces parasitic burden and infectivity [65]. L. donovani also decreases the cellular iron pool, a necessary cofactor for PHDs, preventing the hydroxylation of HIF-1α [126,127] (Figure 2A). Drugs capable of targeting HIF-1α, such as resveratrol and echinomycin, decrease L. amazonesis survival in macrophages [128]. Recent studies have also demonstrated that HIF-dependent upregulation of Vegfa results in lymphangiogenesis, a critical step in the resolution of cutaneous lesions during L. major infection [66,129,130] (Figure 2A). The host cellular hypoxic response may also have leishmanicidal effects. Exposure of macrophages and dendritic cells to hypoxia during infection improves control of parasitic burden [131,132,133]. Mice deficient in HIF-1α in myeloid cell compartments have a more severe disease outcome and a higher parasitic burden, suggesting that HIF-1α expressed by myeloid cells contributes to the innate immune response against L. major [134]. Conversely, other reports suggest that HIF-1α activation has no impact on the macrophage phagocytosis of Leishmania, nor on control of parasitic burden [66]. HIF-1α expression is downregulated in Leishmania-infected hamsters in a species-dependent fashion, suggesting that different Leishmania spp. may elicit different hypoxic responses [135]. Finally, hyper-virulent Leishmania strains reduced HIF-1α expression, whereas hypo-virulent strains increased HIF-1α expression, indicating different strains within the same species can also elicit different hypoxic responses that may contribute to or mitigate virulence [136].

Figure 2.
Cellular hypoxic responses to tissue and blood protozoan parasites. (A) Leishmania donovani-infected macrophages show elevated expression of HIF-1α and HIF target genes miRNA210 and VEGFA. HIF-1α activation in macrophages is in part due to a decrease in intracellular iron, a cofactor of PHDs. (B) Toxoplasma gondii infection is associated with HIF-1α activation and increased expression of IER3, GADD45, and glycolytic enzymes (i.e., HK2) in fibroblasts. ALK4 phosphorylation by T. gondii inhibits PHD2, which further prevents the hydroxylation of HIF-1α. In HEK cells, T. gondii activates HIF-1α signaling to increase the production of HIF target gene products such as EPO. (C) Plasmodium berghei sporozoites activate HIF-1α and upregulate HIF target genes such as Vegf in murine hepatocytes. HIF-1α agonists promote P. berghei survival. (D) Hypoxia target genes HIF-1α, VEGFB, SLC2A3, and ENO1, as well as glycolytic enzymes HK2 and LDHA, are upregulated in stem cell-derived cardiomyocytes infected with Trypanosoma cruzi. Secretion of indolepyruvate by Trypanosoma brucei promotes hydroxylation and subsequent degradation of HIF-1α.
3.2.3. Toxoplasma gondii
Toxoplasma gondii is an obligate intracellular protozoan parasite with a global prevalence between 25% and 30% [137,138]. While T. gondii is generally considered to be an aerobe, it must make adaptations to the environmental conditions of the definitive and intermediate hosts [137]. Indeed, T. gondii experiences numerous oxygenic fluctuations during its life cycle. It first colonizes the feline intestinal tract to replicate oocysts, and after sporulation and ingestion by a human, the infective tachyzoite can travel through the bloodstream to numerous bodily locations, including the central nervous system and skeletal muscles [137]. In the small intestine of felines, the merozoite form exhibits a unique gene expression profile compared to the other life stages of the parasite through the increased transcriptomic and proteomic activity of metabolic pathways, a possible adaptation to the low oxygen of the gut and increased growth requirements [139]. Interestingly, T. gondii encodes a prolyl-hydroxylase (TgPhyA) which hydroxylates S-phase kinase-associated protein 1, a protein that can indirectly sense oxygen levels and hence may assist with survival in various tissues [140,141]. T. gondii also possesses a mitochondrion that can carry out aerobic metabolism via an electron transport chain, as well as a necessary assortment of antioxidant enzymes to protect against self- and host-derived ROS [142,143,144].
In the intermediate host, T. gondii is associated with HIF-1α activation (Figure 2B). In murine embryonic fibroblasts, luciferase assays indicate the upregulation of HIF-1α transcription upon T. gondii infection [67]. Similarly, increased HIF-1α transcription is observed in human foreskin fibroblasts (HFFs) infected with T. gondii, which correlates with increased stable HIF-1α protein [67]. This accumulation of stable protein can be attributed to T. gondii’s ability to inhibit PHD2 via the stimulation of activin-like kinase 4 (ALK4) and subsequent activation of Rho GTPase and JNK MAP kinase pathways, abrogating the hydroxylation of HIF-1α [69,145,146,147]. Increasing HIF-1α production is of great importance to T. gondii’s fitness as it requires HIF-1α for survival and efficient replication under physiological oxygen concentrations (~3% oxygen) [67]. Moreover, T. gondii Cathepsin C1 can upregulate HIF-1α signaling to increase the production of EPO in HEK and HFF cells, a known HIF-dependent gene target [68]. Additionally, the expression of glycolytic enzymes is increased upon T. gondii infection in an HIF-dependent manner [70,148,149] (Table 1). Specifically, HK2 is delocalized from the mitochondrial membrane to the cell cytoplasm, making it more accessible to T. gondii [70,148,149]. The characterization of HIF activation in response to T. gondii, an aerobe, provides an example of a hypoxic response in normoxic conditions, suggesting that T. gondii may utilize host response to enhance its own fitness and virulence.
3.2.4. Plasmodium spp.
Plasmodium spp. are the causative agents of malaria, a life-threatening vector-borne disease that affects nearly 250 million individuals yearly [150]. Plasmodium begins as a sporozoite in the insect vector, and the parasite undergoes schizogony in the liver of the infected vertebrate upon a blood meal [151]. The schizonts then become merozoites, which can infect red blood cells and subsequently digest host hemoglobin [152,153]. During the asexual stage in the human liver, sporozoites act as microaerophiles as the physiologic oxygen concentration is low [115,154,155]. Some reports suggest that sporozoite mitochondrial oxygen consumption and oxidative phosphorylation is reduced during the liver stage due to sufficient glucose availability [156,157]. However, a switch to dependence on oxidative phosphorylation is observed during the sexual life stages as it is exposed to mosquito salivary glands, which may reach up to 21% oxygen, and the capillaries of the human lung, which can approach 13% oxygen [158,159].
Numerous studies have investigated the role of HIF in Plasmodium spp. infections (Figure 2C). Pharmaceutical-induced activation of HIF-1α results in increased survival of P. berghei sporozoites in hepatocytes without altering the growth of the exo-erythrocytic stage of the parasite [160]. Mice infected with P. berghei have increased tissue levels of HIF-1α and VEGF protein, driving angiogenesis as a compensatory mechanism to increased oxygenation in hypoxic infected tissues [71]. Furthermore, insufficient expression of HIF-1α by brain cells has been suggested to contribute to the progression of cerebral malaria [161]. However, increased HIF-1α and VEGFA has not been the consensus in malaria patients. For example, while higher levels of HIF-1α mRNA expression have been identified in placental tissues, VEGFA expression was downregulated in malaria patients [162]. Additionally, post-mortem brain tissue from severe cerebral malaria patients presented no changes in HIF-1α expression but increases in VEGFA, indicating changes in VEGFA may be tissue-dependent or HIF-independent [163].
3.2.5. Trypanosoma spp.
Chagas disease is a vector-borne parasitic infection caused by the parasite Trypanosoma cruzi. T. cruzi colonizes the gut of triatomine insects to generate infectious trypomastigotes which can then be transferred into the mammalian bloodstream during a blood meal [164]. T. cruzi can invade an assortment of nucleated cells but demonstrates an affinity for cardiac and skeletal muscle tissue, and therefore the amastigote is an obligate intracellular parasite [165]. From the insect vector’s gut to cardiac tissues, T. cruzi is exposed to diverse oxygenic states and hence exhibits variable metabolic behaviors that capitalize on fluctuating microenvironmental conditions [166]. In the low-oxygen environment of the triatomine gut, T. cruzi favors an anaerobic mode of metabolism by increasing glycolytic flux to sustain morphological changes [167,168,169]. In the vertebrate host’s bloodstream, trypomastigotes exhibit a preference for oxidative phosphorylation by increasing the activity of the ETC, utilizing oxygen from the blood to sustain an aerobic mode of metabolism [168,170]. T. cruzi can combat oxidative stress by expressing antioxidant enzymes such as peroxiredoxins and trypanothione synthetase [171]. Interestingly, it has been postulated that the presence of ROS influences the proliferation of epimastigotes, suggesting the parasite utilizes environmental oxygen cues to regulate its life cycle [172].
Few reports have characterized the hypoxic signature of T. cruzi infection. Leukocytes from the cardiac tissue of human patients with late-stage trypanosomiasis exhibit increased expression of HIF-1α in conjunction with increased ATP catabolism and parasitic burden [173]. Induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) also show significant enrichment for hypoxia-related genes upon infection with T. cruzi, including HIF-1α, VEGFB, HK2, SLC2A3, ENO1, and LDHA [72] (Figure 1, Table 1). Importantly, reduced expression or inhibition of the glucose transporter GLUT4 in cardiomyocytes lowered parasitic uptake, suggesting T. cruzi can utilize GLUT4 to enter cardiomyocytes [72]. GLUT4 is a passive glucose transporter that is known to have increased action during hypoxia in an HIF-dependent manner, and T. cruzi may hence benefit from the hypoxic response to cellular invasion [174]. However, another report indicates that T. cruzi strain Tulahuen may downregulate HIF-1α in myoblasts, while no alterations to HIF-1α were observed in other strains, suggesting an isolate-dependent phenomenon [175]. Interestingly, T. brucei, the etiologic agent of African trypanomiasis, hydroxylates HIF-1α via the secretion of indolepyruvate, resulting in reduced macrophage glycolysis [176]. This functions as an immune evasion mechanism and prevents macrophages from shifting their metabolism to favor glycolysis, an example of an indirect way in which a parasite can mitigate hypoxic signaling [176]. This observation also supports the hypothesis that HIF-1α can play a role in host immune evasion against protozoan parasites. However, expression of HIF-1α and target genes vegfa, glut1, and il1b is elevated in the median eminence and hypothalamus of both Rag1−/− and wild-type mice infected with T. brucei brucei (Tbb), suggesting different tissues and cell types may alternatively regulate HIF in the presence of the parasite [177].
4. Host Immune Response and Markers of Hypoxia during Protozoan Infections
Parasites elicit a broad array of host immune responses dependent on their mechanism and site of infection. Intracellular protozoans often trigger Th1 immune responses, while extracellular parasites such as Giardia can induce components of Th1, Th2, and Th17 immune responses [178,179]. Several cytokines can promote HIF stabilization independent of oxygen concentration, activating hypoxic responses under normoxic conditions. Proinflammatory cytokines can stabilize HIF via non-canonical pathways and can be reciprocally regulated by HIF. For instance, HIF-dependent regulation of IL-1β, TNFα, INF-γ, IL-33, IL-6, IL-12, and IL-23 illustrates the strong influence HIF has on innate immunity [180,181]. In this section, we will review the main HIF-related cytokines in the context of protozoan infections.
4.1. IL-1β and NLRP3
IL-1β is one of the most important modulators of both inflammation and infection. Notably, IL-1β upregulates HIF-1α protein levels in various human tissues and immune cells, which is confirmed by subsequent HIF-dependent VEGF upregulation [182,183,184,185]. This stabilization of HIF-1α occurs via the IL-1β-driven phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2 or p42/44), activating a mitogen-activated protein kinase (MAPK) pathway responsible for subsequent HIF-1α stabilization [185,186]. Importantly, the regulation of HIF-1α by IL-1β can exhibit bidirectionality as the IL1β gene possesses a canonical HIF-1α binding site upstream of the transcription start site [187]. Knockout of the VHL protein in mice exacerbates IL-1β secretion, further ameliorating this relationship [188]. Accumulation of the TCA metabolite succinate has also been implicated in the HIF–IL-1β axis. During oxygenic stress, ROS can inactivate succinate dehydrogenase, halting further metabolism of succinate [189]. Macrophage-released succinate can inhibit PHDs, promoting the stabilization of HIF-1α and IL-1β signaling [187]. Alternatively, succinate can act extracellularly to promote activation of the HIF–IL-1β axis as human umbilical vein endothelial cells (HUVECs) express a surface succinate receptor (SUCNR1) which, upon recognition of macrophage-produced succinate, can upregulate IL-1β in an HIF-1-dependent manner [190]. As a potent activator of IL-1β secretion via activation of caspase-1, the nod-like receptor protein 3 (NLRP3) inflammasome also has a role in HIF-dependent gene activation [190,191]. In the gut, NLRP3 can bind the mammalian target of rapamycin (mTOR) and promote the development of intestinal inflammation during a state of hypoxia, a result that is counteracted via PHD inhibition [192]. HIF-1α also promotes NLRP3-driven microglial pyroptosis in the brain of mice with traumatic brain injuries, illustrating the tissue-independent pro-inflammatory crosstalk that exists between NLRP3 and HIF [193]. Hence, the production of cytokines can also be an indirect result of HIF-1α-promoted inflammasome assembly.
To date, no studies have demonstrated direct HIF-dependent activation of NLRP3 and the HIF–IL-1β axis by protozoa. E. histolytica peroxiredoxins promote IL-1β secretion and activation of the NLRP3 inflammasome via the activation of toll-like receptor 4 [194,195]. E. histolytica cysteine proteases can increase production of IL-1β by mimicking the action of caspase-1, activating pro-IL-1β independent of the host [196,197]. G. duodenalis can activate the NLRP3 inflammasome, leading to subsequent production of antimicrobial peptides [198]. As G. duodenalis cysteine proteases are able to cleave inflammatory cytokines and chemokines such as CXCL1, CXCL2, CXCL3, CXCL8, CCL2, and CCL20 to dampen local inflammation, this model protozoan parasite offers an interesting avenue for further research into HIF-mediated cytokines [199,200,201]. NLRP3 inflammasome activation and IL-1β secretion can also promote macrophage pyroptosis, a benefit to the parasite that may outweigh the consequential pro-inflammatory response [202,203]. Interestingly, G. duodenalis extracellular vesicles induce the production of IL-1β via modulation of cyclooxygenase 2 (COX2), driving macrophage secretion of the cytokine in a ROS-dependent manner via MAPK/NF-κB signaling [204,205,206]. The increased secretion of IL-1β and concomitant activation of NLRP3 has also been observed upon infection with tissue and blood parasites. For instance, Leishmania spp., T. gondii, and T. cruzi have been shown to activate the NLRP3 inflammasome [207,208,209,210,211,212,213,214]. Moreover, the secretion of indolepyruvate by T. brucei inhibits the expression of pro-IL-1β by bone marrow-derived macrophages in an HIF-1α dependent manner [176]. Leishmania spp. and T. gondii can also suppress Th1 response by secreting an IL-1β antagonist (IL1RN) [211,215,216]. While Plasmodium spp. infection does not cause ubiquitous increases in IL-1β, malarial hemozoin induces IL-1β secretion via activation of the NLRP3 inflammasome [217,218,219]. Further investigation into the role of the HIF–IL-1β axis during host–protozoan interactions will shed light on the physiological significance of this important interactive network.
4.2. TNFα
Tumor necrosis factor alpha (TNFα) is a pleiotropic pro-inflammatory cytokine which regulates inflammation and pathogenesis. TNFα promotes HIF-1 binding to DNA and elicits a hypoxic response in the absence of oxidative stress [182]. This finding is corroborated in rat peritoneal inflammatory cells, which exhibit mirrored increases in HIF-1α protein when cultured in hypoxic conditions or exposed to TNFα prior to cell injury [220]. Importantly, ROS have been identified as key mediators of hypoxic TNFα regulation. In fetal alveolar type II epithelial cells pre-treated with either antioxidants or inhibitors of the mitochondrion complex I, a pertinent element of the electron transport chain and producer of ROS, the TNFα-dependent stabilization of HIF-1α is lost [221]. Alternatively, TNFα relies on NF-κB for the activation of HIF-1α [222]. In TNFα-stimulated HEK cells treated with Iκ-b kinase (IKK) inhibitors, a kinase required for the activation of NF-κB, the transcription and translation of HIF-1α is abrogated [223,224]. IKKs can also be phosphorylated by TNFα, suggesting that TNFα activates HIF-1α expression via an IKK/NF-κB-dependent mechanism [224]. Similar results were observed in murine skeletal muscle cells as TNFα-induced NF-κB activation is required to activate HIF-1α and subsequently promote glycolytic metabolism, confirming TNFα not only activates HIF1α but also elicits a robust hypoxic response [225].
TNFα is a prominent cytokine in the immune response to several protozoan parasites (Figure 1A and Figure 2). TNFα induces a strong inflammatory state and can be cytotoxic to the host during E. histolytica infection, corroborated by higher TNFα serum levels in patients with more severe outcomes [226,227,228,229,230]. Interestingly, E. histolytica trophozoites exhibit chemotaxis towards TNFα, indicating that sites of TNFα may have increased parasitic colonization [231]. Increased production of TNFα can also be beneficial for effective parasite clearance in the context of G. duodenalis [86,206,232,233]. The role of TNFα in Cryptosporidium infections is unclear as parasite infectivity in TNFα-deficient mice is unchanged, while exogenous TNFα promotes oocyst shedding [234,235,236,237,238]. In Leishmaniasis, TNFα enhances disease pathogenesis, but TNFα-deficient mice can exhibit a compensatory mechanism of increased nitric oxide synthase expresssion to further perpetuate parasite killing, indicating this cytokine is not paramount for host defense [239,240]. TNFα also promotes more severe disease outcomes in the context of Plasmodium sp. infections as the absence of TNF receptor 2 (TNFR2) and/or administration of TNFα-neutralizing antibodies leads to an absence of cerebral malaria and fewer liver lesions [241,242,243,244]. In toxoplasmosis, TNFα knockout mice are unable to control the burden of T. gondii and eventually die of necrotizing toxoplasmic encephalitis, stressing the importance of this cytokine for host defense in the brain [245]. Similar findings have been illustrated in models of T. cruzi infection, where transgenic mice capable of neutralizing TNFα have increased infection severity and higher mortality rates compared to wild-type mice [246,247]. The mechanisms involved in the HIF-1α–TNFα interactions in the context of the host inflammatory response to protozoan parasites require further investigation.
4.3. INF-γ
Bolstering the relationship between hypoxia and inflammation, HIF can also be regulated by interferons (IFNs), a complex system of cytokines that orchestrate pro- and anti-inflammatory responses [248]. INF-α, INF-β, and IFN-γ can elicit an HIF-mediated response [249,250,251]. IFN-γ is a powerful immune-modulating cytokine that must be carefully calibrated to assist the host during infection and may promote varying hypoxic responses. IFN-γ increases HIF-1α activation in T84 and Caco-2 IECs when NF-κB is uninhibited [181,252]. The activity of dual oxidase II, a known generator of ROS, is also required for IFN-γ induction of HIF-1α and subsequent VEGF production in pancreatic cell lines [253]. Conversely, HIF-1α activation in Treg cells can also promote secretion of IFN-γ to potentiate Th1 immune responses [254]. Other reports suggest that IFN-γ possesses a potent ability to repress the transcription of HIF-1β, abrogating the hypoxic response in human colonic T84 cells [255]. Interestingly, IFN-γ has exhibited the ability to induce PHD3 in endothelial cells, suggesting IFN-γ may also indirectly exert HIF-1α inhibition [256].
IFN-γ contributes greatly to host protection against E. histolytica as higher secretion of IFN-γ inhibits parasitic ingestion and replication [257,258] (Figure 1A). In cryptosporidiosis, IFN-γ is also required for the clearance of Cryptosporidium [234,259] (Figure 1C). In leishmaniasis, IFN-γ is required for macrophages to facilitate nitrogen oxidation and subsequent intracellular and extracellular killing of L. major amastigotes [239,260] (Figure 2A). Similarly, both IFN-γ and its receptors are required for protection against T. gondii and T. cruzi [261,262,263,264,265] (Figure 2B,D). Although secretion of IFN-γ can be detrimental to the host, Plasmodium spp. infections do not develop into cerebral malaria in IFN-γ receptor-deficient mice or mice receiving IFN-γ-neutralizing antibodies [242,243,244,266] (Figure 2C). Much remains to be learnt about the interactive role of IFN-γ and hypoxia during protozoan infections.
4.4. IL-33
Interleukin-33 (IL-33) possesses an HRE in its promoter, suggesting that it can be activated by HIF [267,268]. In the gut, HIF-1α-driven expression of IL-33 can enhance inflammation in mice with dextran sulfate sodium (DSS)-induced colitis [267]. Expressed constitutively by many tissues, including blood vessels, lymphoid tissues, and epithelial cells, IL-33 acts as a ligand for IL-1 receptor-related protein ST2, found on the surface of immune cells such as mast cells and Th2 cells [269,270,271]. Additionally, IL-33 functions as an alarmin, helping mast cells recognize cellular injury and acting as one of the first responders to parasitic invasion [272,273,274]. IL-33 is inherently linked to the hypoxic response via mTOR, a serine/threonine protein kinase that regulates cell growth, proliferation, and metabolism [275,276]. The mTOR complex is expressed by numerous cell types, and mTOR complex 1 (mTORC1) specifically contributes to cellular homeostasis and modulates glucose metabolism by driving the expression of HIF-1α in a multifaceted manner [277,278,279,280]. IL-33 can activate mTOR, more specifically mTORC1, eliciting a shift favoring glycolytic metabolism and an increase in HIF-dependent VEGFA expression in various immune cells [281,282,283,284,285]. Conversely, HIF-1α can reduce ILC2 responsiveness to IL-33 and even further exacerbate IL-33 production in rheumatoid arthritis, making it a target of drug treatment and indicating that this proinflammatory-hypoxic crosstalk is bidirectional [286,287].
In mice infected with E. histolytica, IL-33 reliably recruits ILC2s to protect against amebic colitis but can also exacerbate amebic liver abscess pathogenesis [288,289]. Mice infected with Cryptosporidium exhibit elevated levels of IL-33 in the small intestine, although C. parvum possesses IL33-silencing RNA transcripts that can be transferred to the host epithelium during invasion to block the IL-33 response [290,291]. In toxoplasmosis, IL-33 plays a role in the pathogenesis of T. gondii in the brain, acting locally to promote astrocyte control of parasitic burden and reducing the risk of encephalitis [292,293]. Blockage of the IL-33/ST2 pathway lessens the severity of ileitis in mice induced by oral T. gondii, illustrating the organ-dependent response of IL-33 [294]. This contradictory role of IL-33 has also been noted during Plasmodium spp. infections. IL-33 prevents development of cerebral malaria (CM); however, the IL-33/ST2 pathway in mice lessens the pathogenicity of experimental cerebral malaria [295,296]. Ultimately, studies in the field have exemplified how IL-33 can either reduce the inflammatory response or exacerbate it depending on the cells it acts on and the location in the body [297]. Research is now warranted to characterize how this response interacts with hypoxia during infection.
5. Conclusions
In summary, HIF can be modulated by protozoa to influence the pathophysiology of parasitic infections. For tissue and blood protozoa, the role of HIF is varied and may help or hinder the parasite in establishing an infection. Little is known about the role of HIF during enteric protozoan parasitic infections, though transcriptomic studies on intestinal epithelial cells clearly point to a hypoxic signature. More research is needed to characterize how pathophysiology may be altered under hypoxic conditions during protozoan infections. Given that HIF can modulate the transcription of key pro-inflammatory mediators, the role of this transcription factor is of great importance when interrogating host immune responses against protozoa. Elucidation of the role of tissue and mucosal oxygen tension and HIF status during these parasitic infections will provide great insight regarding host–parasite interactions and may also have downstream effects on the microbial community present at the sight of infection. Moreover, microbiota dysbiosis associated with protozoan infections may impact hypoxia-related functions since bacterial-derived metabolites can directly activate HIF. Conversely, a hypoxic environment may alter local commensal communities. The effects of hypoxia on mucosal microbiota during infection and mechanisms driving homeostatic modulation will shed light on avenues with great potential for therapeutic development.
Author Contributions
Conceptualization E.D., O.S., A.G.B. and T.A.; writing—original draft preparation, E.D., O.S., A.G.B. and T.A.; writing—review and editing, E.D., O.S., A.G.B. and T.A.; visualization, E.D., O.S., A.G.B. and T.A.; supervision, A.G.B. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef]
- Schaible, B.; Schaffer, K.; Taylor, C.T. Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol. 2010, 174, 235–243. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- He, G.; Shankar, R.A.; Chzhan, M.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. USA 1999, 96, 4586–4591. [Google Scholar] [CrossRef]
- Sheridan, W.G.; Lowndes, R.H.; Young, H.L. Intraoperative tissue oximetry in the human gastrointestinal tract. Am. J. Surg. 1990, 159, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, T.; Paul, T. Oxygen Conformance in Hepatocytes. Am. J. Physiol. 1993, 265 Pt 1, L395–L402. [Google Scholar] [CrossRef] [PubMed]
- Budinger, G.R.; Chandel, N.; Shao, Z.H.; Li, C.Q.; Melmed, A.; Becker, L.B.; Schumacker, P.T. Cellular energy utilization and supply during hypoxia in embryonic cardiac myocytes. Am. J. Physiol.-Lung Cell Mol. Physiol. 1996, 270, L44–L53. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.-H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-J.; Wang, L.-Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential Roles of Hypoxia-Inducible Factor 1α (HIF-1α) and HIF-2α in Hypoxic Gene Regulation. Mol. Cell Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Lemos, R.; Liu, X.; Powis, G. The Hypoxia-Associated Factor Switches Cells from HIF-1α- to HIF-2α-Dependent Signaling Promoting Stem Cell Characteristics, Aggressive Tumor Growth and Invasion. Cancer Res. 2011, 71, 4015–4027. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Moran, S.M.; Hogenesch, J.B.; Wartman, L.; Bradfield, C.A. Molecular Characterization and Chromosomal Localization of a Third α- Class Hypoxia Inducible Factor Subunit, HIF3α. Gene Expr. 1998, 7, 205–213. [Google Scholar] [PubMed]
- Hara, S.; Hamada, J.; Kobayashi, C.; Kondo, Y.; Imura, N. Expression and Characterization of Hypoxia-Inducible Factor (HIF)-3α in Human Kidney: Suppression of HIF-Mediated Gene Expression by HIF-3α. Biochem. Biophys. Res. Commun. 2001, 287, 808–813. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, X.R.; Yang, Y.W.; Lin, H. Hypoxia upregulates hypoxia inducible factor (HIF)-3α expression in lung epithelial cells: Characterization and comparison with HIF-1α. Cell Res. 2006, 16, 548–558. [Google Scholar] [CrossRef]
- Safran, M.; Kaelin, W.G., Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Investig. 2003, 111, 779–783. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Gorman, J.J.; Whelan, D.A.; Whitelaw, M.L.; Bruick, R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002, 16, 1466–1471. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine Hydroxylation of the HIF Transactivation Domain: A Hypoxic Switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 838205. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-inducible Factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial Cell-Specific regulation of Hypoxia-Inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meng, L.; Kim, G.; Mills, T.A.; Benjamin, I.J.; Sherman, M.Y. Heat Shock Transcription Factor Hsf1 Is Involved in Tumor Progression via Regulation of Hypoxia-Inducible Factor 1 and RNA-Binding Protein HuR. Mol. Cell Biol. 2012, 32, 929–940. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2020, 599, 23–37. [Google Scholar] [CrossRef]
- Shepherd, A.P. Metabolic control of intestinal oxygenation and blood flow. Fed. Proc. 1982, 41, 2084–2089. [Google Scholar]
- Matheson, P.J.; Wilson, M.A.; Garrison, R. Regulation of Intestinal Blood Flow. J. Surg. Res. 2000, 93, 182–196. [Google Scholar] [CrossRef]
- Fisher, E.M.; Khan, M.; Salisbury, R.; Kuppusamy, P. Noninvasive Monitoring of Small Intestinal Oxygen in a Rat Model of Chronic Mesenteric Ischemia. Cell Biochem. Biophys. 2013, 67, 451–459. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation Between Intraluminal Oxygen Gradient and Radial Partitioning of Intestinal Microbiota. Gastroenterology 2014, 147, 1055–1063.e8. [Google Scholar] [CrossRef] [PubMed]
- Konjar, Š.; Pavšič, M.; Veldhoen, M. Regulation of Oxygen Homeostasis at the Intestinal Epithelial Barrier Site. Int. J. Mol. Sci. 2021, 22, 9170. [Google Scholar] [CrossRef]
- Taylor, C.T.; Colgan, S.P. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007, 85, 1295–1300. [Google Scholar] [CrossRef]
- Colgan, S.P.; Taylor, C. Hypoxia: An alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 281–287. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Ruan, K.; Song, G.; Ouyang, G. Role of hypoxia in the hallmarks of human cancer. J. Cell Biochem. 2009, 107, 1053–1062. [Google Scholar] [CrossRef]
- Shi, R.; Liao, C.; Zhang, Q. Hypoxia-Driven Effects in Cancer: Characterization, Mechanisms, and Therapeutic Implications. Cells 2021, 10, 678. [Google Scholar] [CrossRef]
- Sebestyén, A.; Kopper, L.; Dankó, T.; Tímár, J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol. Oncol. Res. 2021, 27, 1609802. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, R.A.; Hammond, E.M.; Dorie, M.J.; Welford, S.M.; Giaccia, A.J. DNA Damage during Reoxygenation Elicits a Chk2-Dependent Checkpoint Response. Mol. Cell Biol. 2006, 26, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.A.; Fines, K.; Ng, J.; Traboulsi, D.; Lee, J.; Ihara, E.; Li, Y.; Willmore, W.G.; Chung, D.; Scully, M.M.; et al. Hypoxia-Inducible Factor Signaling Provides Protection in Clostridium difficile-Induced Intestinal Injury. Gastroenterology 2010, 139, 259–269.e3. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Furuta, G.T.; Tomaszewski, J.E.; Johnson, R.S.; Colgan, S.P.; Haase, V.H. Epithelial Hypoxia-Inducible Factor-1 Is Protective in Murine Experimental Colitis. J. Clin. Investig. 2004, 114, 1098–1106. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation Hypoxia-Induced Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Stauffer, W.; Ravdin, J.I. Entamoeba histolytica: An update. Curr. Opin. Infect. Dis. 2003, 16, 479–485. [Google Scholar] [CrossRef]
- Stanley, S.L. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef]
- Pineda, E.; Perdomo, D. Entamoeba histolytica under Oxidative Stress: What Countermeasure Mechanisms Are in Place? Cells 2017, 6, 44. [Google Scholar] [CrossRef]
- Santos, F.; Nequiz, M.; Hernández-Cuevas, N.A.; Hernández, K.; Pineda, E.; Encalada, R.; Guillén, N.; Luis-García, E.; Saralegui, A.; Saavedra, E.; et al. Maintenance of intracellular hypoxia and adequate heat shock response are essential requirements for pathogenicity and virulence of Entamoeba histolytica. Cell Microbiol. 2015, 17, 1037–1051. [Google Scholar] [CrossRef]
- Ghadirian, E.; Denis, M. In vivo activation of macrophages by IFN-γ to kill Entamoeba histolytica trophozoites in vitro. Parasite Immunol. 1992, 14, 397–404. [Google Scholar] [CrossRef]
- Lin, J.Y.; Chadee, K. Macrophage cytotoxicity against Entamoeba histolytica trophozoites is mediated by nitric oxide from L-arginine. J. Immunol. 1992, 148, 3999–4005. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.B.; Ehrenkaufer, G.M.; Saraiva, L.M.; Teixeira, M.; Singh, U. Entamoeba histolytica modulates a complex repertoire of novel genes in response to oxidative and nitrosative stresses: Implications for amebic pathogenesis. Cell Microbiol. 2009, 11, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Chatterjee, N.S.; Sen, P.; Debnath, A.; Pal, A.; Bera, T.; Das, P. Genes induced by a high-oxygen environment in Entamoeba histolytica. Mol. Biochem. Parasitol. 2004, 133, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.G.; Gutierrez, C.E.; Iglesias, A.A.; Guerrero, S.A. Thioredoxin-linked metabolism in Entamoeba histolytica. Free Radic. Biol. Med. 2007, 42, 1496–1505. [Google Scholar] [CrossRef]
- Groneberg, M.; Hoenow, S.; Marggraff, C.; Fehling, H.; Metwally, N.G.; Hansen, C.; Bruchhaus, I.; Tiegs, G.; Sellau, J.; Lotter, H. HIF-1α modulates sex-specific Th17/Treg responses during hepatic amoebiasis. J. Hepatol. 2021, 76, 160–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Stanley, S.L. Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell Microbiol. 2004, 6, 535–554. [Google Scholar] [CrossRef]
- Verma, R.N.; Malik, Z.; Subbarao, N.; Singh, G.P.; Sinha, D.N. Entamoeba histolytica HM-1: IMSS gene expression profiling identifies key hub genes, potential biomarkers, and pathways in Amoebiasis infection: A systematic network meta-analysis. Biosci. Rep. 2022, 42, BSR20220191. [Google Scholar] [CrossRef]
- Roxstrom-Lindquist, K.; Ringqvist, E.; Palm, D.; Svard, S. Giardia lamblia-Induced Changes in Gene Expression in Differentiated Caco-2 Human Intestinal Epithelial Cells. Infect. Immun. 2005, 73, 8204–8208. [Google Scholar] [CrossRef]
- Rojas, L.; Grüttner, J.; Ma’ayeh, S.; Xu, F.; Svärd, S.G. Dual RNA Sequencing Reveals Key Events When Different Giardia Life Cycle Stages Interact with Human Intestinal Epithelial Cells In Vitro. Front. Cell Infect. Microbiol. 2022, 12, 862211. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Sidorenko, N.V.; Matveev, I.V.; Kropotov, A.V.; Vinogradov, A.E. Remodeling of rat cardiomyocytes after neonatal cryptosporidiosis. II. Deformation, excessive polyploidization, and HIF-1α overexpression. Cell Tissue Biol. 2012, 6, 472–484. [Google Scholar] [CrossRef]
- Deng, M.; Lancto, C.A.; Abrahamsen, M.S. Cryptosporidium parvum regulation of human epithelial cell gene expression. Int. J. Parasitol. 2004, 34, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Degrossoli, A.; Bosetto, M.C.; Lima, C.B.C.; Giorgio, S. Expression of hypoxia-inducible factor 1α in mononuclear phagocytes infected with Leishmania amazonensis. Immunol. Lett. 2007, 114, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Degrossoli, A.; Arrais-Silva, W.W.; Colhone, M.C.; Gadelha, F.R.; Joazeiro, P.P.; Giorgio, S. The Influence of Low Oxygen on Macrophage Response to Leishmania Infection. Scand. J. Immunol. 2011, 74, 165–175. [Google Scholar] [CrossRef]
- Araújo, A.P.; Arrais-Silva, W.W.; Giorgio, S. Acta Histochemica Infection by Leishmania amazonensis in Mice: A Potential Model for Chronic Hypoxia. Acta Histochem. 2012, 114, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Das, S.; Kumar, A.; Abhishek, K.; Verma, S.; Mandal, A.; Singh, R.K.; Das, P. Leishmania donovani Activates Hypoxia Inducible Factor-1α and miR-210 for Survival in Macrophages by Downregulation of NF-κB Mediated Pro-inflammatory Immune Response. Front. Microbiol. 2018, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Bettadapura, M.; Roys, H.; Bowlin, A.; Venugopal, G.; Washam, C.L.; Fry, L.; Murdock, S.; Wanjala, H.; Byrum, S.D.; Weinkopff, T. HIF-α Activation Impacts Macrophage Function during Murine Leishmania major Infection. Pathogens 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Spear, W.; Chan, D.; Coppens, I.; Johnson, R.S.; Giaccia, A.; Blader, I.J. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2005, 8, 339–352. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Ou, M.; Ye, X.; Zhang, B.; Wu, T.; Dong, S.; Chen, X.; Liu, H.; Zheng, Z.; et al. Toxoplasma gondii Cathepsin C1 inhibits NF-κB signalling through the positive regulation of the HIF-1α/EPO axis. Acta Trop. 2019, 195, 35–43. [Google Scholar] [CrossRef]
- Syn, G.; Anderson, D.; Blackwell, J.M.; Jamieson, S.E. Toxoplasma gondii Infection Is Associated with Mitochondrial Dysfunction In-Vitro. Front. Cell Infect. Microbiol. 2017, 7, 512. [Google Scholar] [CrossRef]
- Blader, I.J.; Manger, I.D.; Boothroyd, J.C. Microarray Analysis Reveals Previously Unknown Changes in Toxoplasma gondii-infected Human Cells. J. Biol. Chem. 2001, 276, 24223–24231. [Google Scholar] [CrossRef]
- Park, M.-K.; Ko, E.-J.; Jeon, K.-Y.; Kim, H.; Jo, J.-O.; Baek, K.-W.; Kang, Y.-J.; Choi, Y.H.; Hong, Y.; Ock, M.S.; et al. Induction of Angiogenesis by Malarial Infection through Hypoxia Dependent Manner. Korean J. Parasitol. 2019, 57, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Venturini, G.; Alvim, J.M.; Padilha, K.; Toepfer, C.N.; Gorham, J.M.; Wasson, L.K.; Biagi, D.; Schenkman, S.; Carvalho, V.M.; Salgueiro, J.S.; et al. Cardiomyocyte infection by Trypanosoma cruzi promotes innate immune response and glycolysis activation. Front. Cell Infect. Microbiol. 2023, 13, 1098457. [Google Scholar] [CrossRef]
- Ivanov, A.I. Giardia and giardiasis. Bulg. J. Vet. Med. 2010, 13, 65–80. [Google Scholar]
- Cotton, J.A.; Beatty, J.K.; Buret, A.G. Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 2011, 41, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Halliez, M.C.M.; Buret, A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013, 19, 8974–8985. [Google Scholar] [CrossRef]
- Allain, T.; Amat, C.B.; Motta, J.-P.; Manko, A.; Buret, A.G. Interactions of Giardia sp. with the intestinal barrier: Epithelium, mucus, and microbiota. Tissue Barriers 2017, 5, e1274354. [Google Scholar] [CrossRef] [PubMed]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Harris, J.C.; Maroulis, S.; Biagini, G.A.; Wadley, R.B.; Turner, M.P.; Edwards, M.R. The microaerophilic flagellate Giardia intestinalis: Oxygen and its reaction products collapse membrane potential and cause cytotoxicity. Microbiology 2000, 146, 3109–3118. [Google Scholar] [CrossRef]
- Han, J.; Collins, L.J. Reconstruction of Sugar Metabolic Pathways of Giardia lamblia. Int. J. Proteom. 2012, 2012, 980829. [Google Scholar] [CrossRef]
- Lindmark, D.G. Energy metabolism of the anaerobic protozoon Giardia lamblia. Mol. Biochem. Parasitol. 1980, 1, 1–12. [Google Scholar] [CrossRef]
- Brown, D.M.; Upcroft, J.A.; Upcroft, P. Free radical detoxification in Giardia duodenalis. Mol. Biochem. Parasitol. 1995, 72, 47–56. [Google Scholar] [CrossRef]
- Mastronicola, D.; Giuffrè, A.; Testa, F.; Mura, A.; Forte, E.; Bordi, E.; Pucillo, L.P.; Fiori, P.L.; Sarti, P. Giardia intestinalis escapes oxidative stress by colonizing the small intestine: A molecular hypothesis. IUBMB Life 2011, 63, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Scandurra, F.M.; Testa, F.; Forte, E.; Sarti, P.; Brunori, M.; Giuffrè, A. The O2-scavenging Flavodiiron Protein in the Human Parasite Giardia intestinalis. J. Biol. Chem. 2008, 283, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- Townson, S.M.; Hanson, G.R.; Upcroft, J.A.; Upcroft, P. A purified ferredoxin from Giardia duodenalis. JBIC J. Biol. Inorg. Chem. 1994, 220, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Paget, T.A.; Macechko, P.T.; Jarroll, E.L. Metabolic Changes in Giardia intestinalis during Differentiation. J. Parasitol. 1998, 84, 222–226. [Google Scholar] [CrossRef]
- Holthaus, D.; Kraft, M.R.; Krug, S.M.; Wolf, S.; Müller, A.; Betancourt, E.D.; Schorr, M.; Holland, G.; Knauf, F.; Schulzke, J.-D.; et al. Dissection of Barrier Dysfunction in Organoid-Derived Human Intestinal Epithelia Induced by Giardia duodenalis. Gastroenterology 2022, 162, 844–858. [Google Scholar] [CrossRef]
- Ma’Ayeh, S.Y.; Knörr, L.; Sköld, K.; Garnham, A.; Ansell, B.; Jex, A.; Svärd, S.G. Responses of the Differentiated Intestinal Epithelial Cell Line Caco-2 to Infection with the Giardia intestinalis GS Isolate. Front. Cell Infect. Microbiol. 2018, 8, 244. [Google Scholar] [CrossRef]
- Povero, D.; Johnson, S.M.; Liu, J. Hypoxia, hypoxia-inducible gene 2 (HIG2)/HILPDA, and intracellular lipolysis in cancer. Cancer Lett. 2020, 493, 71–79. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium Pathogenicity and Virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- Xu, P.; Widmer, G.; Wang, Y.; Ozaki, L.S.; Alves, J.M.; Serrano, M.G.; Puiu, D.; Manque, P.; Akiyoshi, D.; Mackey, A.J.; et al. The genome of Cryptosporidium hominis. Nature 2004, 431, 1107–1112. [Google Scholar] [CrossRef]
- Barta, J.R.; Thompson, R.A. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006, 22, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bilung, L.M.; Tahar, A.S.; Yunos, N.E.; Apun, K.; Lim, Y.A.-L.; Nillian, E.; Hashim, H.F. Detection of Cryptosporidium and Cyclospora Oocysts from Environmental Water for Drinking and Recreational Activities in Sarawak, Malaysia. BioMed Res. Int. 2017, 2017, 4636420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Chang, Y.; Fu, Y.; Wang, Y.; Zhang, K.; Zhang, S.; Zhang, L. Microbiome-Metabolomics Analysis of the Impacts of Cryptosporidium muris Infection in BALB/C Mice. Microbiol. Spectr. 2022, 11, e02175-22. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.E.; Colgan, S.P. Epithelial Barrier Regulation by Hypoxia-Inducible Factor. Ann. Am. Thorac. Soc. 2017, 14, S233–S236. [Google Scholar] [CrossRef]
- Luo, P.-L.; Wang, Y.-J.; Yang, Y.-Y.; Yang, J.-J. Hypoxia-induced hyperpermeability of rat glomerular endothelial cells involves HIF-2α mediated changes in the expression of occludin and ZO-1. Braz. J. Med. Biol. Res. 2018, 51, 51. [Google Scholar] [CrossRef]
- Lochhead, J.J.; McCaffrey, G.; Quigley, C.E.; Finch, J.; DeMarco, K.M.; Nametz, N.; Davis, T.P. Oxidative Stress Increases Blood-Brain Barrier Permeability and Induces Alterations in Occludin during Hypoxia-Reoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 1625–1636. [Google Scholar] [CrossRef]
- Dowdell, A.S.; Cartwright, I.M.; Goldberg, M.S.; Kostelecky, R.; Ross, T.; Welch, N.; Glover, L.E.; Colgan, S.P. The HIF target ATG9A is essential for epithelial barrier function and tight junction biogenesis. Mol. Biol. Cell 2020, 31, 2249–2258. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef]
- Leroy, A.; Lauwaet, T.; De Bruyne, G.; Cornelissen, M.; Mareel, M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J. 2000, 14, 1139–1146. [Google Scholar] [CrossRef]
- Kissoon-Singh, V.; Moreau, F.; Trusevych, E.; Chadee, K. Entamoeba histolytica Exacerbates Epithelial Tight Junction Permeability and Proinflammatory Responses in Muc2 Mice. Am. J. Pathol. 2013, 182, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, Z.; Hellman, L.; Svärd, S.G. Cleavage specificity of recombinant Giardia intestinalis cysteine proteases: Degradation of immunoglobulins and defensins. Mol. Biochem. Parasitol. 2018, 227, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Allain, T.; Fekete, E.; Buret, A.G. Giardia Cysteine Proteases: The Teeth behind the Smile. Trends Parasitol. 2019, 35, 636–648. [Google Scholar] [CrossRef]
- Allain, T.; Buret, A.G. Chapter Five—Pathogenesis and Post-Infectious Complications in Giardiasis. In Giardia and Giardiasis, Part B; Ortega-Pierres, M.G.B.T.-A., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 107, pp. 173–199. [Google Scholar] [CrossRef]
- Souza, J.B.; Tsantarlis, K.; Tonelli, R.R. Oxygen-dependent regulation of permeability in low resistance intestinal epithelial cells infected with Giardia lamblia. Exp. Parasitol. 2022, 240, 108329. [Google Scholar] [CrossRef]
- Ankri, S. Entamoeba histolytica—Gut Microbiota Interaction: More Than Meets the Eye. Microorganisms 2021, 9, 581. [Google Scholar] [CrossRef]
- Karpe, A.V.; Hutton, M.L.; Mileto, S.J.; James, M.L.; Evans, C.; Shah, R.M.; Ghodke, A.B.; Hillyer, K.E.; Metcalfe, S.S.; Liu, J.-W.; et al. Cryptosporidiosis Modulates the Gut Microbiome and Metabolism in a Murine Infection Model. Metabolites 2021, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Charania, R.; Wade, B.E.; McNair, N.N.; Mead, J.R. Changes in the Microbiome of Cryptosporidium-Infected Mice Correlate to Differences in Susceptibility and Infection Levels. Microorganisms 2020, 8, 879. [Google Scholar] [CrossRef]
- Fekete, E.; Allain, T.; Siddiq, A.; Sosnowski, O.; Buret, A.G. Giardia spp. and the Gut Microbiota: Dangerous Liaisons. Front. Microbiol. 2021, 11, 618106. [Google Scholar] [CrossRef]
- Gerbaba, T.K.; Gupta, P.; Rioux, K.; Hansen, D.; Buret, A.G. Giardia duodenalis-induced alterations of commensal bacteria kill Caenorhabditis elegans: A new model to study microbial-microbial interactions in the gut. Am. J. Physiol.-Gastrointest Liver Physiol. 2015, 308, G550–G561. [Google Scholar] [CrossRef]
- Beatty, J.K.; Akierman, S.V.; Motta, J.-P.; Muise, S.; Workentine, M.L.; Harrison, J.J.; Bhargava, A.; Beck, P.L.; Rioux, K.P.; McKnight, G.W.; et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int. J. Parasitol. 2017, 47, 311–326. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Karl, J.P.; Weber, G.J. Effects of short-chain fatty acids on intestinal function in an enteroid model of hypoxia. Front. Physiol. 2022, 13, 1056233. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, P.; Pascual, J.; Xiao, G.; Lu, H. Effect of Hypoxia and Hyperoxia on Cerebral Blood Flow, Blood Oxygenation, and Oxidative Metabolism. J. Cereb. Blood Flow Metab. 2012, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.B.; Lindberg, U.; Aachmann-Andersen, N.J.; Lisbjerg, K.; Christensen, S.; Law, I.; Rasmussen, P.; Olsen, N.V.; Larsson, H.B.W. Acute hypoxia increases the cerebral metabolic rate—A magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 2015, 36, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Kim, T.; Brumwell, A.N.; Driver, I.H.; Wei, Y.; Tan, V.; Jackson, J.R.; Xu, J.; Lee, D.-K.; Gotts, J.E.; et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017, 19, 904–914. [Google Scholar] [CrossRef]
- Straseski, J.A.; Gibson, A.L.; Thomas-Virnig, C.L.; Allen-Hoffmann, B.L. Oxygen deprivation inhibits basal keratinocyte proliferation in a model of human skin and induces regio-specific changes in the distribution of epidermal adherens junction proteins, aquaporin-3, and glycogen. Wound Repair Regen. 2009, 17, 606–616. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Schlein, Y. Leishmania and Sandflies: Interactions in the life cycle and transmission. Parasitol. Today 1993, 9, 255–258. [Google Scholar] [CrossRef]
- Kima, P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007, 37, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Van Hellemond, J.J.; Van Der Meer, P.; Tielens, A.G.M. Leishmania infantum promastigotes have a poor capacity for anaerobic functioning and depend mainly on respiration for their energy generation. Parasitology 1997, 114, 351–360. [Google Scholar] [CrossRef]
- James, P.E.; Grinberg, O.Y.; Swartz, H.M. Superoxide production by phagocytosing macrophages in relation to the intracellular distribution of oxygen. J. Leukoc. Biol. 1998, 64, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Wilkinson, S.; Mottram, J.C.; Fairlamb, A.H. Evidence that trypanothione reductase is an essential enzyme in Leishmania by targeted replacement of the tryA gene locus. Mol. Microbiol. 1998, 29, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Nandal, A.; Ruiz, J.C.; Subramanian, P.; Ghimire-Rijal, S.; Sinnamon, R.A.; Stemmler, T.L.; Bruick, R.K.; Philpott, C.C. Activation of the HIF Prolyl Hydroxylase by the Iron Chaperones PCBP1 and PCBP2. Cell Metab. 2011, 14, 647–657. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, C.; Biswas, S.; Singh, V.K.; Mukhopadhyay, C.K. Intracellular Pathogen Leishmania donovani Activates Hypoxia Inducible Factor-1 by Dual Mechanism for Survival Advantage within Macrophage. PLoS ONE 2012, 7, e38489. [Google Scholar] [CrossRef]
- Pelegrini, M.D.; Pereira, J.B.; Costa, S.D.S.; Terreros, M.J.S.; Degrossoli, A.; Giorgio, S. Evaluation of hypoxia inducible factor targeting pharmacological drugs as antileishmanial agents. Asian Pac. J. Trop. Med. 2016, 9, 652–657. [Google Scholar] [CrossRef]
- Weinkopff, T.; Roys, H.; Bowlin, A.; Scott, P. Leishmania Infection Induces Macrophage Vascular Endothelial Growth Factor A Production in an ARNT/HIF-Dependent Manner. Infect. Immun. 2019, 87, e00088-19. [Google Scholar] [CrossRef]
- Bowlin, A.; Roys, H.; Wanjala, H.; Bettadapura, M.; Venugopal, G.; Surma, J.; Simon, M.C.; Weinkopff, T. Hypoxia-Inducible Factor Signaling in Macrophages Promotes Lymphangiogenesis in Leishmania major Infection. Infect. Immun. 2021, 89, e00124-21. [Google Scholar] [CrossRef]
- Alonso, D.; Serrano, E.; Bermejo, F.J.; Corral, R.S. HIF-1α-regulated MIF activation and Nox2-dependent ROS generation promote Leishmania amazonensis killing by macrophages under hypoxia. Cell Immunol. 2019, 335, 15–21. [Google Scholar] [CrossRef]
- Colhone, M.C.; Arrais-Silva, W.W.; Picoli, C.; Giorgio, S. Effect of hypoxia on macrophage infection by Leishmania amazonensis. J. Parasitol. 2004, 90, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Bosseto, M.C.; Palma, P.V.B.; Covas, D.T.; Giorgio, S. Hypoxia modulates phenotype, inflammatory response, and leishmanial infection of human dendritic cells. Apmis 2010, 118, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Schatz, V.; Strüssmann, Y.; Mahnke, A.; Schley, G.; Waldner, M.; Ritter, U.; Wild, J.; Willam, C.; Dehne, N.; Brüne, B.; et al. Myeloid Cell-Derived HIF-1α Promotes Control of Leishmania major. J. Immunol. 2016, 197, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.A.; Lera-Nonose, D.S.S.L.; Brustolin, A.A.; Duarte, G.C.; dos Santos, M.C.M.; Lonardoni, M.V.C.; Silveira, T.G.V. Low expression of hypoxia-inducible factor-1α and differential expression of immune mediators during experimental infection with Leishmania (Viannia) spp. Cytokine 2022, 153, 155833. [Google Scholar] [CrossRef]
- Ben-Cheikh, A.; Bali, A.; Guerfali, F.Z.; Atri, C.; Attia, H.; Laouini, D. Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence. Korean J. Parasitol. 2022, 60, 317–325. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Behnke, M.S.; Zhang, T.P.; Dubey, J.P.; Sibley, L.D. Toxoplasma gondii merozoite gene expression analysis with comparison to the life cycle discloses a unique expression state during enteric development. BMC Genom. 2014, 15, 350. [Google Scholar] [CrossRef]
- Liu, T.; Abboud, M.I.; Chowdhury, R.; Tumber, A.; Hardy, A.P.; Lippl, K.; Lohans, C.T.; Pires, E.; Wickens, J.; McDonough, M.A.; et al. Biochemical and biophysical analyses of hypoxia sensing prolyl hydroxylases from Dictyostelium discoideum and Toxoplasma gondii. J. Biol. Chem. 2020, 295, 16545–16561. [Google Scholar] [CrossRef]
- Xu, Y.; Brown, K.M.; Wang, Z.A.; van der Wel, H.; Teygong, C.; Zhang, D.; Blader, I.J.; West, C.M. The Skp1 Protein from Toxoplasma Is Modified by a Cytoplasmic Prolyl 4-Hydroxylase Associated with Oxygen Sensing in the Social Amoeba Dictyostelium. J. Biol. Chem. 2012, 287, 25098–25110. [Google Scholar] [CrossRef]
- Seidi, A.; Muellner-Wong, L.S.; Rajendran, E.; Tjhin, E.T.; Dagley, L.F.; Aw, V.Y.; Faou, P.; Webb, A.I.; Tonkin, C.J.; Van Dooren, G.G. Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. eLife 2018, 7, e38131. [Google Scholar] [CrossRef]
- Pino, P.; Foth, B.J.; Kwok, L.-Y.; Sheiner, L.; Schepers, R.; Soldati, T.; Soldati-Favre, D. Dual Targeting of Antioxidant and Metabolic Enzymes to the Mitochondrion and the Apicoplast of Toxoplasma gondii. PLoS Pathog. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Kwok, L.Y.; Schlüter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Suvorova, E.; Farrell, A.; McLain, A.; Dittmar, A.; Wiley, G.B.; Marth, G.; Gaffney, P.; Gubbels, M.-J.; White, M.; et al. Forward Genetic Screening Identifies a Small Molecule That Blocks Toxoplasma gondii Growth by Inhibiting Both Host- and Parasite-Encoded Kinases. PLoS Pathog. 2014, 10, e1004180. [Google Scholar] [CrossRef] [PubMed]
- Wiley, M.; Sweeney, K.R.; Chan, D.A.; Brown, K.M.; McMurtrey, C.; Howard, E.W.; Giaccia, A.J.; Blader, I.J. Toxoplasma gondii Activates Hypoxia-inducible Factor (HIF) by Stabilizing the HIF-1α Subunit via Type I Activin-like Receptor Kinase Receptor Signaling. J. Biol. Chem. 2010, 285, 26852–26860. [Google Scholar] [CrossRef] [PubMed]
- Lis, A.; Wiley, M.; Vaughan, J.; Gray, P.C.; Blader, I.J. The Activin Receptor, Activin-Like Kinase 4, Mediates Toxoplasma Gondii Activation of Hypoxia Inducible Factor-1. Front. Cell Infect. Microbiol. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Menendez, M.T.; Teygong, C.; Wade, K.; Florimond, C.; Blader, J. Important Hypoxia-Inducible Transcription Factor 1 (HIF-1) Target Gene in Toxoplasma Gondii-Infected Cells. MBio 2015, 6, e00462-15. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.; Gross, U.; Bohne, W. Transcriptional profile of Toxoplasma gondii-infected human fibroblasts as revealed by gene-array hybridization. Mol. Genet. Genom. 2001, 265, 905–912. [Google Scholar] [CrossRef]
- Jagannathan, P.; Kakuru, A. Malaria in 2022: Increasing challenges, cautious optimism. Nat. Commun. 2022, 13, 12–14. [Google Scholar] [CrossRef]
- Sato, S. Plasmodium—A Brief Introduction to the Parasites Causing Human Malaria and Their Basic Biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef]
- Egan, T.J. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J. Inorg. Biochem. 2008, 102, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.G.; Matuschewski, K.; Zhang, M.; Rug, M. Plasmodium falciparum. Trends Parasitol. 2019, 35, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, L.W.; Ashton, S.H.; Trager, W. Plasmodium falciparum: Microaerophilic requirements in human red blood cells. Exp. Parasitol. 1979, 47, 410–418. [Google Scholar] [CrossRef]
- Krungkrai, J. The multiple roles of the mitochondrion of the malarial parasite. Parasitology 2004, 129, 511–524. [Google Scholar] [CrossRef]
- Murphy, A.D.; Doeller, J.E.; Hearn, B.; Lang-Unnasch, N. Plasmodium falciparum: Cyanide-Resistant Oxygen Consumption. Exp. Parasitol. 1997, 87, 112–120. [Google Scholar] [CrossRef]
- Vaidya, A.B.; Mather, M.W. Mitochondrial Evolution and Functions in Malaria Parasites. Annu. Rev. Microbiol. 2009, 63, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Torrentino-Madamet, M.; Fall, B.; Benoit, N.; Camara, C.; Amalvict, R.; Fall, M.; Dionne, P.; Fall, K.B.; Nakoulima, A.; Diatta, B.; et al. Limited polymorphisms in k13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012–2013. Malar. J. 2014, 13, 472. [Google Scholar] [CrossRef]
- Tsai, A.G.; Johnson, P.G.; Intaglietta, M. Oxygen Gradients in the Microcirculation. Physiol. Rev. 2003, 83, 933–963. [Google Scholar] [CrossRef]
- Ng, S.; March, S.; Galstian, A.; Hanson, K.; Carvalho, T.; Mota, M.M.; Bhatia, S.N. Hypoxia promotes liver stage malaria infection in primary human hepatocytes in vitro. Dis. Model. Mech. 2013, 7, 215–224. [Google Scholar] [CrossRef]
- Hempel, C.; Combes, V.; Hunt, N.H.; Kurtzhals, J.A.L.; Grau, G.E.R. CNS Hypoxia Is More Pronounced in Murine Cerebral than Noncerebral Malaria and Is Reversed by Erythropoietin. Am. J. Pathol. 2011, 179, 1939–1950. [Google Scholar] [CrossRef]
- Boeuf, P.; Tan, A.; Romagosa, C.; Radford, J.; Mwapasa, V.; Molyneux, M.E.; Meshnick, S.R.; Hunt, N.H.; Rogerson, S.J. Placental Hypoxia during Placental Malaria. J. Infect. Dis. 2008, 197, 757–765. [Google Scholar] [CrossRef]
- Medana, I.M.; Day, N.P.J.; Roberts, R.; Sachanonta, N.; Turley, H.; Pongponratn, E.; Hien, T.T.; White, N.J.; Turner, G.D.H. Induction of the vascular endothelial growth factor pathway in the brain of adults with fatal falciparum malaria is a non-specific response to severe disease. Histopathology 2010, 57, 282–294. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Andrews, N.W. Host cell invasion by Trypanosoma cruzi: A unique strategy that promotes persistence. FEMS Microbiol. Rev. 2012, 36, 734–747. [Google Scholar] [CrossRef]
- Macedo, A.; Pena, S. Genetic Variability of Trypanosoma cruzi: Implications for the Pathogenesis of Chagas Disease. Parasitol. Today 1998, 14, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.D.F.P.; Guarneri, A.A.; Silber, A.M. The Influence of Environmental Cues on the Development of Trypanosoma cruzi in Triatominae Vector. Front. Cell Infect. Microbiol. 2020, 10, 27. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Bourguignon, S.C.; Gonçalves, P.S.; Lechuga, G.C.; Provance, D.W. Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms. Pathogens 2022, 11, 268. [Google Scholar] [CrossRef]
- Saraiva, F.M.S.; Cosentino-Gomes, D.; Inacio, J.D.F.; Almeida-Amaral, E.E.; Louzada-Neto, O.; Rossini, A.; Nogueira, N.P.; Meyer-Fernandes, J.R.; Paes, M.C. Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism. Pathogens 2022, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Berná, L.; Chiribao, M.L.; Greif, G.; Rodriguez, M.; Alvarez-Valin, F.; Robello, C. Transcriptomic analysis reveals metabolic switches and surface remodeling as key processes for stage transition in Trypanosoma cruzi. PeerJ 2017, 5, e3017. [Google Scholar] [CrossRef]
- Gonçalves, R.L.S.; Barreto, R.F.S.M.; Polycarpo, C.R.; Gadelha, F.R.; Castro, S.L.; Oliveira, M.F. A comparative assessment of mitochondrial function in epimastigotes and bloodstream trypomastigotes of Trypanosoma cruzi. J. Bioenerg. Biomembr. 2011, 43, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, L.; Peluffo, G.; Alvarez, M.N.; Martínez, A.; Radi, R. Trypanosoma cruzi Antioxidant Enzymes as Virulence Factors in Chagas Disease. Antioxid. Redox Signal. 2013, 19, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, N.P.; Saraiva, F.M.S.; Sultano, P.E.; Cunha, P.R.B.B.; Laranja, G.A.T.; Justo, G.A.; Sabino, K.C.C.; Coelho, M.G.P.; Rossini, A.; Atella, G.C.; et al. Proliferation and Differentiation of Trypanosoma cruzi inside Its Vector Have a New Trigger: Redox Status. PLoS ONE 2015, 10, e0116712. [Google Scholar] [CrossRef]
- Eberhardt, N.; Sanmarco, L.M.; Bergero, G.; Theumer, M.G.; García, M.C.; Ponce, N.E.; Cano, R.C.; Aoki, M.P. Deficiency of CD73 activity promotes protective cardiac immunity against Trypanosoma cruzi infection but permissive environment in visceral adipose tissue. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1866, 165592. [Google Scholar] [CrossRef]
- Wallberg-Henriksson, J.R.Z.H.; Wallberg-Henriksson, J.H. GLUT4: A key player regulating glucose homeostasis? Insights from transgenic and knockout mice. Mol. Membr. Biol. 2001, 18, 205–211. [Google Scholar] [CrossRef]
- Adesse, D.; Iacobas, S.; Meirelles, M.d.N.; Tanowitz, H.B.; Spray, D.C.; Iacobas, D.A.; Garzoni, L.R. Transcriptomic Signatures of Alterations in a Myoblast Cell Line Infected with Four Distinct Strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2010, 82, 846–854. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; Corcoran, S.E.; Barry, P.J.G.; McFarland, J.; Crès, C.; Curtis, A.M.; Franklin, E.; Corr, S.C.; Mok, K.H.; Cummins, E.P.; et al. Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion. Proc. Natl. Acad. Sci. USA 2016, 113, E7778–E7787. [Google Scholar] [CrossRef]
- Olivera, G.C.; Vetter, L.; Tesoriero, C.; Del Gallo, F.; Hedberg, G.; Basile, J.; Rottenberg, M.E. Role of T cells during the cerebral infection with Trypanosoma brucei. PLoS Negl. Trop. Dis. 2021, 15, e0009764. [Google Scholar] [CrossRef] [PubMed]
- Engwerda, C.R.; Ng, S.; Bunn, P.T. The Regulation of CD4+ T Cell Responses during Protozoan Infections. Front. Immunol. 2014, 5, 498. [Google Scholar] [CrossRef] [PubMed]
- Serradell, M.C.; Gargantini, P.R.; Saura, A.; Oms, S.R.; Rupil, L.L.; Berod, L.; Sparwasser, T.; Luján, H.D. Cytokines, Antibodies, and Histopathological Profiles during Giardia Infection and Variant-Specific Surface Protein-Based Vaccination. Infect. Immun. 2018, 86, e00773-17. [Google Scholar] [CrossRef]
- Malkov, M.I.; Lee, C.T.; Taylor, C.T. Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines. Cells 2021, 10, 2340. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M.; Sun, L.; Xiao, W.; Yang, X.; Sun, L.; Zhang, C.; Ma, Y.; Yang, H.; Liu, Y.; et al. Interferon-γ-Induced Intestinal Epithelial Barrier Dysfunction by NF-κB/HIF-1α Pathway. J. Interf. Cytokine Res. 2014, 34, 195–203. [Google Scholar] [CrossRef]
- Hellwig-Burgel, T.; Rutkowski, K.; Metzen, E.; Fandrey, J.; Jelkmann, W. Interleukin-1β and Tumor Necrosis Factor-Stimulate DNA Binding of Hypoxia-Inducible Factor-1. Blood 1999, 94, 1561–1567. [Google Scholar] [CrossRef]
- Jung, Y.J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. IL-1beta-Mediated up-Regulation of HIF-1alpha via an NFkap-paB/COX-2 Pathway Identifies HIF-1 as a Critical Link between Inflammation and Oncogenesis. FASEB J. 2003, 17, 2115–2117. [Google Scholar] [CrossRef]
- Sartori-Cintra, A.R.; de Mara, C.S.; Argolo, D.L.; Coimbra, I.B. Regulation of hypoxia-inducible factor-1α (HIF-1α) expression by interleukin-1β (IL-1β), insulin-like growth factors I (IGF-I) and II (IGF-II) in human osteoarthritic chondrocytes. Clinics 2012, 67, 35–40. [Google Scholar] [CrossRef]
- Qian, D.; Lin, H.-Y.; Wang, H.-M.; Zhang, X.; Liu, D.-L.; Li, Q.-L.; Zhu, C. Normoxic Induction of the Hypoxic-Inducible Factor-1α by Interleukin-1β Involves the Extracellular Signal-Regulated Kinase 1/2 Pathway in Normal Human Cytotrophoblast Cells1. Biol. Reprod. 2004, 70, 1822–1827. [Google Scholar] [CrossRef]
- Berra, E.; Pagès, G.; Pouysségur, J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000, 19, 139–145. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is a Danger Signal That Induces IL-1β via HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Shah, Y.M.; Ito, S.; Morimura, K.; Chen, C.; Yim, S.; Haase, V.H.; Gonzalez, F.J. Hypoxia-Inducible Factor Augments Experimental Colitis through an MIF–Dependent Inflammatory Signaling Cascade. Gastroenterology 2008, 134, 2036–2048.e3. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Wittig, I.; Brüne, B. TMEM126B deficiency reduces mitochondrial SDH oxidation by LPS, attenuating HIF-1α stabilization and IL-1β expression. Redox Biol. 2018, 20, 204–216. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Zhao, Y.; Zhang, Y.; Li, H.; Zhang, A.; Wang, X.; Wang, W.; Hou, Y.; Wang, J. Succinate/IL-1β Signaling Axis Promotes the Inflammatory Progression of Endothelial and Exacerbates Atherosclerosis. Front. Immunol. 2022, 13, 817572. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Geng, X.; Warren, J.; Cosky, E.E.P.; Kaura, S.; Stone, C.; Li, F.; Ding, Y. Hypoxia Inducible Factor-1α (HIF-1α) Mediates NLRP3 Inflammasome-Dependent-Pyroptotic and Apoptotic Cell Death Following Ischemic Stroke. Neuroscience 2020, 448, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Cosin-Roger, J.; Simmen, S.; Melhem, H.; Atrott, K.; Frey-Wagner, I.; Hausmann, M.; de Vallière, C.; Spalinger, M.R.; Spielmann, P.; Wenger, R.H.; et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat. Commun. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Guan, S.; Wang, Z.; Ni, H.; Ding, D.; Xu, W.; Li, G. HIF-1α aggravated traumatic brain injury by NLRP3 inflammasome-mediated pyroptosis and activation of microglia. J. Chem. Neuroanat. 2021, 116, 101994. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, L.; Moreau, F.; Cornick, S.; Chadee, K. The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction. PLoS Pathog. 2015, 11, e1004887. [Google Scholar] [CrossRef]
- Li, X.; Feng, M.; Zhao, Y.; Zhang, Y.; Zhou, R.; Zhou, H.; Pang, Z.; Tachibana, H.; Cheng, X. A Novel TLR4-Binding Domain of Peroxiredoxin from Entamoeba histolytica Triggers NLRP3 Inflammasome Activation in Macrophages. Front. Immunol. 2021, 12, 758451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, L.; Wang, L.; Seydel, K.B.; Li, E.; Ankri, S.; Mirelman, D.; Stanley, S.L. Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol. Microbiol. 2000, 37, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Kim, S.-H.; Sajid, M.; Eckmann, L.; Dinarello, C.A.; McKerrow, J.H.; Reed, S.L. A Surface Amebic Cysteine Proteinase Inactivates Interleukin-18. Infect. Immun. 2003, 71, 1274–1280. [Google Scholar] [CrossRef]
- Manko-Prykhoda, A.; Allain, T.; Motta, J.-P.; Cotton, J.A.; Feener, T.; Oyeyemi, A.; Bindra, S.; Vallance, B.A.; Wallace, J.L.; Beck, P.; et al. Giardia spp. promote the production of antimicrobial peptides and attenuate disease severity induced by attaching and effacing enteropathogens via the induction of the NLRP3 inflammasome. Int. J. Parasitol. 2020, 50, 263–275. [Google Scholar] [CrossRef]
- Cotton, J.A.; Motta, J.-P.; Schenck, L.P.; Hirota, S.A.; Beck, P.L.; Buret, A.G. Giardia duodenalis Infection Reduces Granulocyte Infiltration in an in vivo Model of Bacterial Toxin-Induced Colitis and Attenuates Inflammation in Human Intestinal Tissue. PLoS ONE 2014, 9, e109087. [Google Scholar] [CrossRef]
- Cotton, J.A.; Bhargava, A.; Ferraz, J.G.; Yates, R.M.; Beck, P.L.; Buret, A.G. Giardia duodenalis Cathepsin B Proteases Degrade Intestinal Epithelial Interleukin-8 and Attenuate Interleukin-8-Induced Neutrophil Chemotaxis. Infect. Immun. 2014, 82, 2772–2787. [Google Scholar] [CrossRef]
- Liu, J.; Ma’Ayeh, S.; Peirasmaki, D.; Lundström-Stadelmann, B.; Hellman, L.; Svärd, S.G. Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines. Virulence 2018, 9, 879–894. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.; Fang, R.; Zhu, W.; Wu, J.; Li, X.; Patankar, J.V.; Li, W. Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination. Cells 2021, 10, 3425. [Google Scholar] [CrossRef]
- Riestra, A.M.; Valderrama, J.A.; Patras, K.A.; Booth, S.D.; Quek, X.Y.; Tsai, C.-M.; Nizet, V. Trichomonas vaginalis Induces NLRP3 Inflammasome Activation and Pyroptotic Cell Death in Human Macrophages. J. Innate Immun. 2018, 11, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Wang, X.; Dong, J.; Zhang, N.; Li, X.; Li, J.; Zhang, X.; Gong, P. Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLoS Negl. Trop. Dis. 2021, 15, e0009304. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Wang, X.; Li, J.; Dong, J.; Zhang, N.; Li, X.; Li, S.; Sun, M.; Zhang, X.; et al. Giardia duodenalis extracellular vesicles regulate the proinflammatory immune response in mouse macrophages in vitro via the MAPK, AKT and NF-κB pathways. Parasites Vectors 2021, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Liu, M.; Qin, X.; Yu, X.; Zhao, H.; Li, X.; Li, W. COX-2 is required to mediate crosstalk of ROS-dependent activation of MAPK/NF-κB signaling with pro-inflammatory response and defense-related NO enhancement during challenge of macrophage-like cell line with Giardia duodenalis. PLoS Negl. Trop. Dis. 2022, 16, e0010402. [Google Scholar] [CrossRef]
- Gurung, P.; Karki, R.; Vogel, P.; Watanabe, M.; Bix, M.; Lamkanfi, M.; Kanneganti, T.-D. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J. Clin. Investig. 2015, 125, 1329–1338. [Google Scholar] [CrossRef]
- Santos, D.; Campos, T.M.; Saldanha, M.; Oliveira, S.C.; Nascimento, M.; Zamboni, D.; Machado, P.R.; Arruda, S.; Scott, P.; Carvalho, E.M.; et al. IL-1β Production by Intermediate Monocytes Is Associated with Immunopathology in Cutaneous Leishmaniasis. J. Investig. Dermatol. 2018, 138, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.O.; Carvalho, A.M.; Clark, M.L.; Carvalho, L.P.; Beiting, D.P.; Brodsky, I.E.; Carvalho, E.M.; Scott, P. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog. 2017, 13, e1006196. [Google Scholar] [CrossRef] [PubMed]
- Charmoy, M.; Hurrell, B.P.; Romano, A.; Lee, S.H.; Ribeiro-Gomes, F.; Riteau, N.; Mayer-Barber, K.; Tacchini-Cottier, F.; Sacks, D.L. The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur. J. Immunol. 2016, 46, 897–911. [Google Scholar] [CrossRef]
- Gov, L.; Schneider, C.A.; Lima, T.S.; Pandori, W.; Lodoen, M.B. NLRP3 and Potassium Efflux Drive Rapid IL-1β Release from Primary Human Monocytes during Toxoplasma gondii Infection. J. Immunol. 2017, 199, 2855–2864. [Google Scholar] [CrossRef]
- Pandori, W.J.; Lima, T.S.; Mallya, S.; Kao, T.H.; Gov, L.; Lodoen, M.B. Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes. PLoS Pathog. 2019, 15, e1007923. [Google Scholar] [CrossRef] [PubMed]
- da Silva, H.B.; de Salles, M.; Panatieri, R.H.; Boscardin, S.B.; Rodríguez-Málaga, S.M.; Álvarez, J.M.; Lima, M.R.D. IFN-γ-Induced Priming Maintains Long-Term Strain-Transcending Immunity against Blood-Stage Plasmodium chabaudi Malaria. J. Immunol. 2013, 191, 5160–5169. [Google Scholar] [CrossRef] [PubMed]
- de Pinho, R.T.; da Silva, W.S.; Côrtes, L.M.D.C.; Sousa, P.D.S.V.; Soares, R.O.D.A.; Alves, C.R. Production of MMP-9 and inflammatory cytokines by Trypanosoma cruzi-infected macrophages. Exp. Parasitol. 2014, 147, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lamberet, A.; Rostan, O.; Dion, S.; Jan, A.; Guegan, H.; Manuel, C.; Samson, M.; Gangneux, J.-P.; Robert-Gangneux, F. IL-33/ST2 axis is involved in disease progression in the spleen during Leishmania donovani infection. Parasites Vectors 2020, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Rostan, O.; Gangneux, J.-P.; Piquet-Pellorce, C.; Manuel, C.; McKenzie, A.N.J.; Guiguen, C.; Samson, M.; Robert-Gangneux, F. The IL-33/ST2 Axis Is Associated with Human Visceral Leishmaniasis and Suppresses Th1 Responses in the Livers of BALB/c Mice Infected with Leishmania donovani. mBio 2013, 4, e00383-13. [Google Scholar] [CrossRef]
- Shio, M.T.; Eisenbarth, S.C.; Savaria, M.; Vinet, A.F.; Bellemare, M.-J.; Harder, K.W.; Sutterwala, F.S.; Bohle, D.S.; Descoteaux, A.; Flavell, R.A.; et al. Malarial Hemozoin Activates the NLRP3 Inflammasome through Lyn and Syk Kinases. PLoS Pathog. 2009, 5, e1000559. [Google Scholar] [CrossRef]
- Ouma, C.; Davenport, G.C.; Awandare, G.A.; Keller, C.C.; Were, T.; Otieno, M.F.; Vulule, J.M.; Martinson, J.; Ong’echa, J.M.; Ferrell, R.E.; et al. Polymorphic Variability in the Interleukin (IL)-1β Promoter Conditions Susceptibility to Severe Malarial Anemia and Functional Changes in IL-1β Production. J. Infect. Dis. 2008, 198, 1219–1226. [Google Scholar] [CrossRef]
- Guha, R.; Mathioudaki, A.; Doumbo, S.; Doumtabe, D.; Skinner, J.; Arora, G.; Siddiqui, S.; Li, S.; Kayentao, K.; Ongoiba, A.; et al. Plasmodium falciparum malaria drives epigenetic reprogramming of human monocytes toward a regulatory phenotype. PLoS Pathog. 2021, 17, e1009430. [Google Scholar] [CrossRef]
- Albina, J.E.; Mastrofrancesco, B.; Vessella, J.A.; Louis, C.A.; Henry, W.L., Jr.; Reichner, J.S. HIF-1 expression in healing wounds: HIF-1α induction in primary inflammatory cells by TNF-α. Am. J. Physiol. Cell Physiol. 2001, 281, C1971–C1977. [Google Scholar] [CrossRef]
- Haddad, J.J.; Land, S.C. A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 2001, 505, 269–274. [Google Scholar] [CrossRef]
- Taylor, C.T. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J. 2007, 409, 19–26. [Google Scholar] [CrossRef]
- van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Suzuki, S.; Kawasaki, N.; Nakano, H.; Okazaki, T.; Chino, A.; Doi, T.; Saiki, I. Tumor Necrosis Factor-α-induced IKK Phosphorylation of NF-κB p65 on Serine 536 Is Mediated through the TRAF2, TRAF5, and TAK1 Signaling Pathway. J. Biol. Chem. 2003, 278, 36916–36923. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.V.; Gosker, H.; Verhees, K.K.; Langen, R.C.J.; Schols, A.M.W.J. TNF-α-Induced NF-κB Activation Stimulates Skeletal Muscle Glycolytic Metabolism through Activation of HIF-1α. Endocrinology 2015, 156, 1770–1781. [Google Scholar] [CrossRef]
- Peterson, K.M.; Mondal, D.; Petri, W.A.; Duggal, P.; Haque, R.; Shu, J. Association between TNF-α and Entamoeba histolytica Diarrhea. Am. J. Trop. Med. Hyg. 2010, 82, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Helk, E.; Bernin, H.; Ernst, T.; Ittrich, H.; Jacobs, T.; Heeren, J.; Tacke, F.; Tannich, E.; Lotter, H. TNFα-Mediated Liver Destruction by Kupffer Cells and Ly6Chi Monocytes during Entamoeba histolytica Infection. PLoS Pathog. 2013, 9, e1003096. [Google Scholar] [CrossRef]
- Noor, Z.; Watanabe, K.; Abhyankar, M.M.; Burgess, S.L.; Buonomo, E.L.; Cowardin, C.A.; Petri, W.A. Role of Eosinophils and Tumor Necrosis Factor Alpha in Interleukin-25-Mediated Protection from Amebic Colitis. mBio 2017, 8, e02329-16. [Google Scholar] [CrossRef]
- Moonah, S.N.; Jiang, N.M.; Jr, W.A.P. Host Immune Response to Intestinal Amebiasis. PLoS Pathog. 2013, 9, e1003489. [Google Scholar] [CrossRef]
- Lin, J.Y.; Seguin, R.; Keller, K.; Chadee, K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect. Immun. 1994, 62, 1534–1541. [Google Scholar] [CrossRef]
- Blazquez, S.; Zimmer, C.; Guigon, G.; Olivo-Marin, J.-C.; Guillén, N.; Labruyère, E. Human Tumor Necrosis Factor is a Chemoattractant for the Parasite Entamoeba histolytica. Infect. Immun. 2006, 74, 1222–1232. [Google Scholar] [CrossRef]
- Zhou, P.; Li, E.; Shea-Donohue, T.; Singer, S.M. Tumour necrosis factor α contributes to protection against Giardia lamblia infection in mice. Parasite Immunol. 2007, 29, 367–374. [Google Scholar] [CrossRef]
- Muñoz-Cruz, S.; Gomez-García, A.; Matadamas-Martínez, F.; Alvarado-Torres, J.A.; Meza-Cervantez, P.; Arriaga-Pizano, L.; Yépez-Mulia, L. Giardia lamblia: Identification of molecules that contribute to direct mast cell activation. Parasitol. Res. 2018, 117, 2555–2567. [Google Scholar] [CrossRef]
- Lean, I.-S.; McDonald, S.A.C.; Bajaj-Elliott, M.; Pollok, R.C.G.; Farthing, M.J.G.; McDonald, V. Interleukin-4 and Transforming Growth Factor β Have Opposing Regulatory Effects on Gamma Interferon-Mediated Inhibition of Cryptosporidium parvum Reproduction. Infect. Immun. 2003, 71, 4580–4585. [Google Scholar] [CrossRef]
- Lacroix, S.; Mancassola, R.; Naciri, M.; Laurent, F. Cryptosporidium parvum-Specific Mucosal Immune Response in C57BL/6 Neonatal and Gamma Interferon-Deficient Mice: Role of Tumor Necrosis Factor Alpha in Protection. Infect. Immun. 2001, 69, 1635–1642. [Google Scholar] [CrossRef]
- Robinson, P.; Okhuysen, P.C.; Chappell, C.L.; Lewis, D.E.; Shahab, I.; Janecki, A.; White, A.C. Expression of Tumor Necrosis Factor Alpha and Interleukin 1β in Jejuna of Volunteers after Experimental Challenge with Cryptosporidium parvum Correlates with Exposure but Not with Symptoms. Infect. Immun. 2001, 69, 1172–1174. [Google Scholar] [CrossRef]
- de Sablet, T.; Potiron, L.; Marquis, M.; Bussière, F.I.; Lacroix-Lamandé, S.; Laurent, F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes. Cell Microbiol. 2016, 18, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.-S.; Lacroix-Lamandé, S.; Laurent, F.; McDonald, V. Role of Tumor Necrosis Factor Alpha in Development of Immunity against Cryptosporidium parvum Infection. Infect. Immun. 2006, 74, 4379–4382. [Google Scholar] [CrossRef]
- Green, S.J.; Crawford, R.M.; Hockmeyer, J.T.; Meltzer, M.S.; Nacy, C.A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 1990, 145, 4290–4297. [Google Scholar] [CrossRef] [PubMed]
- Nashleanas, M.; Kanaly, S.; Scott, P. Control of Leishmania major Infection in Mice Lacking TNF Receptors. J. Immunol. 1999, 160, 5506–5513. [Google Scholar] [CrossRef]
- Lucas, R.; Juillard, P.; Decoster, E.; Redard, M.; Burger, D.; Donati, Y.; Giroud, C.; Monso-Hinard, C.; De Kesel, T.; Buurman, W.A.; et al. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur. J. Immunol. 1997, 27, 1719–1725. [Google Scholar] [CrossRef]
- Jacobs, P.; Radzioch, D.; Stevenson, M.M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect. Immun. 1996, 64, 535–541. [Google Scholar] [CrossRef]
- Seixas, E.; Oliveira, P.; Nunes, J.F.M.; Coutinho, A. An experimental model for fatal malaria due to TNF-α-dependent hepatic damage. Parasitology 2008, 135, 683–690. [Google Scholar] [CrossRef]
- Grau, G.E.; Heremans, H.; Piguet, P.F.; Pointaire, P.; Lambert, P.H.; Billiau, A.; Vassalli, P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1989, 86, 5572–5574. [Google Scholar] [CrossRef] [PubMed]
- Schluter, D.; Kwok, L.-Y.; Lutjen, S.; Soltek, S.; Hoffmann, S.; Korner, H.; Deckert, M. Both Lymphotoxin-α and TNF Are Crucial for Control of Toxoplasma gondii in the Central Nervous System. J. Immunol. 2003, 170, 6172–6182. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.S.; Garcia, I.; Vicentelli, M.H.; Vassalli, P.; Minoprio, P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect. Immun. 1997, 65, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Montalvão, F.; Almeida, G.M.; Silva, E.M.; Borges, V.M.; Vasconcellos, R.; Takiya, C.M.; Lopes, M.F.; Nunes, M.P.; DosReis, G.A. Apoptotic lymphocytes treated with IgG from Trypanosoma cruzi infection increase TNF-α secretion and reduce parasite replication in macrophages. Eur. J. Immunol. 2010, 40, 417–425. [Google Scholar] [CrossRef]
- Arnaiz, E.; Harris, A.L. Role of Hypoxia in the Interferon Response. Front. Immunol. 2022, 13, 821816. [Google Scholar] [CrossRef]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 15623–15628. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Hsiao, H.-F.; Chen, T.-W.; Li, T.-K. Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J. Exp. Clin. Cancer Res. 2018, 37, 70. [Google Scholar] [CrossRef]
- Parra-Izquierdo, I.; Castaños-Mollor, I.; López, J.; Gómez, C.; Román, J.A.S.; Crespo, M.S.; García-Rodríguez, C. Lipopolysaccharide and interferon-γ team up to activate HIF-1α via STAT1 in normoxia and exhibit sex differences in human aortic valve interstitial cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2168–2179. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Wang, P.; Wang, F. Blockade of hypoxia-inducible factor-1α by YC-1 attenuates interferon-γ and tumor necrosis factor-α-induced intestinal epithelial barrier dysfunction. Cytokine 2011, 56, 581–588. [Google Scholar] [CrossRef]
- Wu, Y.; Meitzler, J.L.; Antony, S.; Juhasz, A.; Lu, J.; Jiang, G.; Liu, H.; Hollingshead, M.; Haines, D.C.; Butcher, D.; et al. Dual oxidase 2 and pancreatic adenocarcinoma: IFN-γ-mediated dual oxidase 2 overexpression results in H2O2-induced, ERK-associated up-regulation of HIF-1α and VEGF-A. Oncotarget 2016, 7, 68412–68433. [Google Scholar] [CrossRef]
- Lee, J.H.; Elly, C.; Park, Y.; Liu, Y.-C. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1α to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity 2015, 42, 1062–1074. [Google Scholar] [CrossRef]
- Glover, L.E.; Colgan, S.P. Hypoxia and Metabolic Factors That Influence Inflammatory Bowel Disease Pathogenesis. Gastroenterology 2011, 140, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.A.; Yatsula, B.; Maier, C.L.; Sadler, T.J.; Whittaker, L.W.; Pober, J.S. Interferon-Gamma Induces Prolyl Hydroxylase (PHD)3 Through a STAT1-Dependent Mechanism in Human Endothelial Cells. Arter. Thromb. Vasc. Biol. 2009, 29, 1363–1369. [Google Scholar] [CrossRef]
- Moraes, L.C.A.; França, E.L.; Pessoa, R.S.; Fagundes, D.L.G.; Gil Hernandes, M.; Ribeiro, V.P.; Gomes, M.A.; Honorio-França, A.C. The effect of IFN-γ and TGF-β in the functional activity of mononuclear cells in the presence of Entamoeba histolytica. Parasites Vectors 2015, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, C.; Ramos, C.; Ortiz-Ortiz, L. Effects of gamma interferon on syntheses of DNA and proteins by Entamoeba histolytica. Infect. Immun. 1989, 57, 2771–2775. [Google Scholar] [CrossRef]
- You, X.; Mead, J.R. Characterization of experimental Cryptosporidium parvum infection in IFN-γ knockout mice. Parasitology 1998, 117, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Flandin, J.-F.; Chano, F.; Descoteaux, A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-γ-primed macrophages. Eur. J. Immunol. 2006, 36, 411–420. [Google Scholar] [CrossRef]
- Yap, B.G.S.; Sher, A. Effector Cells of Both Nonhemopoietic and Hemopoietic Origin are required for Interferon (IFN)- γ- and Tumor Necrosis Factor (TNF)- α -dependent Host Resistance to the Intracellular Pathogen, Toxoplasma gondii. J. Exp. Med. 1999, 189, 1083–1091. [Google Scholar] [CrossRef]
- Suzuki, Y.; Orellana, M.A.; Schreiber, R.D.; Remington, J.S. Interferon-γ: The Major Mediator of Resistance against Toxoplasma gondii. Science 1988, 240, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Gekker, G.; Hu, S.; Peterson, P.K. Human microglial cell defense against Toxoplasma gondii. The Role of Cytokines. J. Immunol. 1994, 152, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.K.; Chiu, F.-C.; Weiss, L.M. Effect of Cytokines on Growth of Toxoplasma gondii in Murine Astrocytes. Infect. Immun. 1998, 66, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C.; Kohler, G.; Muller, U.; Mossmann, H.; Schaub, G.A.; Brombacher, F. Defective Nitric Oxide Effector Functions Lead to Extreme Susceptibility of Trypanosoma cruzi-Infected Mice Deficient in Gamma Interferon Receptor or Inducible Nitric Oxide Synthase. Infect. Immun. 1998, 66, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Potter, S.M.; Rosa, D.S.; Mauduit, M.; Grüner, A.C.; Kayibanda, M.; Mitchell, A.J.; Hunt, N.H.; Rénia, L. Control of pathogenic CD8+ T cell migration to the brain by IFN-γ during experimental cerebral malaria. Parasite Immunol. 2008, 30, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, C.; Wu, W.; Zhou, G.; Liu, F.; Cong, Y.; Liu, Z. Hypoxia inducible factor-1α-induced interleukin-33 expression in intestinal epithelia contributes to mucosal homeostasis in inflammatory bowel disease. Clin. Exp. Immunol. 2017, 187, 428–440. [Google Scholar] [CrossRef]
- Neumann, K.; Schiller, B.; Tiegs, G. NLRP3 Inflammasome and IL-33: Novel Players in Sterile Liver Inflammation. Int. J. Mol. Sci. 2018, 19, 2732. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.-P. The IL-1-Like Cytokine IL-33 Is Constitutively Expressed in the Nucleus of Endothelial Cells and Epithelial Cells In Vivo: A Novel ‘Alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.-A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.-P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Lunderius-Andersson, C.; Enoksson, M.; Nilsson, G. Mast Cells Respond to Cell Injury through the Recognition of IL-33. Front. Immunol. 2012, 3, 82. [Google Scholar] [CrossRef]
- Ryan, N.; Anderson, K.; Volpedo, G.; Varikuti, S.; Satoskar, M.; Satoskar, S.; Oghumu, S. The IL-33/ST2 Axis in Immune Responses against Parasitic Disease: Potential Therapeutic Applications. Front. Cell Infect. Microbiol. 2020, 10, 153. [Google Scholar] [CrossRef]
- Lechner, C.J.; Komander, K.; Hegewald, J.; Huang, X.; Gantin, R.G.; Soboslay, P.T.; Agossou, A.; Banla, M.; Köhler, C. Cytokine and chemokine responses to helminth and protozoan parasites and to fungus and mite allergens in neonates, children, adults, and the elderly. Immun. Ageing 2013, 10, 29. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Setiawan, J.; Konno, T.; Ihara, N.; Okamoto, S.; Saito, Y.; Murata, Y.; Noda, T.; Matozaki, T. Regulation of colonic epithelial cell homeostasis by mTORC1. Sci. Rep. 2020, 10, 13810. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2014, 34, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004, 10, 594–601. [Google Scholar] [CrossRef]
- Land, S.C.; Tee, A.R. Hypoxia-inducible Factor 1α Is Regulated by the Mammalian Target of Rapamycin (mTOR) via an mTOR Signaling Motif. J. Biol. Chem. 2007, 282, 20534–20543. [Google Scholar] [CrossRef]
- Xu, E.A.H.; Li, D.; Ma, J.; Zhao, Y.; Xu, L.; Tian, R.; Liu, Y.; Sun, L.; Su, J. The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization. Cancer Biol. Med. 2021, 18, 172–183. [Google Scholar] [CrossRef]
- Salmond, R.J.; Mirchandani, A.S.; Besnard, A.-G.; Bain, C.C.; Thomson, N.C.; Liew, F.Y. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J. Allergy Clin. Immunol. 2012, 130, 1159–1166.e6. [Google Scholar] [CrossRef] [PubMed]
- Nian, J.-B.; Zeng, M.; Zheng, J.; Zeng, L.-Y.; Fu, Z.; Huang, Q.-J.; Wei, X. Epithelial cells expressed IL-33 to promote degranulation of mast cells through inhibition on ST2/PI3K/mTOR-mediated autophagy in allergic rhinitis. Cell Cycle 2020, 19, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, J.; Ma, R.; Hao, J.; Liang, Y.; Zhao, J.; Zhang, A.; Meng, H.; Lu, J. Interleukin-33: Metabolic checkpoints, metabolic processes, and epigenetic regulation in immune cells. Front. Immunol. 2022, 13, 900826. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Chen, S.; Tian, B.; Huang, K.; Corrigan, C.J.; Ying, S.; Wang, W.; Wang, C. IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1α and VEGF Expression in Vascular Endothelial Cells. Ebiomedicine 2018, 33, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, D.; Zhang, X.; Wan, Q.; Zhang, W.; Zheng, M.; Zou, L.; Elly, C.; Lee, J.H.; Liu, Y.-C. E3 Ligase VHL Promotes Group 2 Innate Lymphoid Cell Maturation and Function via Glycolysis Inhibition and Induction of Interleukin-33 Receptor. Immunity 2018, 48, 258–270.e5. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Dias, T.H. Hypoxia-Inducible Factor (HIF) as a Target for Novel Therapies in Rheumatoid Arthritis. Front. Pharmacol. 2016, 7, 184. [Google Scholar] [CrossRef]
- Uddin, J.; Leslie, J.L.; Burgess, S.L.; Oakland, N.; Thompson, B.; Abhyankar, M.; Revilla, J.; Frisbee, A.; Donlan, A.N.; Kumar, P.; et al. The IL-33-ILC2 pathway protects from amebic colitis. Mucosal Immunol. 2022, 15, 165–175. [Google Scholar] [CrossRef]
- Nakamura, R.; Yoshizawa, A.; Moriyasu, T.; Deloer, S.; Senba, M.; Kikuchi, M.; Koyasu, S.; Moro, K.; Hamano, S. Group 2 Innate Lymphoid Cells Exacerbate Amebic Liver Abscess in Mice. iScience 2020, 23, 101544. [Google Scholar] [CrossRef]
- Bedi, B.; McNair, N.N.; Mead, J.R. Dendritic cells play a role in host susceptibility to Cryptosporidium parvum infection. Immunol. Lett. 2014, 158, 42–51. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, A.-Y.; Ma, S.; Chen, X.; Strauss-Soukup, J.K.; Chen, X.-M. Delivery of parasite Cdg7_Flc_0990 RNA transcript into intestinal epithelial cells during Cryptosporidium parvum infection suppresses host cell gene transcription through epigenetic mechanisms. Cell Microbiol. 2017, 19, e12760. [Google Scholar] [CrossRef]
- Still, K.M.; Batista, S.J.; O’brien, C.A.; Oyesola, O.O.; Früh, S.P.; Webb, L.M.; Smirnov, I.; Kovacs, M.A.; Cowan, M.N.; Hayes, N.W.; et al. Astrocytes promote a protective immune response to brain Toxoplasma gondii infection via IL-33-ST2 signaling. PLoS Pathog. 2020, 16, e1009027. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Roberts, F.; Nickdel, M.B.; Brombacher, F.; McKenzie, A.N.J.; Henriquez, F.L.; Alexander, J.; Roberts, C.W. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur. J. Immunol. 2010, 40, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ryffel, B.; Huang, F.; Robinet, P.; Panek, C.; Couillin, I.; Erard, F.; Piotet, J.; Le Bert, M.; Mackowiak, C.; Arias, M.T.; et al. Blockade of IL-33R/ST2 Signaling Attenuates Toxoplasma gondii Ileitis Depending on IL-22 Expression. Front. Immunol. 2019, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.-G.; Guabiraba, R.; Niedbala, W.; Palomo, J.; Reverchon, F.; Shaw, T.N.; Couper, K.N.; Ryffel, B.; Liew, F.Y. IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells. PLoS Pathog. 2015, 11, e1004607. [Google Scholar] [CrossRef]
- Palomo, J.; Reverchon, F.; Piotet, J.; Besnard, A.-G.; Couturier-Maillard, A.; Maillet, I.; Tefit, M.; Erard, F.; Mazier, D.; Ryffel, B.; et al. Critical role of IL-33 receptor ST2 in experimental cerebral malaria development. Eur. J. Immunol. 2015, 45, 1354–1365. [Google Scholar] [CrossRef]
- Glineur, C.; Leleu, I.; Pied, S. The IL-33/ST2 Pathway in Cerebral Malaria. Int. J. Mol. Sci. 2022, 23, 13457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).