Entomopathogenic Fungi: An Eco-Friendly Synthesis of Sustainable Nanoparticles and Their Nanopesticide Properties
Abstract
1. Introduction
2. What Are Entomopathogenic Fungi?
3. Effect of Different Entomopathogenic Fungi against Pest
3.1. Secondary Metabolites of Entomopathogenic Fungi and Their Role in Pest Infection
3.2. Mode of Action of Entomopathogenic Fungi against Insect Pest
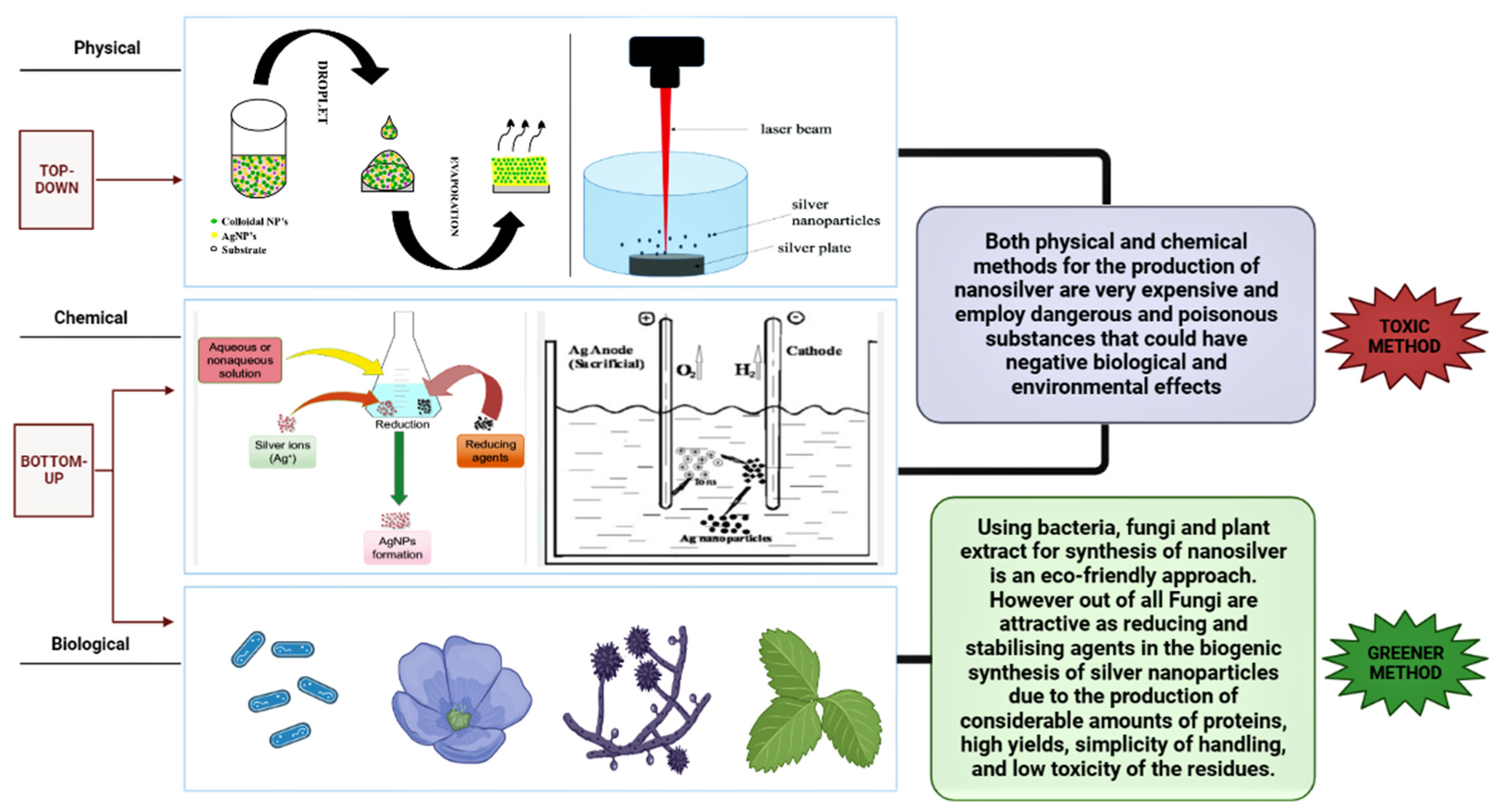
4. Different Routes of Synthesis of Nanoparticles
The Greener Synthesis of Metal Nanoparticles
5. Synthesis of Metal Nanoparticles by Entomopathogenic Fungi
5.1. Selenium Nanoparticles
5.2. Copper Nanoparticles
5.3. Zinc Nanoparticles
5.4. Biogenic Synthesis of Silver Nanoparticles Mediated by Fungi
6. Effects of Mycosynthesized Silver Nanoparticles: A Greener Approach in Controlling Pest Species
Mode of Action of Mycosynthesized Nanoparticles
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhaliwal, G.S.; Jindal, V.; Dhawan, A.K. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2007, 37, 1–7. [Google Scholar]
- Sharma, S.; Kooner, R.; Arora, R. Insect pests and crop losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Springer: Singapore, 2017; pp. 45–66. [Google Scholar]
- Lawler, S.P. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicol. Environ. Saf. 2017, 139, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K.; Lance, W.; Hemsarth, H. Synergism of the IGRs methoprene and pyriproxyfen against larval cat fleas (Siphonaptera: Pulicidae). J. Med. Entomol. 2016, 53, 629–633. [Google Scholar] [CrossRef]
- Tunaz, H.; Uygun, N. Insect growth regulators for insect pest control. Turk. J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Madhu, S.K.; Shaukath, A.K.; Vijayan, V.A. Efficacy of bioactive compounds from Curcuma aromatica against mosquito larvae. Acta Trop. 2010, 113, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Guo, L.; Maimaiti, Y.; Mijit, M.; Qiu, D. Entomopathogenic fungi as microbial biocontrol agent. Mol. Plant Breed. 2012, 3, 63–79. [Google Scholar] [CrossRef]
- Jaihan, P.; Sangdee, K.; Sangdee, A. Selection of entomopathogenic fungus for biological control of chili anthracnose disease caused by Colletotrichum spp. Eur. J. Plant Pathol. 2016, 146, 551–564. [Google Scholar] [CrossRef]
- Araújo, J.P.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016, 94, 1–39. [Google Scholar]
- Castro, T.; Mayerhofer, J.; Enkerli, J. Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric. Ecosyst. Environ. 2016, 233, 361–369. [Google Scholar] [CrossRef]
- Rı’os-Moreno, A.; Garrido-Jurado, I.; Resquín-Romero, G.; Arroyo-Manzanares, N.; Arce, L.; Quesada-Moraga, E. Destruxin A production by Metarhizium brunneum strains during transient endophytic colonisation of Solanum tuberosum. Biocontrol Sci. Technol. 2016, 26, 1574–1585. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Lovett, B.; Leger, R.J.S. The insect pathogens. Microbiol. Spectr. 2017, 5, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Litwin, A.; Nowak, M.; Różalska, S. Entomopathogenic fungi: Unconventional applications. Rev. Environ. Sci. Bio/Technol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of entomopathogenic fungi. In Integrated Pest Management; Abrol, D.P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 169–191. [Google Scholar]
- Donzelli, B.G.G.; Krasnoff, S.B.; Churchill, A.C.L. Identification of a hybrid PKS–NRPS required for the biosynthesis of NG-391 in Metarhizium robertsii. Curr. Genet. 2010, 56, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Smith, A.M.; Duan, H.; Rhyner, M.N.; Ruan, G.; Nie, S. A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys. Chem. Chem. Phys. 2006, 8, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Roy, I.; Sengupta, S.; Debnath, N. Novel applications of solid and liquid formulations of nanoparticles against insect pests and pathogens. Thin Solid Films 2010, 519, 1252–1257. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Duraisamy, P.; Govindarajan, M.; Buhroo, A.A.; Prasad, R. Nano biofungicides: Emerging trend in insect pest control. In Advances and Applications through Fungal Nanobiotechnology; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 307–319. [Google Scholar]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 2200356. [Google Scholar] [CrossRef]
- Cantu, A.A. Nanoparticles in forensic science. In Optics and Photonics for Counterterrorism and Crime Fighting IV; SPIE: Bellingham, WA, USA, 2008; Volume 7119, p. 71190F. [Google Scholar]
- Li, H.; Li, F.; Wang, L.; Sheng, J.; Xin, Z.; Zhao, L.; Xiao, H.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd). Food Chem. 2009, 114, 547–552. [Google Scholar] [CrossRef]
- Yokesh Babu, M.; Janaki Devi, V.; Ramakritinan, C.M.; Umarani, R.; Taredahalli, N.; Kumaraguru, A.K. Application of biosynthesized silver nanoparticles in agricultural and marine pest control. Curr. Nanosci. 2014, 10, 374–381. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Hazaa, M.; Alm-Eldin, M.; Ibrahim, A.E.; Elbarky, N.; Salama, M.; Sayed, R.; Sayed, W. Biosynthesis of Silver Nanoparticles using Borago officinslis leaf extract, characterization and larvicidal activity against cotton leaf worm, Spodoptera littoralis (Bosid). Int. J. Trop. Insect Sci. 2021, 41, 145–156. [Google Scholar] [CrossRef]
- Sinha, K.K.; Choudhary, A.K.; Kumari, P. Entomopathogenic fungi. In Ecofriendly Pest Management for Food Security; Academic Press: Cambridge, MA, USA, 2016; pp. 475–505. [Google Scholar]
- Lin, B.X. Use of Beauveria bassiana against the Sweet Potato Weevil. Acta Entomol. Sin. 1956, 6, 539540. (In Chinese) [Google Scholar]
- Xu, Q.F. Study on Soybean Moth Control by Beauveria bassiana. Acta Entomol. Sin. 1959, 9, 203215. (In Chinese) [Google Scholar]
- Quesada-Moraga, E.; Carrasco-Diaz, J.A.; Santiago-Álvarez, C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J. Appl. Entomol. 2006, 130, 442–452. [Google Scholar] [CrossRef]
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemushampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthropod Rev. 2009, 2, 129–147. [Google Scholar]
- Badii, M.H.; Abreu, J.L. Control biológicouna forma sustentable de control de plagas. Int. J. Good Consci. 2006, 1, 82–89. [Google Scholar]
- Behie, S.W.; Bidochka, M.J. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: An additional branch of the soil nitrogen cycle. Appl. Environ. Microbiol. 2014, 80, 1553–1560. [Google Scholar] [CrossRef]
- Delgado, P.A.M.; Murcia, O.P. Hongosentomopatógenos: Umaalternativa para la obtención de Biopesticidas. Ambiente Agua 2011, 6, 77–90. [Google Scholar] [CrossRef]
- Samson, R.A.; Evans, H.C.; Latgé, J.P. Atlas of Entomopathogenic Fungi; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Roberts, D.W.; Humber, R.A. Entomogenous fungi. Biol. Conidial Fungi 1981, 2, e236. [Google Scholar]
- Evans, H.C.; Elliot, S.L.; Hughes, D.P. Ophiocordyceps unilateralis: A keystone species for unraveling ecosystem functioning and biodiversity of fungi in tropical forests? Commun. Integr. Biol. 2011, 4, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Klinger, E.G.; James, R.R.; Youssef, N.N.; Welker, D.L. A multi-gene phylogeny provides additional insight into the relationships between several Ascosphaera species. J. Invertebr. Pathol. 2013, 112, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tanzini, M.; Alves, S.; Setten, A.; Augusto, N. Compatibilidad de agent estensoactivos com Beauveria bassiana y Metarhizium anisopliae. Manejo Integr. Plagas 2001, 59, 15–18. [Google Scholar]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019, 18, 101079. [Google Scholar] [CrossRef]
- Deka, B.; Baruah, C.; Babu, A. Entomopathogenic microorganisms: Their role in insect pest management. Egypt. J. Biol. Pest Control 2021, 31, 121. [Google Scholar] [CrossRef]
- Umaru, F.F.; Simarani, K. Efficacy of Entomopathogenic Fungal Formulations against Elasmolomus pallens (Dallas) (Hemiptera: Rhyparochromidae) and Their Extracellular Enzymatic Activities. Toxins 2022, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.F.; Fermin, G.A.; Roye, M.E. Viruses infecting papaya (Carica papaya L.): Etiology, pathogenesis, and molecular biology. Plant Viruses 2007, 1, 178–188. [Google Scholar]
- Chakrabarti, S.; Raychaudhuri, D.N. New and little known aphids (Homoptera: Aphididae) from Kumaon Himalaya, India. Entomon 1978, 3, 95–103. [Google Scholar]
- Mukherjee, A.; Debnath, P.; Ghosh, S.K.; Medda, P.K. Biological control of papaya aphid (Aphis gossypii Glover) using entomopathogenic fungi. Vegetos 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Sani, I.; Ismail, S.I.; Abdullah, S.; Jalinas, J.; Jamian, S.; Saad, N. A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 2020, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, C.C.; Willett, D.S. The Lesser Chestnut Weevil (Curculio sayi): Damage and Management with Biological Control Using Entomopathogenic Fungi and Entomopathogenic Nematodes. Insects 2022, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, B.G.G.; Krasnoff, S.B. Molecular genetics of secondary chemistry in Metarhizium Fungi. In Advances in Genetics; Lovett, B., Leger, R.J.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, pp. 365–436. [Google Scholar]
- Gibson, D.M.; Donzelli, B.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef]
- Pedrini, N. The entomopathogenic fungus Beauveria bassiana shows its toxic side within insects: Expression of genes encoding secondary metabolites during pathogenesis. J. Fungi 2022, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, A.; Bandani, A.R.; Talaei-Hassanlouei, R.; Malagoli, D. Cellular immune reactions of the sunn pest, Eurygaster integriceps, to the entomopathogenic fungus, Beauveria bassiana and its secondary metabolites. J. Insect Sci. 2011, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Freed, S.; Feng-Liang, J.; Naeem, M.; Shun-Xiang, R.; Hussian, M. Toxicity of proteins secreted by entomopathogenic fungi against Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Agric. Biol. 2012, 14, 291–295. [Google Scholar]
- Vinayaga Moorthi, P.; Balasubramanian, C.; Selvarani, S.; Radha, A. Efficacy of sub lethal concentration of entomopathogenic fungi on the feeding and reproduction of Spodoptera litura. Springerplus 2015, 4, 681. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, M.; Mostafa, E.H.; Ismail, N.A.E.R. Secondary metabolites of the entomopathogenic fungus, Cladosporium cladosporioides and its relation to toxicity of cotton aphid, Aphis gossypii (Glov.). Int. J. 2019, 5, 115–120. [Google Scholar]
- Woo, R.M.; Park, M.G.; Choi, J.Y.; Park, D.H.; Kim, J.Y.; Wang, M.; Kim, H.J.; Woo, S.D.; Kim, J.S.; Je, Y.H. Insecticidal and insect growth regulatory activities of secondary metabolites from entomopathogenic fungi, Lecanicillium attenuatum. J. Appl. Entomol. 2020, 144, 655–663. [Google Scholar] [CrossRef]
- Arunthirumeni, M.; Vinitha, G.; Shivakumar, M.S. Antifeedant and larvicidal activity of bioactive compounds isolated from entomopathogenic fungi Penicillium sp. for the control of agricultural and medically important insect pest (Spodoptera litura and Culex quinquefasciatus). Parasitol. Int. 2023, 92, 102688. [Google Scholar] [CrossRef]
- Sevim, A.; Sevim, E.; Demirbağ, Z. General biology of entomopathogenic fungi and their potential to control pest species in Turkey (Entomopatojenik fungusların genel biyolojileri ve Türkiye’de zararlı böceklerin mücadelesinde kullanılma potansiyelleri). Erzincan Üniv. Bilim. Enst. Derg. 2015, 8, 115–147. [Google Scholar]
- Andersen, S.O. Biochemistry of insect cuticle. Annu. Rev. Entomol. 1979, 24, 29–59. [Google Scholar] [CrossRef]
- Ross, H.H. A Textbook of Entomology; John Wiley and Sons: Hoboken, NJ, USA, 1948. [Google Scholar]
- Glare, T. Entomopathogenic Fungi and Their Role in Regulation of Insect Populations. In Insect Control; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Electron-microscope studies of the histopathology of fungal infections by Metarrhizium anisopliae. Misc. Publ. Entomol. Soc. Am. 1973, 9, 112–119. [Google Scholar]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef] [PubMed]
- St. Leger, R.J. The role of cuticle-degrading proteases in fungal pathogenesis of insects. Can. J. Bot. 1995, 73, 1119–1125. [Google Scholar] [CrossRef]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Zhang, S.; Keyhani, N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N.; Zhang, S.; Juárez, M.P.; Keyhani, N.O. Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology 2010, 156, 2549–2557. [Google Scholar] [CrossRef]
- Suzuki, A.; Kawakami, K.; Tamura, S. Detection of destruxins in silkworm larvae infected with Metarrhizium anisopliae. Agric. Biol. Chem. 1971, 35, 1641–1643. [Google Scholar]
- Eyal, J.; Mabud, M.A.; Fischbein, K.L.; Walter, J.F.; Osborne, L.S.; Landa, Z. Assessment of Beauveria bassiana Nov. EO-1 strain, which produces a red pigment for microbial control. Appl. Biochem. Biotechnol. 1994, 44, 65–80. [Google Scholar] [CrossRef]
- Sweet, M.J.; Chessher, A.; Singleton, I. Metal-based nanoparticles; size, function, and areas for advancement in applied microbiology. Adv. Appl. Microbiol. 2012, 80, 113–142. [Google Scholar] [PubMed]
- Beltrán Pineda, M.E.; Lizarazo Forero, L.M.; Sierra, Y.C.A. Mycosynthesis of silver nanoparticles: A review. BioMetals 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of nanostructures with bottom-up approach and their utility in diagnostics, therapeutics, and others. In Environmental Chemical and Medical Sensors; Springer: Singapore, 2018; pp. 167–198. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Gupta, R. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–201. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Fekri, S.; Kouhi, M.; Akbarzadeh, A.; Nasrabadi, H.; Nikasa, P.; Joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bioapplications and properties. Crit. Rev. Microbiol. 2014, 42, 173–180. [Google Scholar]
- Keat, C.; Aziz, A.; Eid, A.; Elmarzugi, A. Biosynthesis of nanoparticles and silver nanoparticle. Bioresour. Bioprocess. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kudesia, N.; Najitha Banu, A.; Raut, A.M.; Wahengbam, J. Biofabricated Nanoparticles: A Greener Approach towards Insect Control. In Advances in Integrated Pest Management Technology: Innovative and Applied Aspects; Springer International Publishing: Cham, Switzerland, 2019; pp. 391–419. [Google Scholar]
- Gezaf, S.A.; Hamedo, H.A.; Ibrahim, A.A.; Mossa, M.I. Mycosynthesis of silver nanoparticles by endophytic Fungi: Mechanism, characterization techniques and their applications. Microb. Biosyst. 2022, 7, 48–65. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-inspired green nanoparticles: Synthesis, mechanism, and antibacterial application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef]
- Velázquez-Robledo, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Hernández-Morales, A.; Aguirre, J.; Casas-Flores, S.; Herrera-Estrella, A. Role of the 4-phosphopantetheinyl transferase of Trichoderma virens in secondary metabolism and induction of plant defense responses. Mol. Plant Microbe Interact. 2011, 24, 1459–1471. [Google Scholar] [CrossRef]
- Iranifam, M.; Fathinia, M.; Rad, T.S.; Hanifehpour, Y.; Khataee, A.R.; Joo, S.W. A novel selenium nanoparticles-enhanced chemiluminescence system for determination of dinitrobutylphenol. Talanta 2013, 107, 263–269. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Herrera Estrella, A.; López-Bucio, J. The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil 2014, 379, 261–274. [Google Scholar] [CrossRef]
- Berini, F.; Caccia, S.; Franzetti, E.; Congiu, T.; Marinelli, F.; Casartelli, M.; Tettamanti, G. Effects of Trichoderma viride chitinases on the peritrophic matrix of Lepidoptera. Pest Manag. Sci. 2016, 72, 980–989. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.-X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. J. Sci. Rep. 2017, 7, 1771. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Appels, F.V.; van Wees, S.C. Impact of salicylic acid-and jasmonic acid-regulated defences on root colonization by Trichoderma harzianum T-78. Plant Signal. Behav. 2017, 12, e1345404. [Google Scholar] [CrossRef] [PubMed]
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M.S. Biocontrol efficacy of mycosynthesized selenium nanoparticle using Trichoderma sp. on insect pest Spodoptera litura. J. Clust. Sci. 2022, 33, 1645–1653. [Google Scholar] [CrossRef]
- Pimentel, D.J. Amounts of Pesticides Reaching Target Pests: Environmental Impacts and Ethics. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Sengottayan, S.N. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar]
- Senthil-Nathan, S. A Review of Biopesticides and Their Mode of Action against Insect Pests. In Environmental Sustainability; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar]
- Dhivya, K.; Vengateswari, G.; Arunthirumeni, M.; Karthi, S.; Senthil-Nathan, S.; Shivakumar, M.S. Bioprospecting of Prosopis juliflora (Sw.) DC seed pod extract effect on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Physiol. Mol. Plant Pathol. 2018, 101, 45–53. [Google Scholar] [CrossRef]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Kora, A.J. Copper-based nanopesticides. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevirer: Amsterdam, The Netherlands, 2022; pp. 133–153. [Google Scholar]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Rafeeq, C.M.; Paul, E.; Saagar, E.V.; Ali, P.M. Mycosynthesis of zinc oxide nanoparticles using Pleurotus floridanus and optimization of process parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Ullah, K.; Almutairi, S.M.; AlMunqedhi, B.M.; Ragab abdelGawwad, M. Mycosynthesis, Characterization of Zinc Oxide Nanoparticles, and Its Assessment in Various Biological Activities. Crystals 2023, 13, 171. [Google Scholar] [CrossRef]
- Shobha, B.; Lakshmeesha, T.R.; Ansari, M.A.; Almatroudi, A.; Alzohairy, M.A.; Basavaraju, S.; Chowdappa, S. Mycosynthesis of ZnO nanoparticles using Trichoderma spp. isolated from rhizosphere soils and its synergistic antibacterial effect against Xanthomonas oryzae pv. oryzae. J. Fungi 2020, 6, 181. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Banu, A.N.; Kudesia, N.; Raut, A.M.; Pakrudheen, I.; Wahengbam, J. Toxicity, bioaccumulation, and transformation of silver nanoparticles in aqua biota: A review. Environ. Chem. Lett. 2021, 19, 4275–4296. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2869–2877. [Google Scholar] [CrossRef]
- Amerasan, D.; Nataraj, T.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Nicoletti, M.; Benelli, G. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J. Pest Sci. 2016, 89, 249–256. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Physiochemical properties of Trichoderma longibrachiatum DSMZ 16517-synthesized silver nanoparticles for the mitigation of halotolerant sulphate-reducing bacteria. J. Appl. Microbiol. 2019, 126, 138–154. [Google Scholar] [CrossRef]
- Gupta, P.; Rai, N.; Verma, A.; Saikia, D.; Singh, S.P.; Kumar, R.; Singh, S.K.; Kumar, D.; Gautam, V. Green-Based Approach to Synthesize Silver Nanoparticles Using the Fungal Endophyte Penicillium oxalicum and Their Antimicrobial, Antioxidant, and In Vitro Anticancer Potential. ACS Omega 2022, 7, 46653–46673. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Ping, L.Y.; Marcato, P.D.; Alves, O.L.; Silva, M.C.; Ruiz, R.C.; Melo, I.S.; Tasic, L.; De Souza, A.O. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microbiol. Biotechnol. 2013, 97, 775–782. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Roy, S.; Mukherjee, T.; Chakraborty, S.; Das, T.K. Biosynthesis, characterisation & antifungal activity of silver nanoparticles synthesized by the fungus Aspergillus foetidus MTCC8876. Dig. J. Nanomater. Biostruct. 2013, 8, 197–205. [Google Scholar]
- Banu, A.N.; Balasubramanian, C. Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol. Res. 2014, 113, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Kamil, D.; Prameeladevi, T.; Ganesh, S.; Prabhakaran, N.; Nareshkumar, R.; Thomas, S.P. Green synthesis of silver nanoparticles by entomopathogenic fungus Beauveria bassiana and their bioefficacy against mustard aphid (Lipaphis erysimi Kalt.). Indian J. Exp. Biol. 2017, 5, 555–561. [Google Scholar]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Hayat, P.; Khan, I.; Rehman, A.; Jamil, T.; Hayat, A.; Rehman, M.U.; Ullah, N.; Sarwar, A.; Alharbi, A.A.; Dablool, A.S.; et al. Myogenesis and Analysis of Antimicrobial Potential of Silver Nanoparticles (AgNPs) against Pathogenic Bacteria. Molecules 2023, 28, 637. [Google Scholar] [CrossRef]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran. J. Pharm. Res. IJPR 2013, 12, 289. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2013, 28, 313–318. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Padmanabhan, M.N. Effect of mycosynthesized silver nanoparticles from filtrate of Trichoderma harzianum against larvae and pupa of dengue vector Aedes aegypti L. Environ. Sci. Pollut. Res. 2014, 21, 4624–4633. [Google Scholar] [CrossRef]
- Chinnaperumal, K.; Govindasamy, B.; Paramasivam, D.; Dilipkumar, A.; Dhayalan, A.; Vadivel, A.; Pachiappan, P. Bio-pesticidal effects of Trichoderma viride formulated titanium dioxide nanoparticle and their physiological and biochemical changes on Helicoverpa armigera (Hub.). Pestic. Biochem. Physiol. 2018, 149, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.K.R.; Bharani, R.S.A. Biocompatible silver nanoparticles-loaded fungal metabolites nanoconjugate (agnp–fm) preparation for the noteworthy pesticidal activity. Natl. Acad. Sci. Lett. 2021, 44, 511–517. [Google Scholar] [CrossRef]
- Yosri, M.; Abdel-Aziz, M.M.; Sayed, R.M. Larvicidal potential of irradiated myco-insecticide from Metarhizium anisopliae and larvicidal synergistic effect with its mycosynthesized titanium nanoparticles (TiNPs). J. Radiat. Res. Appl. Sci. 2018, 11, 328–334. [Google Scholar] [CrossRef]
- Khooshe-Bast, Z.; Sahebzadeh, N.; Ghaffari-Moghaddam, M.; Mirshekar, A. Insecticidal effects of zinc oxide nanoparticles and Beauveria bassiana TS11 on Trialeurodes vaporariorum (Westwood, 1856) (Hemiptera: Aleyrodidae). Acta Agric. Slov. 2016, 107, 299–309. [Google Scholar] [CrossRef]
- Santos, T.S.; Passos, E.M.D.; Seabra, M.G.D.J.; Souto, E.B.; Severino, P.; Mendonça, M.D.C. Entomopathogenic fungi biomass production and extracellular biosynthesis of silver nanoparticles for bioinsecticide action. Appl. Sci. 2021, 11, 2465. [Google Scholar] [CrossRef]
- Shukla, G.; Gaurav, S.S.; Singh, A.; Rani, P. Synthesis of mycogenic silver nanoparticles by Fusarium pallidoroseum and evaluation of its larvicidal effect against white grubs (Holotrichia sp.). Mater. Today Proc. 2022, 49, 3517–3527. [Google Scholar] [CrossRef]
- Kamalakannan, S.; Gobinath, C.; Ananth, S. Synthesis and characterization of fungus mediated silver nanoparticle for toxicity on filarial vector, Culex quinquefasciatus. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 124–132. [Google Scholar]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wang, X.; Qiu, B.; Cuthbertson, A.G.; Du, C.; Wu, J.; Ali, S. Isaria fumosorosea-based zero-valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisia tabaci (Gennadius). Pest Manag. Sci. 2019, 75, 2174–2181. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, K.; Cuthbertson, A.G.; Du, C.; Ali, S. Toxicity and biological effects of Beauveria brongniartii Fe0 nanoparticles against Spodoptera litura (Fabricius). Insects 2020, 11, 895. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Madasamy, M.; Radhika, S.A. Insecticidal activity of bio-silver and gold nanoparticles against Pericallia ricini Fab. (Lepidaptera: Archidae). J. Biopestic. 2016, 9, 63. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Jiang, X.; Miclăus, T.; Chunying, C.; Autrup, H.; Beer, C. Silver nanoparticles—Wolves in sheep’s clothing? Toxicol. Res. 2015, 4, 563–575. [Google Scholar] [CrossRef]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2021, 44, 1–11. [Google Scholar] [CrossRef]
- Lade, B.D.; Gogle, D.P. Nano-biopesticides: Synthesis and applications in plant safety. Nanobiotechnol. Appl. Plant Prot. 2019, 2, 169–189. [Google Scholar]
- Stadler, T.; López García, G.P.; Gitto, J.G.; Buteler, M. Nanostructured alumina: Biocidal properties and mechanism of action of a novel insecticide powder. Bull. Insectol. 2017, 70, 17–25. [Google Scholar]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum-insecticidal activity and mode of action. Environ. Sci. Pollut. Res. Int. 2018, 25, 18802–18812. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Ramamoorthy, M.; Lyon, D.; Jones, K.; Duttaroy, A. Mechanism of silver nanoparticles action on insect pigmentation reveals intervention of copper homeostasis. PLoS ONE 2013, 8, e53186. [Google Scholar] [CrossRef]
- Sultana, N.; Raul, P.K.; Goswami, D.; Das, B.; Gogoi, H.K.; Raju, P.S. Nanoweapon: Control of mosquito breeding using carbon-dot-silver nanohybrid as a biolarvicide. Environ. Chem. Lett. 2018, 16, 1017–1023. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Choi, J. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat. Toxicol. 2011, 101, 550–560. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Pandey, A.; Chandra, S.; Chauhan, L.K.S.; Narayan, G.; Chowdhuri, D.K. Cellular internalization and stress response of ingested amorphous silica nanoparticles in the midgut of Drosophila melanogaster. Biochim. Biophys. Acta 2013, 1830, 2256–2266. [Google Scholar] [CrossRef] [PubMed]



| Entomopathogenic Fungi Used | Particle Size | Type of Synthesis | Time Taken for Synthesis | Reference |
|---|---|---|---|---|
| Aspergillus tubingensis | 35 nm | Extracellular | 96 h | [112] |
| Penicillium citrinum | 109 nm | Extracellular | 24 h | [113] |
| Aspergillus foetidus | 20 nm | Extracellular | 24 h | [114] |
| Isaria fumosorosea | 51 nm | Extracellular | 72 h | [115] |
| Metarhizium anisopliae | 28 nm | Extracellular | 72 h | [109] |
| Beauveria bassaina | 20 nm | Extracellular | 120 h | [116] |
| Trichoderma longibrachiatum | 24 nm | Extracellular | 72 h | [117] |
| Penicillium oxalicum | 150 nm | Extracellular | 96 h | [118] |
| Fusarium oxysporum | 25 nm | Extracellular | 48 h | [119] |
| Fusarium oxysporum | 5 nm | Extracellular | 72 h | [120] |
| Coriolus versicolor | 444 nm | Intracellular | 96 h | [121] |
| Aspergillus fumigatus | 25 nm | Extracellular | [122] |
| Entomopathogenic Fungi | Metal Nanoparticles | Targeted Pest | LC50 | Time Taken | Reference |
|---|---|---|---|---|---|
| Penecillium verucosum | Ag | Culex quinquefasciatus | 4.91, 5.16, 5.95, 7.83 ppm | 24 h | [130] |
| Cochliobolus lunatus | Ag | Aedes aegypti | 1.29, 1.48, 1.58 ppm | 24 h | [131] |
| Beauveria bassiana | ZnO | Trialeurodes vaporariorum | 7.35 ppm | 240 h | [127] |
| Isaria fumosorosea | Fe | Bemisia tabaci | 19.17, 26.10, 37.71 ppm | 75 h | [132] |
| Beauveria brongniartii | Fe | Spodoptera litura | 59 ppm | 70 h | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bihal, R.; Al-Khayri, J.M.; Banu, A.N.; Kudesia, N.; Ahmed, F.K.; Sarkar, R.; Arora, A.; Abd-Elsalam, K.A. Entomopathogenic Fungi: An Eco-Friendly Synthesis of Sustainable Nanoparticles and Their Nanopesticide Properties. Microorganisms 2023, 11, 1617. https://doi.org/10.3390/microorganisms11061617
Bihal R, Al-Khayri JM, Banu AN, Kudesia N, Ahmed FK, Sarkar R, Arora A, Abd-Elsalam KA. Entomopathogenic Fungi: An Eco-Friendly Synthesis of Sustainable Nanoparticles and Their Nanopesticide Properties. Microorganisms. 2023; 11(6):1617. https://doi.org/10.3390/microorganisms11061617
Chicago/Turabian StyleBihal, Ritu, Jameel M. Al-Khayri, A. Najitha Banu, Natasha Kudesia, Farah K. Ahmed, Rudradeb Sarkar, Akshit Arora, and Kamel A. Abd-Elsalam. 2023. "Entomopathogenic Fungi: An Eco-Friendly Synthesis of Sustainable Nanoparticles and Their Nanopesticide Properties" Microorganisms 11, no. 6: 1617. https://doi.org/10.3390/microorganisms11061617
APA StyleBihal, R., Al-Khayri, J. M., Banu, A. N., Kudesia, N., Ahmed, F. K., Sarkar, R., Arora, A., & Abd-Elsalam, K. A. (2023). Entomopathogenic Fungi: An Eco-Friendly Synthesis of Sustainable Nanoparticles and Their Nanopesticide Properties. Microorganisms, 11(6), 1617. https://doi.org/10.3390/microorganisms11061617








