Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diets, and Experimental Design
2.3. Sample Collection and Analysis
2.4. Volatile Fatty Acids Measurement
2.5. DNA Extraction and Sequencing
2.6. Statistical Data Analysis
3. Results
3.1. Heat Stress Determination
3.2. Lactation Performance and Ruminal TVFAs
3.3. Composition and Diversity of the Rumen Microbiota
3.4. The Impact of Cr-Pro and Ca-Pro on Rumen Microbial Composition
3.5. Gene Function Prediction of Rumen Microbial Communities
3.6. Blood Oxidative Stress Index and Cytokines
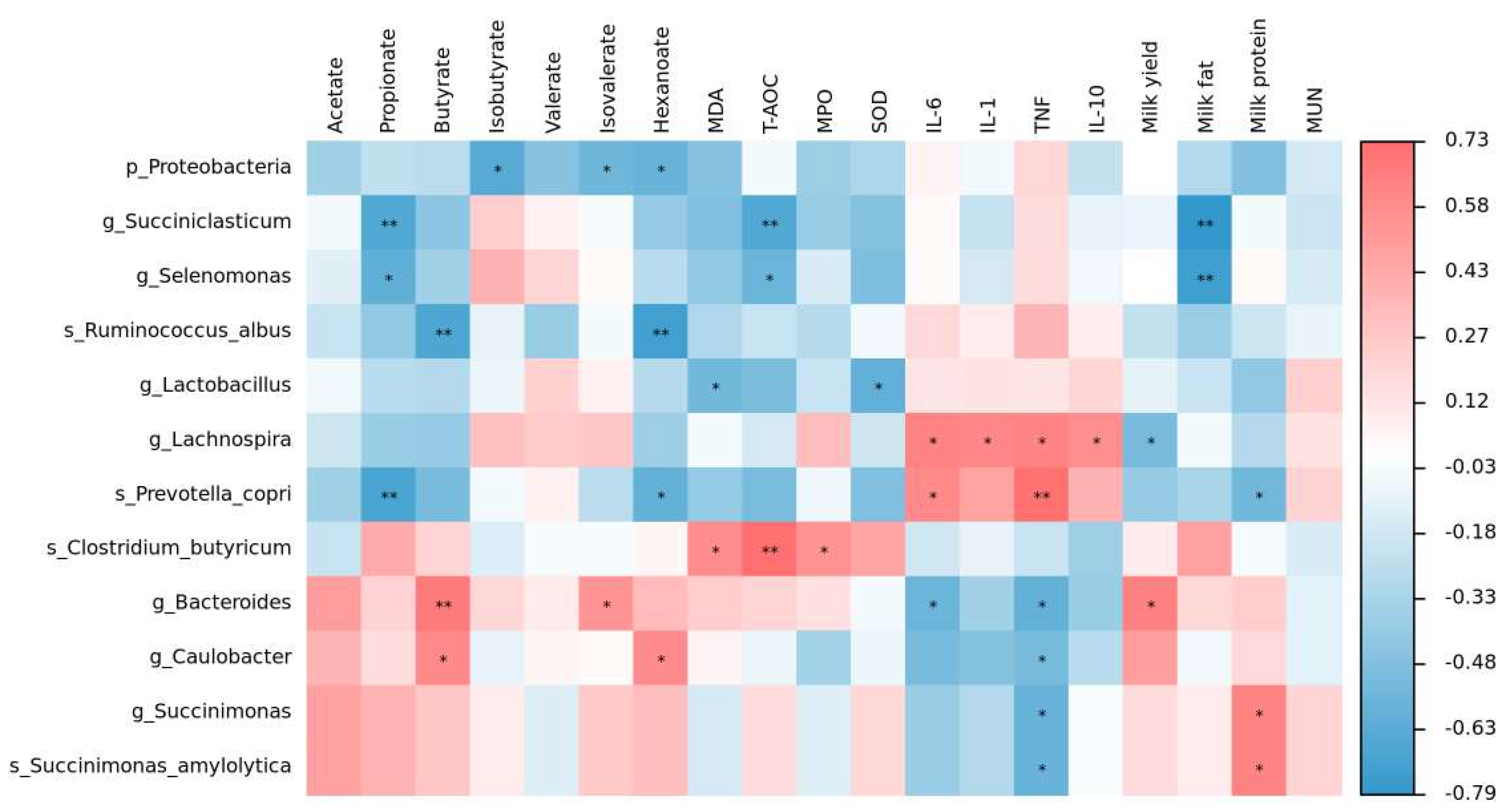
3.7. The Relationship between the Rumen Microbiota, TVFAs, Oxidative Stress Index, Cytokines, and Lactation Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fabris, T.F.; Laporta, J.; Skibiel, A.L.; Corra, F.N.; Senn, B.D.; Wohlgemuth, S.E.; Dahl, G.E. Effect of heat stress during early, late, and entire dry period on dairy cattle. J. Dairy Sci. 2019, 102, 5467–5656. [Google Scholar] [CrossRef]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.I.; Lee, S.S. Heat Stress: Effects on Rumen Microbes and Host Physiology, and Strategies to Alleviate the Negative Impacts on Lactating Dairy Cows. Front. Microbiol. 2022, 13, 804562. [Google Scholar] [CrossRef]
- Park, T.; Ma, L.; Gao, S.; Bu, D.; Yu, Z. Heat stress impacts the multi-domain ruminal microbiota and some of the functional features independent of its effect on feed intake in lactating dairy cows. J. Anim. Sci. Biotechnol. 2022, 13, 71. [Google Scholar] [CrossRef]
- Tao, S.; Rivas, R.M.O.; Marins, T.N.; Chen, Y.-C.; Gao, J.; Bernard, J.K. Impact of heat stress on lactational performance of dairy cows. Theriogenology 2020, 150, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of Heat Stress on Bacterial Composition and Metabolism in the Rumen of Lactating Dairy Cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Chen, W.; Zhang, Y.; Jiang, Y.; Meng, Q.; Zhou, Z. Growth performance and development of internal organ, and gastrointestinal tract of calf supplementation with calcium propionate at various stages of growth period. PLoS ONE 2017, 12, e0179940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Chen, W.B.; Wu, X.; Zhang, Y.W.; Jiang, Y.M.; Meng, Q.X.; Zhou, Z.M. Calcium propionate supplementation improves development of rumen epithelium in calves via stimulating G protein-coupled receptors. Animal 2018, 12, 2284–2291. [Google Scholar] [CrossRef]
- Zhang, F.; Nan, X.; Wang, H.; Guo, Y.; Xiong, B. Research on the Applications of Calcium Propionate in Dairy Cows: A Review. Animals 2020, 10, 1336. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Guo, G.; Yang, W.Z.; Dong, K.H.; Huang, Y.X.; Yang, X.M.; He, D.C. Effects of calcium propionate on rumen fermentation, urinary excretion of purine derivatives and feed digestibility in steers. J. Agric. Sci. 2009, 147, 201. [Google Scholar] [CrossRef]
- Vincent, J.B. Recent advances in nutritional biochemistry of trivalent Chromium. Proc. Nutr. Soc. 2004, 63, 41–47. [Google Scholar] [CrossRef]
- Pechova, A.; Pavlata, L. Chromium as an essential nutrient: A review. Veterinární Med. 2007, 52, 1–18. [Google Scholar] [CrossRef]
- Bunting, L.D.; Tarifa, T.A.; Crochet, B.T.; Fernandez, J.M.; Depew, C.; Lovejoy, J.C. Effects of Dietary Inclusion of Chromium Propionate and Calcium Propionate on Glucose Disposal and Gastrointestinal Development in Dairy Calves. J. Dairy Sci. 2000, 83, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Mcart, J.A.A.; Ryan, C.M.; Gilbert, R.O.; Nydam, D.V.; Valdez, F.; Griswold, K.E.; Overton, T.R. Effects of chromium propionate supplementation during the periparturient period and early lactation on metabolism, performance, and cytological endometritis in dairy cows. J. Dairy Sci. 2014, 97, 6400–6410. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.E.; Fellner, V.; Mcleod, S.J.; Fry, R.S.; Krafka, K.; Lamptey, A.; Spears, J.W. Effects of supplementing dairy cows with chromium propionate on milk and tissue chromium concentrations. J. Dairy Sci. 2010, 93, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Sadri, H.; Rahmani, H.R.; Khorvash, M.; Ghorbani, G.R.; Bruckmaier, R.M. Chromium supplementation and substitution of barley grain with corn: Effects on metabolite and hormonal responses in periparturient dairy cows. J. Anim. Physiol. Anim. Nutr. 2011, 96, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.A.; Socha, M.T.; Tomlinson, D.J. Supplementing Intensively Grazed Late-Gestation and Early-Lactation Dairy Cattle with Chromium. J. Dairy Sci. 2004, 87, 4269–4277. [Google Scholar] [CrossRef]
- Al-Saiady, M.Y.; Al-Shaikh, M.A.; Al-Mufarrej, S.I.; Al-Showeimi, T.A.; Mogawer, H.H.; Dirrar, A. Effect of chelated chromium supplementation on lactation performance and blood parameters of Holstein cows under heat stress. Anim. Feed. Sci. Technol. 2004, 117, 223–233. [Google Scholar] [CrossRef]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Gaughan, J.B. Physiological responses of Australian Merino wethers exposed to high heat load. J. Anim. Sci. 2012, 90, 212. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L.J.A. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, E.J.; Kvidera, S.K.; Seibert, J.T.; Horst, E.A.; Abuajamieh, M.; Al-Qaisi, M.; Lei, S.; Ross, J.W.; Johnson, C.D.; Kremer, B.; et al. Effects of dietary chromium propionate on growth performance, metabolism, and immune biomarkers in heat-stressed finishing pigs. J. Anim. Sci. 2018, 97, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Luo, Q.H.; Xiao, F.; Lin, X.; Spears, J.W. Research Note: Responses of growth performance, immune traits and small intestinal morphology to dietary supplementation of chromium propionate in heat stressed broilers. J. Poult. Sci. 2020, 99, 5070–5073. [Google Scholar] [CrossRef]
- Chen, X.L.; Zeng, Y.B.; Liu, L.X.; Song, Q.L.; Zou, Z.; Wei, Q.; Song, W.J. Effects of dietary chromium propionate on laying performance, egg quality, serum biochemical parameters and antioxidant status of laying ducks under heat stress. Animal 2020, 15, 100081. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Rashid, M.A.; Malik, M.I. Effects of sodium bicarbonate and chromium propionate supplementation on growth performance, blood indices of Beetal bucks under heat stress. Trop. Anim. Health Prod. 2021, 53, 496. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Peng, W.; Liu, J.; Xu, G.; Wang, D. Effect of chromium methionine supplementation on lactation performance, hepatic respiratory rate and anti-oxidative capacity in early-lactating dairy cows. Animal 2021, 15, 100326. [Google Scholar] [CrossRef]
- Martins, W.D.C.; Cunha, S.H.M.; Boscarato, A.G.; De Lima, J.S.; Junior, J.D.E.; Uliana, G.C.; Pedrini, M.; Alberton, L.R. Calcium Propionate Increased Milk Parameters in Holstein Cows. Acta Sci. Vet. 2019, 47. [Google Scholar] [CrossRef]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Liu, X.; Sha, Y.; Dingkao, R.; Zhang, W.; Luo, Y. Interactions Between Rumen Microbes, VFAs, and Host Genes Regulate Nutrient Absorption and Epithelial Barrier Function During Cold Season Nutritional Stress in Tibetan Sheep. Front. Microbiol. 2020, 11, 593062. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Wang, Y.; Jiang, L.; Xiong, B. Ruminal Degradation of Rumen-Protected Glucose Influences the Ruminal Microbiota and Metabolites in Early-Lactation Dairy Cows. Appl. Environ. Microbiol. 2021, 4, e01908–e01920. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-H.; Kim, S.-B.; Son, J.-K.; Lee, J.-H.; Joo, S.-S.; Gu, B.-H.; Park, T.; Park, B.-Y.; Kim, E.-T. Differential Dynamics of the Ruminal Microbiome of Jersey Cows in a Heat Stress Environment. Animals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Patra, A.K.; Kar, I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Biotechnol. 2021, 63, 211–247. [Google Scholar] [CrossRef]
- Zhong, Y.; Xue, M.; Liu, J. Composition of Rumen Bacterial Community in Dairy Cows with Different Levels of Somatic Cell Counts. Front. Microbiol. 2018, 9, 3217. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, K.; Rushdi, H.E.; Zhang, M.; Fu, T.; Gao, T.; Yang, L.; Liu, S.; Lin, F. Heat Stress Induces Shifts in the Rumen Bacteria and Metabolome of Buffalo. Animals 2022, 12, 1300. [Google Scholar] [CrossRef]
- Abdelmegeid, M.K.; Elolimy, A.A.; Zhou, Z.; Lopreiato, V.; McCann, J.C.; Loor, J.J. Rumen-protected methionine during the peripartal period in dairy cows and its effects on abundance of major species of ruminal bacteria. J. Anim. Sci. Biotechnol. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of Oxidative Status in Plasma and Erythrocytes of Transition Dairy Cows During Hot Season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Yahong; Cheng; Qianting; Mai; Xin; Zeng; Huiling; Wang; Yao; Xiao. Propionate relieves pentylenetetrazol-induced seizures, consequent mitochondrial disruption, neuron necrosis and neurological deficits in mice-ScienceDirect. Biochem. Pharmacol. 2019, 169, 113607. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; El-Seedy, Y.Y.; Gomaa, N.A. Cytokine Response and Oxidative Stress Status in Dairy Cows with Acute Clinical Mastitis. J. Dairy Vet. Anim. Res. 2015, 3, 9–13. [Google Scholar]
- Burton, J.L.; Nonnecke, B.J.; Dubeski, P.L.; Elsasser, T.H.; Mallard, B.A. Effects of Supplemental Chromium on Production of Cytokines by Mitogen-Stimulated Bovine Peripheral Blood Mononuclear Cells. J. Dairy Sci. 1996, 79, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Mallard, B.A.; Mowat, D.N. Effects of chromium on health status, blood neutrophil phagocytosis and in vitro lymphocyte blastogenesis of dairy cows. Vet. Immunol. Immunopathol. 1996, 52, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Porporato, P.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.M.; Martin, J.C.; Scott, K.P.; Calderon, P.B.; Feron, O.; Muccioli, G.G.; et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, G.; Li, X.; Guan, Y.; Wang, Y.; Yuan, X.; Sun, G.; Wang, Z.; Li, X. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet. Res. 2018, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Yuan, X.; Sun, G.; Shen, B.; Xu, F.; Fan, G.; Jin, M.; Li, X.; Liu, G. Berberine inhibits lipopolysaccharide-induced expression of inflammatory cytokines by suppressing TLR4-mediated NF-kB and MAPK signaling pathways in rumen epithelial cells of Holstein calves. J. Dairy Res. 2019, 86, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Vissenaekens, H.; Criel, H.; Grootaert, C.; Raes, K.; Smagghe, G.; Van Camp, J. Flavonoids and cellular stress: A complex interplay affecting human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 8535–8566. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 21, 175. [Google Scholar] [CrossRef]
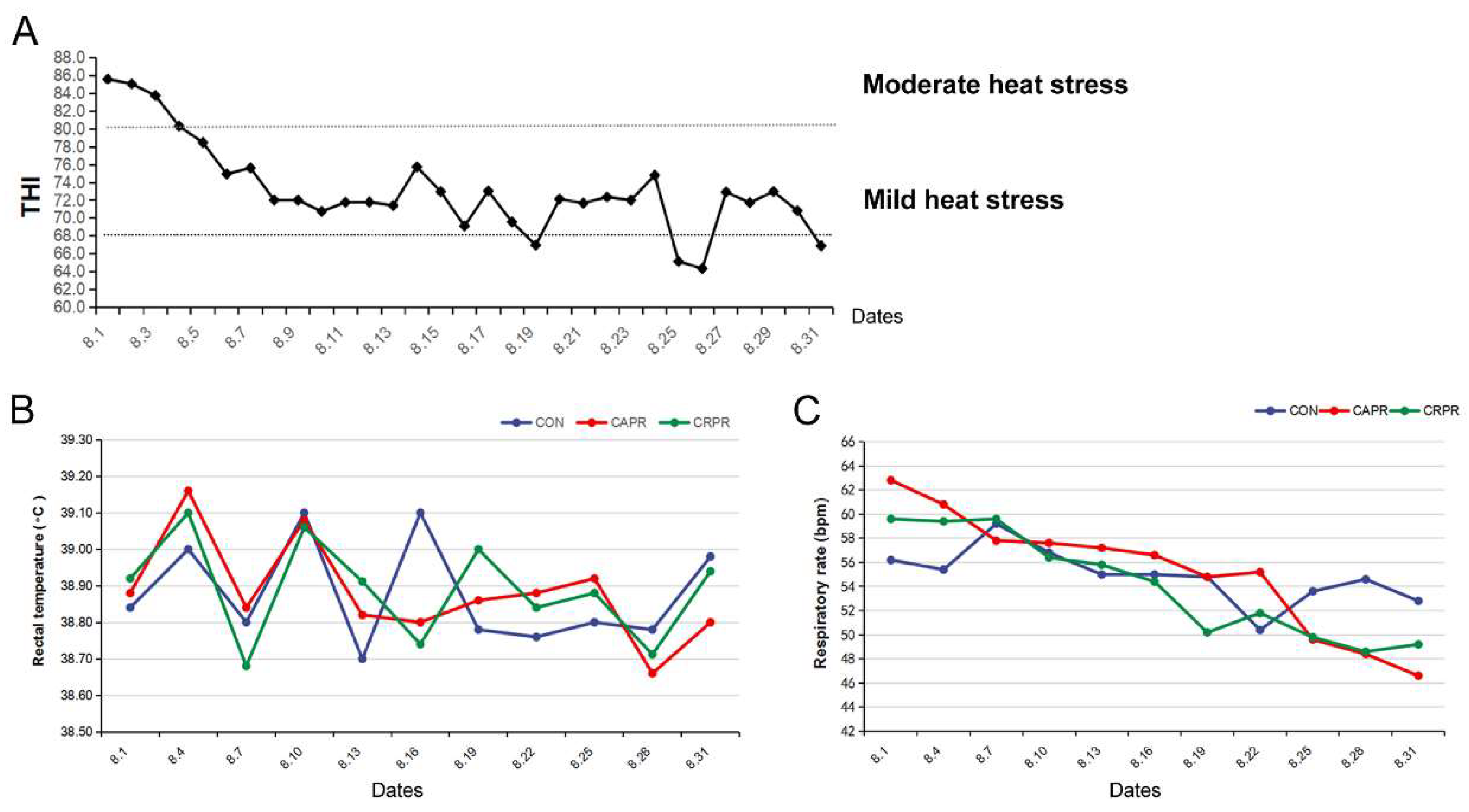
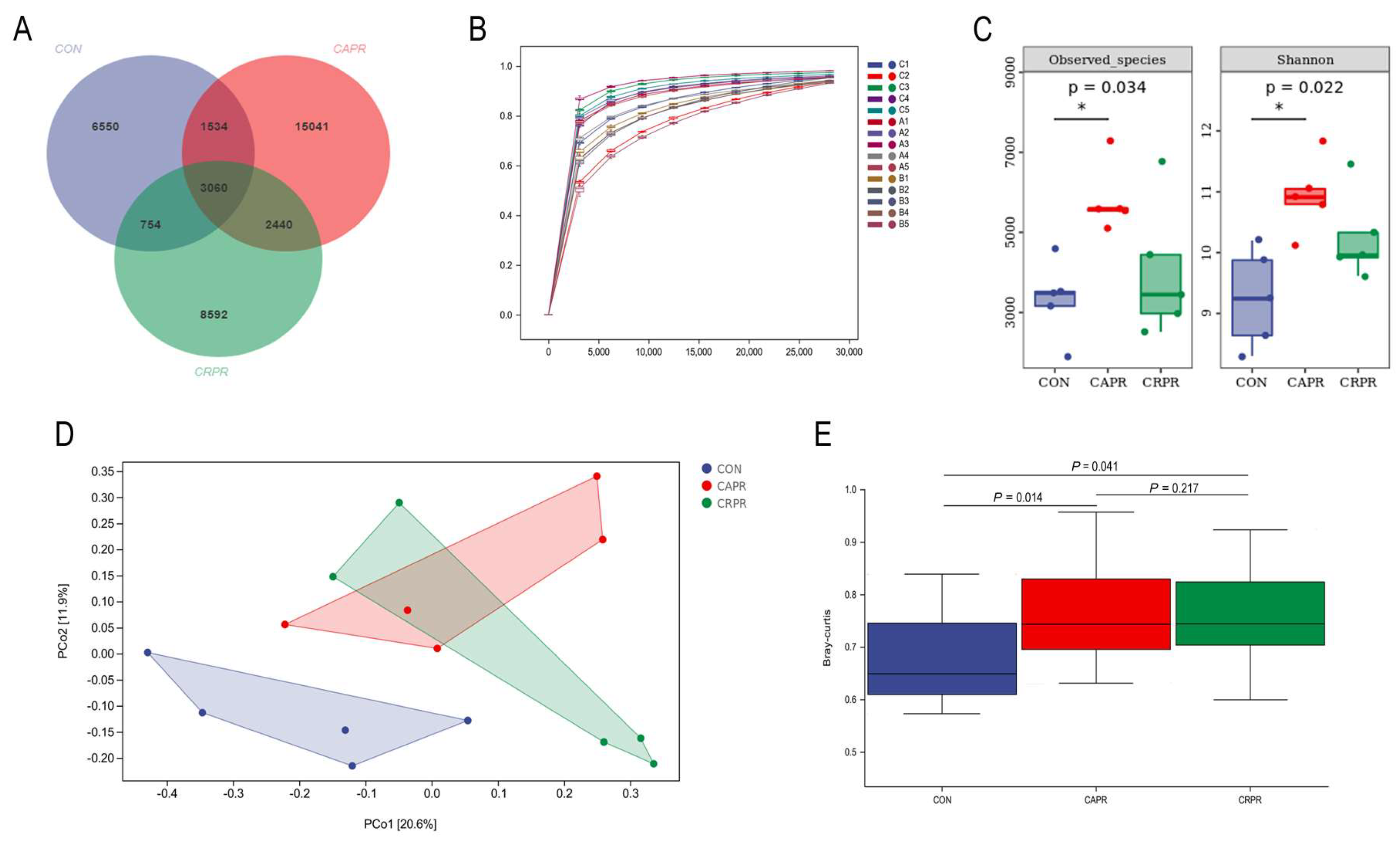
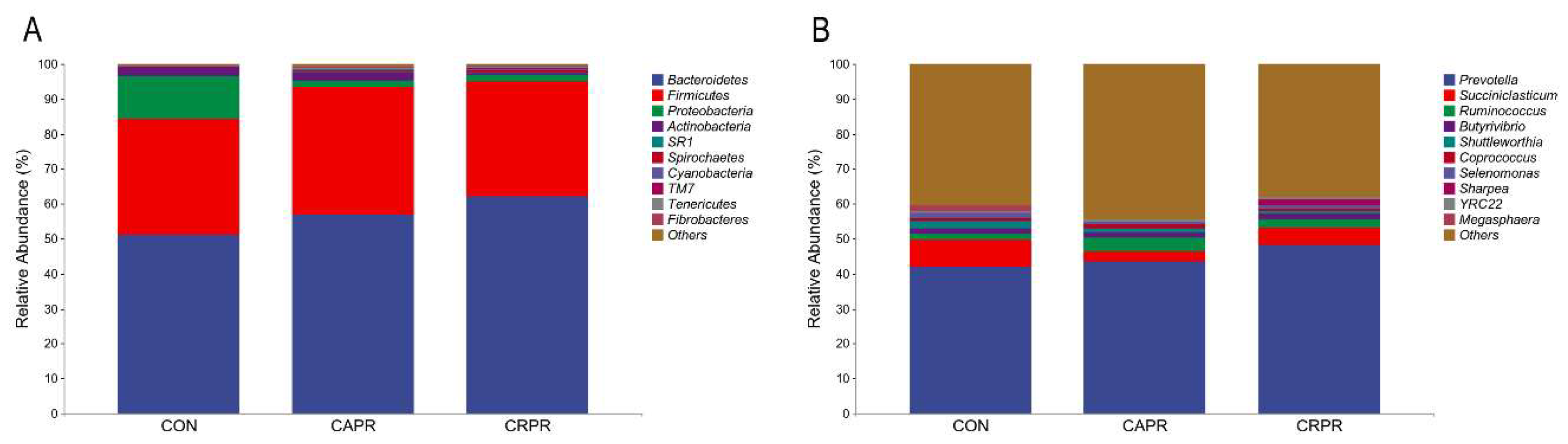

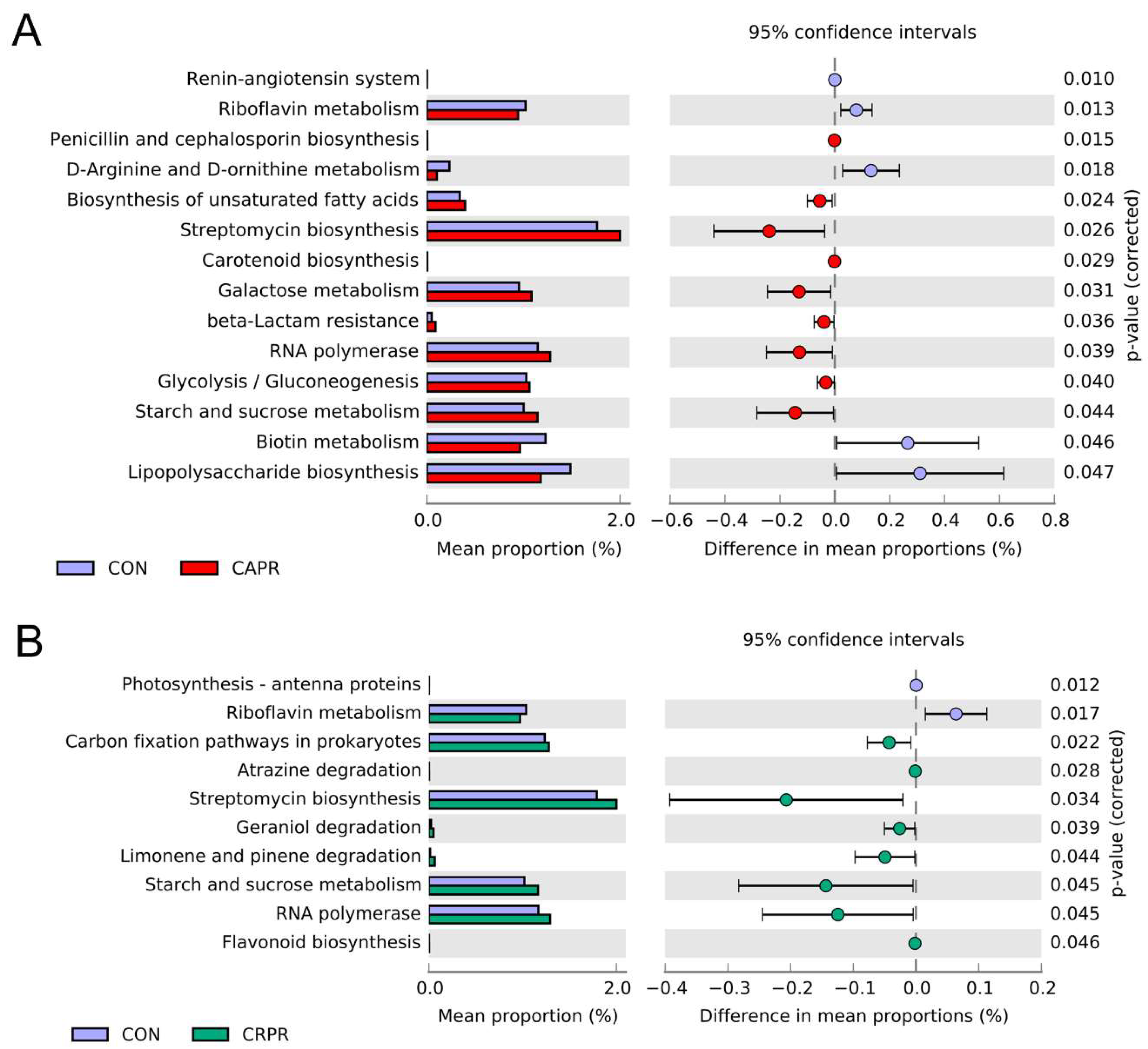
| Feed Composition | (% of TMR) | Nutrient Composition | (% of DM) 2 |
|---|---|---|---|
| Leymus chinensis | 2.80 | CP | 16.48 |
| RUP | 1.17 | ME (mCal/kg) | 2.68 |
| Corn silage | 63.08 | NEL (mCal/kg) | 1.72 |
| Premix 1 | 5.84 | ADF | 17.31 |
| Corn | 12.85 | NDF | 29.52 |
| Soybean meal | 6.78 | Crude fat | 4.75 |
| Corn germ meal | 3.97 | Ca | 0.79 |
| Cottonseed | 3.51 | K | 1.09 |
| Total | 100.00 | Na | 0.42 |
| Treatment 1 | |||||
|---|---|---|---|---|---|
| Items | CON | CAPR | CRPR | SEM | p-Value |
| Milk yield (kg/d) | 38.09 b | 40.60 b | 51.35 a | 2.17 | 0.018 |
| Milk fat (%) | 3.13 | 3.75 | 3.43 | 0.20 | 0.487 |
| Milk protein (%) | 3.14 | 3.31 | 3.26 | 0.07 | 0.585 |
| Milk fat:protein | 0.99 | 1.14 | 1.06 | 0.06 | 0.626 |
| MUN 2 (mg/dL) | 16.52 | 17.92 | 18.82 | 0.80 | 0.521 |
| Ruminal TVFAs 3, mM | |||||
| Total | 46.61 b | 50.98 ab | 61.78 a | 2.60 | 0.034 |
| Acetate | 23.71 b | 22.70 b | 30.21 a | 1.50 | 0.073 |
| Propionate | 11.99 b | 16.63 ab | 17.51 a | 1.08 | 0.067 |
| Butyrate | 7.19 b | 7.73 ab | 9.85 a | 0.51 | 0.066 |
| Isobutyrate | 0.69 | 0.69 | 0.70 | 0.04 | 0.993 |
| Valerate | 1.22 | 1.06 | 1.19 | 0.06 | 0.548 |
| Isovalerate | 1.46 | 1.55 | 1.74 | 0.09 | 0.496 |
| Hexanoate | 0.34 | 0.63 | 0.57 | 0.08 | 0.289 |
| A:P Ratio 4 | 2.02 | 1.41 | 1.79 | 0.13 | 0.173 |
| Abundance 1 (%) | ||||||
|---|---|---|---|---|---|---|
| Phylum | Genus | CON | CAPR | CRPR | SEM | p-Value |
| Bacteroidetes | 51.14 | 56.73 | 62.04 | 2.49 | 0.212 | |
| Prevotella | 42.05 | 43.37 | 48.28 | 2.54 | 0.608 | |
| unidentified_Bacteroidales | 1.98 b | 5.21 a | 4.60 ab | 0.59 | 0.049 | |
| YRC22 | 0.58 | 0.82 | 0.62 | 0.10 | 0.642 | |
| Firmicutes | 33.12 | 36.69 | 32.80 | 2.62 | 0.821 | |
| unidentified Ruminococcaceae | 3.60 b | 7.89 a | 7.26 ab | 0.90 | 0.100 | |
| unidentified_Clostridiales | 3.87 | 9.27 | 4.74 | 1.31 | 0.275 | |
| Succiniclasticum | 7.62 a | 2.99 b | 4.86 ab | 0.78 | 0.037 | |
| Ruminococcus | 1.81 | 3.99 | 2.34 | 0.61 | 0.334 | |
| Butyrivibrio | 1.40 | 1.34 | 1.58 | 0.16 | 0.841 | |
| Shuttleworthia | 2.18 | 1.15 | 0.83 | 0.34 | 0.357 | |
| unidentified Lachnospiraceae | 1.20 | 1.49 | 1.24 | 0.12 | 0.571 | |
| Coprococcus | 0.98 | 1.23 | 0.79 | 0.14 | 0.651 | |
| Selenomonas | 1.23 a | 0.53 b | 0.67 ab | 0.15 | 0.105 | |
| Sharpea | 0.13 | 0.14 | 1.82 | 0.59 | 0.883 | |
| Megasphaera | 1.58 | 0.03 | 0.02 | 0.51 | 0.320 | |
| Proteobacteria | 12.33 | 1.93 | 1.94 | 2.49 | 0.102 | |
| unidentified Succinivibrionaceae | 11.97 | 1.63 | 1.39 | 2.50 | 0.065 | |
| Actinobacteria | 2.15 | 2.35 | 0.56 | 0.67 | 0.519 | |
| unidentified Bifidobacteriaceae | 1.79 | 2.11 | 0.28 | 0.64 | 0.496 | |
| Treatment 1 | |||||
|---|---|---|---|---|---|
| Items | CON | CAPR | CRPR | SEM | p-Value |
| MDA 2 (nM/mL) | 4.52 b | 6.13 a | 5.23 ab | 0.27 | 0.032 |
| T-AOC 3 (U/mL) | 13.76 b | 17.32 a | 16.08 ab | 0.59 | 0.030 |
| SOD 4 (U/mL) | 22.93 b | 24.48 a | 23.72 ab | 0.32 | 0.131 |
| MPO 5 (U/L) | 199.82 b | 238.41 a | 216.28 ab | 7.22 | 0.081 |
| Blood Cytokine (pg/mL) | |||||
| TNF-α 6 | 271.94 a | 244.68 b | 142.13 c | 11.37 | 0.000 |
| IL-1β 7 | 674.42 a | 619.93 ab | 576.21 b | 13.28 | 0.006 |
| IL-6 8 | 251.57 a | 229.67 b | 209.96 c | 4.74 | 0.000 |
| IL-10 9 | 104.45 a | 96.69 ab | 92.26 b | 2.16 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Shen, B.; Huang, Y.; Kong, Y.; Tan, P.; Zhou, Y.; Yang, J.; Xu, C.; Wang, J. Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows. Microorganisms 2023, 11, 1625. https://doi.org/10.3390/microorganisms11071625
Zhao C, Shen B, Huang Y, Kong Y, Tan P, Zhou Y, Yang J, Xu C, Wang J. Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows. Microorganisms. 2023; 11(7):1625. https://doi.org/10.3390/microorganisms11071625
Chicago/Turabian StyleZhao, Chenxu, Bingyu Shen, Yan Huang, Yezi Kong, Panpan Tan, Yi Zhou, Jiaqi Yang, Chuang Xu, and Jianguo Wang. 2023. "Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows" Microorganisms 11, no. 7: 1625. https://doi.org/10.3390/microorganisms11071625
APA StyleZhao, C., Shen, B., Huang, Y., Kong, Y., Tan, P., Zhou, Y., Yang, J., Xu, C., & Wang, J. (2023). Effects of Chromium Propionate and Calcium Propionate on Lactation Performance and Rumen Microbiota in Postpartum Heat-Stressed Holstein Dairy Cows. Microorganisms, 11(7), 1625. https://doi.org/10.3390/microorganisms11071625







