Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize, Rhizobium, and Soil
2.2. Seed Treatment and Germination Test
2.3. RNA Isolation, cDNA Synthesis, and Next-Generation Sequencing
2.4. DNA Extraction and 16S rDNA Gene Amplicon Sequencing
2.5. Statistical Analysis
3. Results
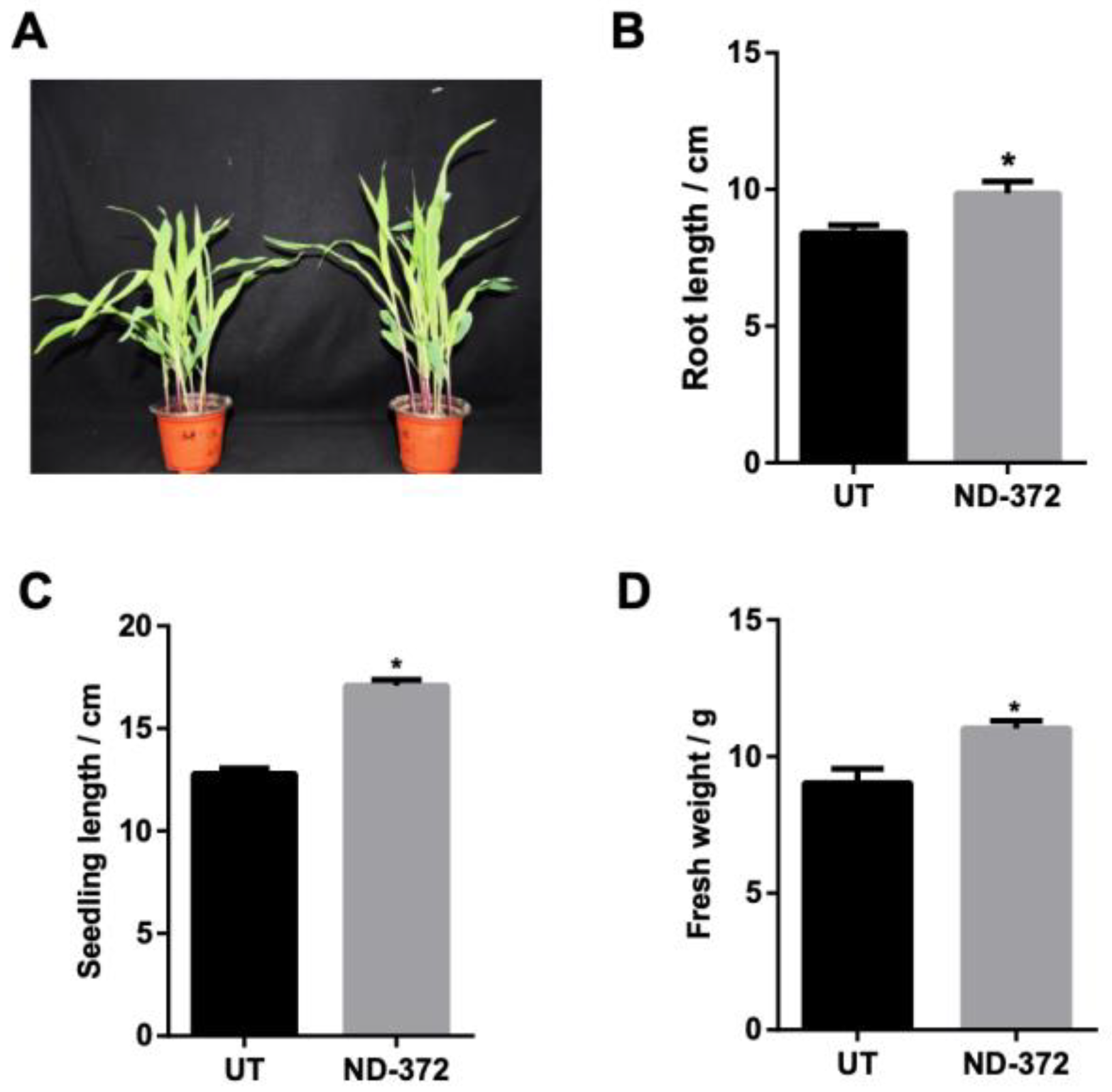
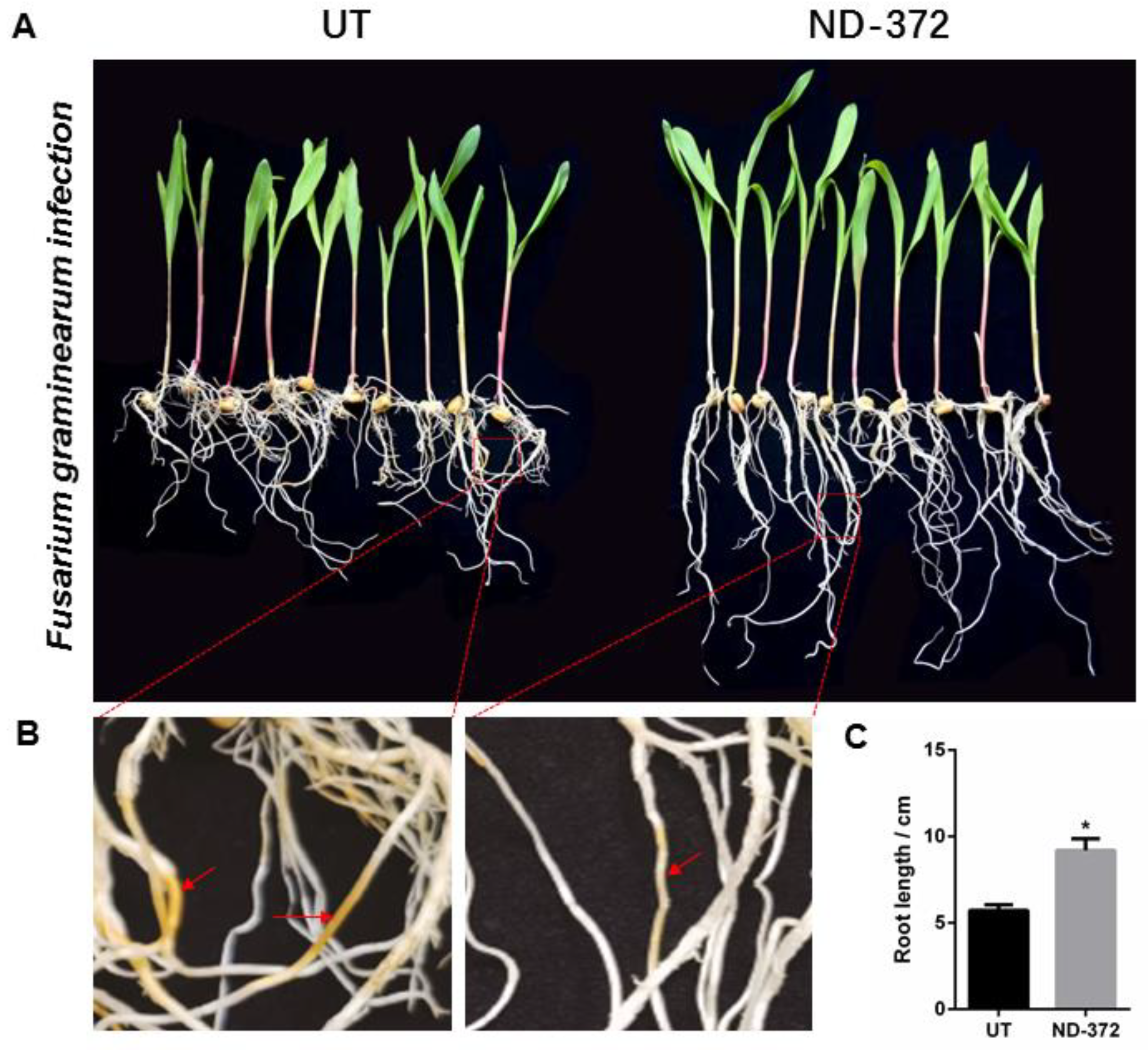
3.1. B. japonicum Treatment Promoted Maize Growth
3.2. The Expression of Maize Growth-Related Genes Was Induced by B. japonicum
3.3. B. japonicum Treatment Increased the Abundance of Maize Rhizosphere Bacteria
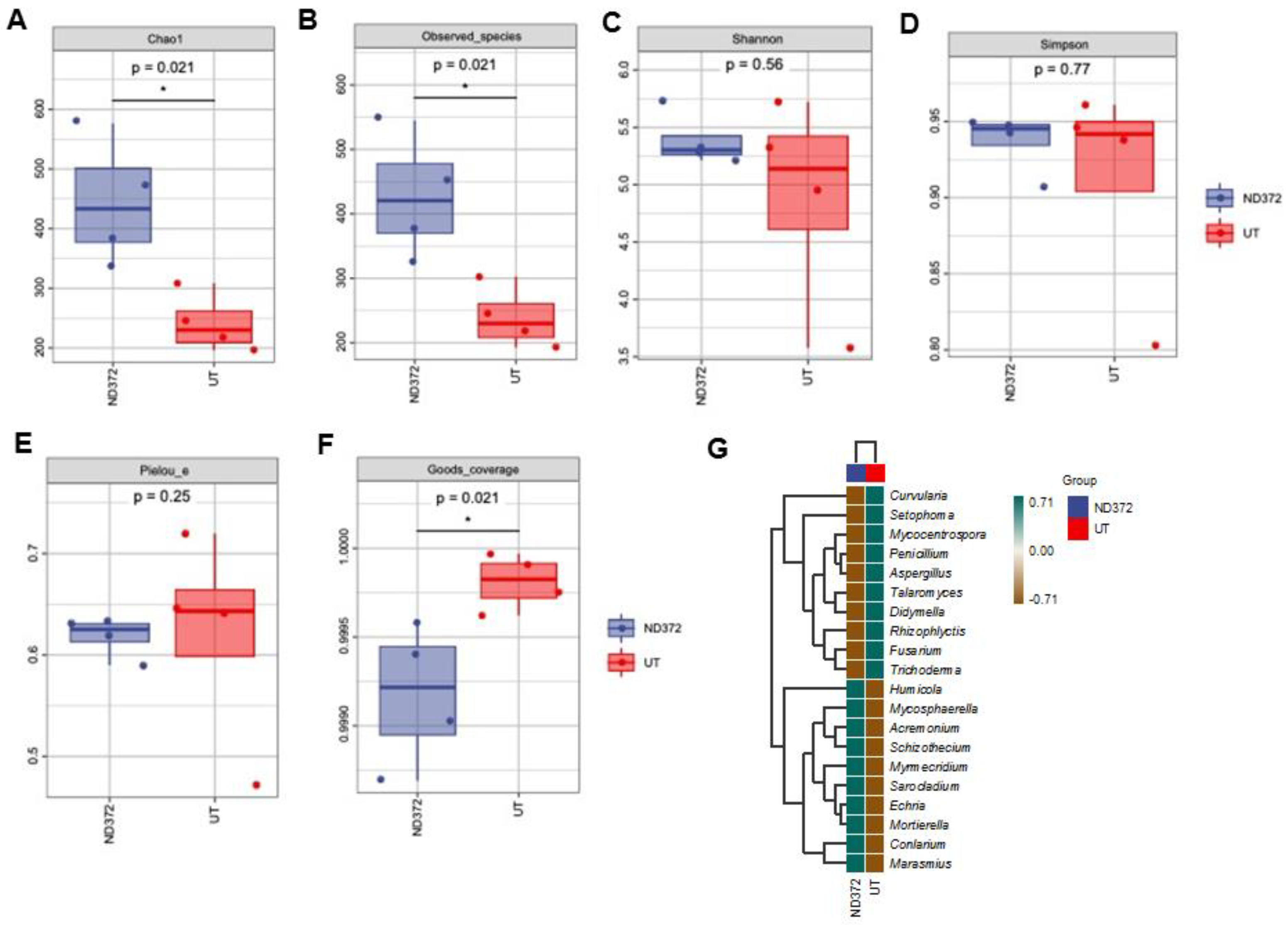
3.4. B. japonicum Treatment Affected the Variation of Maize Rhizosphere Fungi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Effect of specific plant-growth-promoting rhizobacteria (PGPR) on growth and uptake of neonicotinoid insecticide thiamethoxam in corn (Zea mays L.) Seedlings. Pest Manag. Sci. 2015, 71, 1258–1266. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Z.; Li, X.; Zhao, Y.; Zhao, B.; Wu, G.; Ma, X.; Wang, H.; Xie, Y.; Li, Q.; et al. Genome-wide selection and genetic improvement during modern maize breeding. Nat. Genet. 2020, 52, 565–571. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, X.; Chen, W.; Zhang, R.; Li, Z.; Wen, W.; Warburton, M.L.; Li, J.; Li, H.; Yang, X. Population genomics of Zea species identifies selection signatures during maize domestication and adaptation. BMC Plant Biol. 2022, 22, 72. [Google Scholar] [CrossRef]
- Eakin, H.; Perales, H.; Appendini, K.; Sweeney, S. Selling Maize in Mexico: The persistence of peasant farming in an era of global markets. Dev. Chang. 2014, 45, 133–155. [Google Scholar] [CrossRef]
- Kistler, L.; Maezumi, S.Y.; Gregorio de Souza, J.; Przelomska, N.A.S.; Malaquias Costa, F.; Smith, O.; Loiselle, H.; Ramos-Madrigal, J.; Wales, N.; Ribeiro, E.R.; et al. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science 2018, 362, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.-M.; Elliott, G.N.; Bontemps, C.; Estrada-de los Santos, P.; Gross, E.; dos Reis, F.B.; Sprent, J.I.; et al. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant-Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef] [Green Version]
- Rogel, M.A.; Ormeño-Orrillo, E.; Martinez Romero, E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011, 34, 96–104. [Google Scholar] [CrossRef]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial associations with legumes. CRC Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Gao, J.-S.; Cao, Y.-H.; Sheirdil, R.A.; Wang, X.-C.; Zhang, L. Rhizobium oryzicola sp. nov., potential plant-growth-promoting endophytic bacteria isolated from rice roots. Int. J. Syst. Evol. Microbiol. 2015, 65, 2931–2936. [Google Scholar] [CrossRef]
- Celador-Lera, L.; Menéndez, E.; Peix, A.; Igual, J.M.; Velázquez, E.; Rivas, R. Rhizobium zeae sp. nov., isolated from maize (Zea mays L.) Roots. Int. J. Syst. Evol. Microbiol. 2017, 67, 2306–2311. [Google Scholar] [CrossRef]
- Mano, H.; Morisaki, H. Endophytic bacteria in the rice plant. Microbes Environ. 2008, 23, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, M.D.l.E.; García-Fraile, P.; Rivas, R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B.; Yanni, Y.G.; Rolfe, B.G. Rhizobial inoculation influences seedling vigor and yield of rice. Agron. J. 2000, 92, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.X.; Tavares, M.J.; Franck, J.; Ali, S.; Glick, B.R.; Rossi, M.J. ACC deaminase plays a major role in Pseudomonas fluorescens yss6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch. Microbiol. 2019, 201, 817–822. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B.; Squartini, A.; Zanardo, M.; Zidan, M.I.; Elsadany, A.E.Y. Assessment of the natural endophytic association between Rhizobium and wheat and its ability to increase wheat production in the Nile delta. Plant Soil 2016, 407, 367–383. [Google Scholar] [CrossRef]
- Tilak, K.V.B.R.; Ranganayaki, N.; Manoharachari, C. Synergistic effects of plant-growth promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeonpea (Cajanus cajan). Eur. J. Soil Sci. 2006, 57, 67–71. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food. Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Chandra, A.; Chandra, P.; Tripathi, P. Whole genome sequence insight of two plant growth-promoting bacteria (B. subtilis BS87 and B. megaterium BM89) isolated and characterized from sugarcane rhizosphere depicting better crop yield potentiality. Microbiol. Res. 2021, 247, 126733. [Google Scholar] [CrossRef]
- Ouyabe, M.; Tanaka, N.; Shiwa, Y.; Fujita, N.; Kikuno, H.; Babil, P.; Shiwachi, H. Rhizobium dioscoreae sp. nov., a plant growth-promoting bacterium isolated from yam (Dioscorea species). Int. J. Syst. Evol. Microbiol. 2020, 70, 5054–5062. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Wang, H.; Feng, D. Genome-wide identification and expression analysis of the tayucca gene family in wheat. Mol. Biol. Rep. 2021, 48, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, Y.; Zeng, S.; Yan, J.; Wang, E.; Luo, L. Expression of a novel PSK-encoding gene from soybean improves seed growth and yield in transgenic plants. Planta 2019, 249, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, Y.; Tian, Z.; Fu, G.; Pei, X.; Pan, Z.; Nazir, M.F.; Song, S.; Li, H.; Wang, X.; et al. Ghgasa10-1 promotes the cell elongation in fiber development through the phytohormones IAA-induced. BMC Plant Biol. 2021, 21, 448. [Google Scholar] [CrossRef]
- Chen, W.; Chen, L.; Zhang, X.; Yang, N.; Guo, J.; Wang, M.; Ji, S.; Zhao, X.; Yin, P.; Cai, L.; et al. Convergent selection of a WD40 protein that enhances grain yield in maize and rice. Science 2022, 375, eabg7985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, H.; Li, T.; Wu, J.; Nagarajan, R.; Lei, L.; Powers, C.; Kan, C.-C.; Hua, W.; Liu, Z.; et al. Tacol-B5 modifies spike architecture and enhances grain yield in wheat. Science 2022, 376, 180–183. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Wang, Z.; Zhang, Z.; Wu, Z. Identifying key regulatory genes of maize root growth and development by RNA sequencing. Genomics 2020, 112, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottova, D.; Kristufek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hao, Z.; Li, K.; Sha, Y.; Wang, E.; Sui, X.; Mi, G.; Tian, C.; Chen, W. Effectsof growth-promoting rhizobacteria on maize growth and rhizosphere microbial community under conservation tillage in Northeast China. Microbiol. Biotechnol. 2021, 14, 535–550. [Google Scholar] [CrossRef]
- Ge, C.; Tang, C.; Zhu, Y.-X.; Wang, G.-F. Genome-wide identification of the maize 2OGD superfamily genes and their response to Fusarium verticillioides and Fusarium graminearum. Gene 2021, 764, 145078. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, L.; Yin, Z.; Liang, Y.; Chen, L.; Zou, S.; Dong, H. The golgin protein RUD3 regulates Fusarium graminearum growth and virulence. Appl. Environ. Microbiol. 2021, 87, e02522-20. [Google Scholar] [CrossRef]
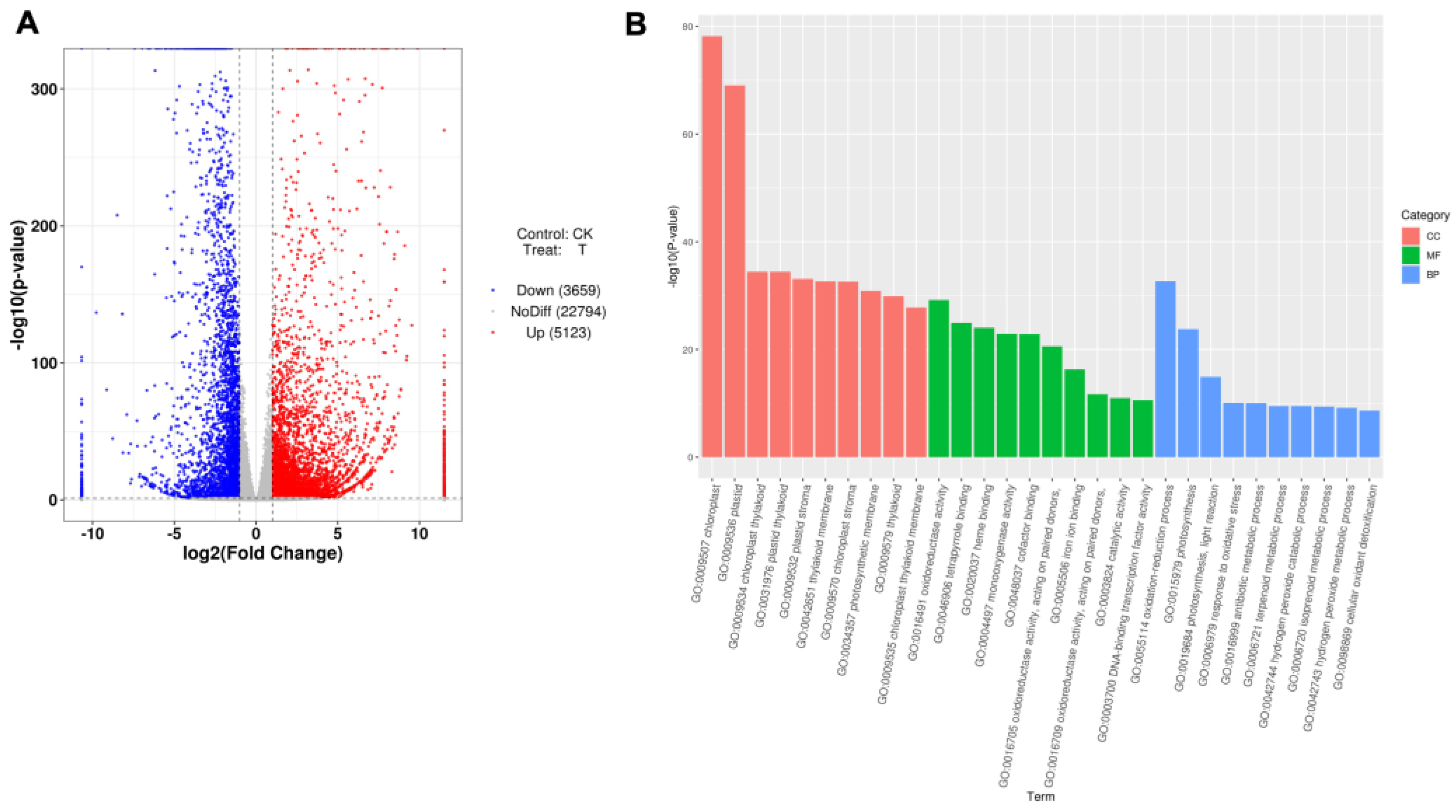

| Control | Treat | Up-Regulated | Down-Regulated | Total |
|---|---|---|---|---|
| UT | T (ND-372) | 5123 | 3659 | 8782 |
| Gene ID | Foldchange (ND-372/UT) | Potential Function |
|---|---|---|
| Zm00001eb397820 | 433.63 | multicellular organism development |
| Zm00001eb125420 | 168.58 | multicellular organism development |
| Zm00001eb244140 | 167.19 | positive regulation of organ growth |
| Zm00001eb204390 | 136.68 | vegetative to reproductive phase transition of meristem |
| Zm00001eb147260 | 105.73 | structure development |
| Zm00001eb123060 | 88.90 | multicellular organism development |
| Zm00001eb054040 | 67.45 | oxidation-reduction process of development |
| Zm00001eb074640 | 56.91 | response to auxin |
| Zm00001eb236120 | 56.38 | meristem initiation |
| Zm00001eb110420 | 56.36 | plant organ development |
| Zm00001eb248500 | 45.89 | syncytium formation |
| Zm00001eb243730 | 33.32 | system development |
| Zm00001eb330990 | 29.49 | lateral root formation |
| Zm00001eb335320 | 28.74 | cytokinin-activated signaling pathway |
| Zm00001eb308610 | 21.35 | embryo development ending in seed dormancy |
| Zm00001eb012940 | 21.09 | multicellular organism development |
| SampleID | Input | Filtered | Denoised | Merged | Nonchimeric | Nonsingleton |
|---|---|---|---|---|---|---|
| ND372_1 | 134,425 | 125,195 | 116,500 | 80,010 | 67,478 | 62,262 |
| ND372_2 | 147,860 | 1365,91 | 126,988 | 84,603 | 69,399 | 63,651 |
| ND372_3 | 146,836 | 129,701 | 120,458 | 79,713 | 65,519 | 60,463 |
| ND372_4 | 138,138 | 128,174 | 120,050 | 82,681 | 69,203 | 64,372 |
| UT_1 | 138,595 | 128,379 | 121,750 | 90,777 | 75,797 | 72,260 |
| UT_2 | 135,707 | 124,918 | 118,907 | 91,637 | 74,928 | 71,524 |
| UT_3 | 141,972 | 130,331 | 122,674 | 87,220 | 72,159 | 68,014 |
| UT_4 | 146,266 | 134,626 | 126,237 | 87,319 | 69,539 | 64,766 |
| SampleID | Input | Filtered | Denoised | Merged | Nonchimeric | Nonsingleton |
|---|---|---|---|---|---|---|
| ND_372_1 | 192,013 | 153,449 | 152,606 | 150,526 | 148,915 | 148,915 |
| ND_372_2 | 152,905 | 129,712 | 128,835 | 127,487 | 124,810 | 124,809 |
| ND_372_3 | 108,237 | 81,117 | 80,415 | 79,215 | 77,885 | 77,885 |
| ND_372_4 | 120,495 | 102,122 | 101,522 | 100,854 | 97,385 | 97,383 |
| UT_1 | 88,801 | 77,634 | 77,138 | 75,670 | 72,733 | 72733 |
| UT_2 | 89,368 | 63,851 | 63,321 | 61,711 | 56,830 | 56,830 |
| UT_3 | 96,514 | 82,242 | 81,509 | 80,691 | 78,083 | 78,083 |
| UT_4 | 64,372 | 53,757 | 53,034 | 52,104 | 48,766 | 48,766 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chi, Y.; Su, X.; Ye, Z.; Ren, X. Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes. Microorganisms 2023, 11, 1654. https://doi.org/10.3390/microorganisms11071654
Li Z, Chi Y, Su X, Ye Z, Ren X. Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes. Microorganisms. 2023; 11(7):1654. https://doi.org/10.3390/microorganisms11071654
Chicago/Turabian StyleLi, Zhao, Yu Chi, Xianyan Su, Zhenghe Ye, and Xuexiang Ren. 2023. "Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes" Microorganisms 11, no. 7: 1654. https://doi.org/10.3390/microorganisms11071654




