Abstract
Two formulations of Alltech Crop Science products (ACS), a proprietary blend of fermentation products and plant extracts with micronutrients (ACS5075), and a microbial based product (ACS3048), were tested to understand (1) their impact on the tomato plant immune response and (2) whether they are priming a resistance response in plants against root knot nematodes (RKN). Research findings reported previously indicate that tomato plants pre-treated with ACS5075 and ACS3048 were found less sensitive to Meloidogyne javanica infection. In the current study, the expression of six defence-related genes (PR-1, PR-3, PR-5T, ACO, CAT and JERF 3), relative to a housekeeping gene, were monitored via RT-PCR. Results suggest that the treatment with ACS5075 enhanced ACO and PR-1 gene expression levels, both post- treatment and post-infection with M. javanica. Reduced M. javanica infestation that was reported in the previous study could be attributed to the increased expression of these genes in the ACS5075-treated plants. Tomato plants treated with ACS3048, but without RKN infection, also demonstrated higher levels of ACO and PR-1 gene expression. Subsequently, 2D-gel electrophoresis was performed to study the differential protein expression in leaf tissues of treated tomato plants in an effort to elucidate a possible mechanism of action for these products. Protein spot 1 was identified as ‘disease resistance protein RPP13-like’, protein spot 2 as ‘phosphatidylinositol 4-phosphate 5-kinase 2’, spot 3 as ‘protein SABRE like’ and protein spot 4 as ‘uncharacterized protein’. Overall research findings indicate that the ACS products could be used as plant immunity-boosting agents, as they play a significant role in the expression of certain genes and proteins associated with plant defence.
1. Introduction
Plant parasitic nematode (PPN) infections cause serious crop losses worldwide and therefore are a threat to world agriculture and economy. They are widely spread with a broad host range causing yield losses of about 30% in susceptible crop varieties (tomatoes, eggplants and melons; [1]). Plant parasitic nematodes are known to manifest different diseases, such as root-knots in tomatoes, cysts in potatoes, white tip disease in rice, stunted growth in wheat and many more [2]. Root-knot nematodes (RKN) are PPN that are most prevalent and highly damaging [3]. The four predominant species are Meloidogyne arenaria, M. incognita, M. javanica and M. hapla [2]. De Waele and Elsen [4] have reported that the difficulty in controlling the damage caused by Meloidogyne species is due to their broad host range and high reproductive rate.
The application of synthetic chemical nematicides has been known to have various environmental and cost implications for growers [5]. They also have long term impacts on human health, ground waters and animals [1], and for this reason, many synthetic chemical nematicides have been banned [6]. Biofumigation has been practiced as one of the successful approaches in the past few years to manage PPNs [7]; however, one major limitation with this approach is that the efficacy of the fumigant deteriorates over time due to various environmental factors, such as temperature, humidity and soil characteristics [7]. Some research groups have reported the application of extracts from cruciferous plants [8], such as rape, mustard, canola, cabbage and broccoli [8,9,10], for PPN management, but these treatments have been shown to cause root decay and eventually lead to rotting of the plant biomass, resulting in unpleasant odours in the fields [8]. Other successful approaches include the application of organic amendments, bionematicides and fermentation products to control PPN infestation. However, before applying any such products a thorough investigation is essential to study their impact on other non-targeted organisms and overall soil health. It is also essential to study the mode of action of various products before field application. Therefore, there is still scope for research and development to investigate sustainable approaches to manage PPN infestations.
Priming is a mechanism or approach through which a plant’s defence mode is activated to prepare the plant to respond better to an abiotic or biotic factor and thus to improve crop productivity [11]. The process of inducing plant defence to confer resistance against broad range of pathogens is referred to as systemic acquired resistance (SAR). The immune system in plants is generally associated with or regulated by low molecular weight phytohormones, such as salicylic acid (SA), jasmonic acid (JA) and ethylene (ET). Systemic acquired resistance is regulated by SA, in collaboration with pathogenesis-related (PR) genes to provide long-term resistance in plants against biotrophic pathogens. Jasmonic acid and ET are among other basic plant hormones that activate rhizobacteria-induced systemic resistance (ISR) in plants [12].
The treatment of a plant with an elicitor or compounds, such as hormones, fermentation mixtures, soil health products or nematicides, help trigger its priming phase and therefore prepares the plant to encounter an infection. Chester [13] initially reported the induction of plant defence through externally applied elicitor molecules. Indeed, this hypothesis was later proven correct through the application of SA to tobacco plants, which enhanced the expression of PR-genes and provided plant resistance against tobacco mosaic virus (TMV; [14]). The application of Bacillus firmus (I-1582 isolates) to tomato plants before transplantation leads to plant systemic resistance and reduced development of Meloidogyne eggs [15]. There are several PR-genes that become overexpressed in plants when treated with certain elicitor compounds [12]. This overexpression further helps in the activation of plant defence and thus mediates resistance against plant pathogens.
Previous research findings outlined the impact that ACS products had on reducing RKN infestation by inhibiting egg mass formation on tomato roots and also on improvements in tomato plant growth, by enhancing biomass production of the treated plants [16,17]. The objective of this study was to establish what impact the ACS products have on the plant immune response and whether they are priming a resistance response in plants against RKN. The expression of six defence-related genes, relative to the actin housekeeping gene (Table 1), was monitored via RT-PCR using samples of both leaf and root tissue of treated plants with and without nematode infection. Out of the six genes studied, three were PR-genes (PR-1, PR-3, PR-5T), while the other three genes were ACO, CAT and JERF 3. Protein expression in the leaf tissues of tomato plants treated with ACS products was also examined in an effort to elucidate a possible mechanism of action for the products.

Table 1.
List of defence-related genes studied and the specific primers used in reverse transcriptase- real time polymerase chain reaction (RT-PCR).
2. Materials and Methods
2.1. Sourcing, Culturing and Inoculation of Nematodes to Tomato Plants
Dr. E. A. Tzortzakakis, Hellenic Agricultural Organization—DEMETER, Crete, Greece—kindly provided tomato (Solanum lycopersicum cv. Moneymaker) roots that were infected with M. javanica. These roots were carefully used for further culturing of the RKN species within a South East Technological University (SETU) plant growth room. Roots of 3–4-week-old tomato seedlings were infected with 5–7 egg masses/plant around the roots. In the plant growth room, all the plants were maintained at a constant temperature of 26 ± 2 °C, 70 ± 10% relative humidity (RH), and a 14 h day/10 h night cycle. To prevent white fly, aphid, spider mite and other insect infestations, organic insecticide (Neudorff® Organic Bug and Larvae killer) was applied (1%) once every three weeks on the leaves and stems. After a 8–12-week interval, tomato roots were examined for nematode galling damage. Using a sterile scalpel and forceps, egg masses were removed from the roots and kept at 9 °C to prevent juvenile hatching. All RKN work, including all growing, experimentation and waste disposal, was performed with permission from the Plant Health Division of the Irish Department of Agriculture, Food, and the Marine (DAFM) and under rigorous quarantine and confinement regulations.
2.2. Treatment of Tomato Plants with ACS Products and NemguardTM
Tomato (cv. Moneymaker) seeds were germinated under environmentally controlled glasshouse conditions at 32 ± 2 °C and a natural day/night cycle. The seeds were first sown on sterilized garden soil with the following characteristics: pH 7.6 ± 0.02, electrical conductivity, 540 µs/cm; clay (%), 8 ± 0.02; silt (%), 19 ± 0.5; sand (%), 73 ± 3.9; Na, 12.58 (mg Kg−1); P, 2.8 (mg Kg−1); K, 30.59 (mg Kg−1). The seedlings were grown for a period of 3–4 weeks until they attained a height of approximately 6–10 cm before being transplanted into individual cups (4.5 cm × 12 cm) for all further experiments. The cups contained approximately 150–200 g of sterilized soil and compost mixture in a ratio of 1:1.
Two sets of experiments were performed; in the first set, 4-week-old tomato plants were treated individually with 3% (v/v) ACS5075 or 3 g ACS3048 in separate cups only once and were harvested after 3, 5, 9 and 15 days. For treatment with ACS5075, 50 mL of a 3% solution was prepared using distilled water, and this solution was added directly into the pot containing soil with one tomato plant in it. For treatment with ACS3048, 3 g of the powdered product was added as layers in between the soil in each cup, and then, one tomato plant was planted in each cup. Tomato plants without any treatment were considered the untreated control (UC) for this set of experiments. The ACS concentrations were selected based on previous results from entomopathogenic nematode work [16] and an RKN study [17]. Six replications (individual plants) were established per treatment and per time interval. All plants at 3 days post-treatment (3 dpt) were individually uprooted to proceed with RNA extraction and then RT-PCR. Subsequently, plants were uprooted after each dpt to proceed with RNA extraction and then RT-PCR; the same was performed for the control plants as well.
Leaf and root tissue, separately, was ground to a fine powder using liquid nitrogen with a sterile porcelain pestle and mortar, and 100 mg per sample was immediately used for RNA extraction or kept at −80 °C until it was used. Twelve tissue samples (two from each plant; leaf and root) were collected for each time point and treatment.
In the second set of experiments, tomato plants were also grown for a period of 4 weeks and after attaining an approximate height of 10 cm, plants were randomly selected and individually received the following treatments: six tomato plants grown without any treatment or nematode inoculation were considered the untreated + uninoculated control plants (UC). Six more tomato plants were infected with approximately 500 freshly hatched M. javanica juveniles (J2)/plant. Infection was carried out by gently releasing the nematodes into three holes made in the soil around the plant roots using a micropipette. These six plants were only infected but not treated with any products and therefore were considered inoculated untreated plants (IU). Six more tomato plants were initially treated with 3% ACS5075 and 3 g ACS3048 one week before infecting them with approximately 500 J2/plant. The infection in these plants was conducted as outlined above. These six plants were considered treated + inoculated test plants (T). Plants that were infected with RKN J2 were gently removed from the pots after 3, 7 and 15 dpi (days post-inoculation) to check the development of egg masses on the roots. The development of egg masses was observed only after 15 dpi, and therefore, all the plants (IU, UC and T) were harvested after 15 dpi, when leaf and root samples were taken for RNA isolation. All treatments were conducted with six replications, and a total of 12 tissue samples (two from each plant; leaf and root) were collected per treatment.
2.3. RNA Extraction and Quantitative Real-Time Reverse PCR
Leaf and root tissues, collected post-treatment and post-inoculation, were either used immediately or stored at −80 °C until use. RNA was extracted from the leaf and root tissues (approx. 100 mg/sample) for all the collected samples using the RNA-easy plant mini kit (Qiagen, Manchester, UK). The quality of the extracted RNA was verified by performing electrophoresis on 1.0% agarose gels, under denaturing conditions, with gels containing 2.2 M formaldehyde, and visualized using ethidium bromide stain. The extracted RNA samples were stored at −80 °C.
The expression of six defence-related genes relative to the actin housekeeping gene (Table 1) was monitored via RT-PCR (Roche LightCycler® 96 System, Switzerland) from samples of both leaf and root tissue of the plants. Expression of the genes was studied after 3, 5, 9 and 15 dpt and 15 dpi. PCR mixtures (20 μL final volume) contained RNase-free water, 1 μL (0.4 μM) each of forward and reverse primers, 5 μL (200–250 ng/μL) of RNA template (approximately 1000 ng/reaction) and 4 μL (5 × concentration) of EvoScript RNA SYBR® Green I Master Mix (Roche Life Science, Switzerland). PCR cycling conditions were as follows: 60 °C for 15 min (reverse transcription); 95 °C for 10 min (pre-incubation and initial denaturation); 40–45 cycles (amplification) of 95 °C for 10 s, 58 °C for 30 s, 95 °C for 60 s, 25 °C for 60 s, 95 °C continuous (melting curve) and the final step at 40 °C for 30 s (cooling) following the manufacturer’s instructions.
Actin was used as the housekeeping gene, as its expression in tomato tissues does not vary after nematode infestation [12]. Primers sets are described in Table 1. The relative fold-changes in gene expression were calculated using the 2−ΔΔCT method [19]. The cycle threshold (CT values) that were generated by the Roche machine were recorded individually. ΔCT values were calculated by deducting the individual CT values from that of the CT values obtained for the respective Actin gene for each sample [ΔCT(a target sample) − ΔCT(Actin)]. Using a reference gene (Actin) as a standard, the ultimate outcome of this method is represented as the mean relative fold-change in target gene expression in a target sample compared to that in the untreated control, which acted as the calibrating sample.
2.4. Treatment of Tomato Plants with ACS Products, Protein Extraction, SDS-PAGE and 2D-Gel Electrophoresis
Tomato plants were grown in greenhouse conditions as described previously. Four-week-old tomato plants were treated with ACS5075, ACS3048 and NemguardTM at 3% (v/v), 3 g and 13.3 mg/L, respectively, for 9 days. NemguardTM is a commercial organic nematicide containing 45% garlic extract. After 9 days of treatment, shoot tissues were harvested, ground to a fine powder using liquid nitrogen in a sterile porcelain pestle and mortar and were stored at −80 °C, if not immediately used, for protein extraction. Total protein was extracted from 0.5 g of tissues/sample using the Sigma Total protein extraction kit® (USA), following the manufacturer’s instructions. Protein concentrations were estimated using a NanoDropTM. All the treatments were conducted in triplicate.
Initially, approximately 4–5 mg of protein was loaded in each well in a 4–20% Mini-PROTEAN® TGX™ Precast Protein Gel (Biorad, USA) to analyse and separate proteins present in the samples based on molecular weight using SDS-PAGE. Subsequently, extracted protein samples were also analysed via separation through two dimensions, isoelectric point (pI) and molecular weight (MW), by performing 2D gel electrophoresis using the Invitrogen ZOOM® IPGRunner™ system (Carlsbad, CA, USA), following the manufacturer’s instructions [20]. Approximately 400 μg (155 μL of the total volume diluted with rehydration buffer) of total protein per sample was loaded onto each ZOOM® strip (pH 3–12) for protein separation based on the isoelectric point. Upon rehydration overnight, the second-dimension separation based on molecular weight was performed using an Invitrogen™ NuPAGE™ 4–12% Bis-Tris ZOOM™ protein gel, 1.0 mm, IPG-well. Once the samples were run, the gels were stained using the Coomassie staining procedure [21]. After 2 h of staining, gels were destained with methanol and glacial acetic acid. Subsequently, protein differences in the gels were observed using the white background of a white/UV transilluminator [21].
2.5. Protein Spot Excision from the 2D-Gels and N-Terminal Protein Sequencing
Upon completion of the staining and de-staining procedure of all the individual gels, the gels were carefully placed onto the gel documentation system to observe the protein spots using white light transmission. The unique protein spots were identified by comparing the gels containing untreated control protein samples with the gels containing protein samples from the plants treated with ACS5075, ACS3048 and NemguardTM. The unique protein spots were carefully excised from the gels using a sterile scalpel and were suspended into 1 mL of storage buffer (5% glacial acetic acid) in 1.5 mL centrifuge tubes. The protein spots were labelled based on their molecular weight. These protein spots were collected from multiple runs and were sent for identification to Alta Biosciences in the United Kingdom. Automated N-terminal sequence analysis was carried out on each of the samples.
The amino acid sequences were blasted against the available sequences in the NCBI database using Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome) (Accessed date: 20 April 2022). The sequences that showed query coverage of 100%, with a lower E-value and being in S. lycopersicum, were considered the expected protein from each spot.
2.6. Statistical Analysis
In the first set of experiments, results recorded from RT-PCR using RNA samples from tomato plants (n = 6) that were not infected with RKN were presented as means ± the standard error (SE). These values were calculated using 2−ΔΔCt and indicate gene expression changes in tissue from ACS-treated plants relative to those from untreated control (UC) plants and the actin housekeeping gene. Each value is derived from a single RNA extraction. The y-axis in Figure 1 and Figure 2 indicates the mean fold-change compared to UC. It is a ratio. If the ratio was equal to 1, it means that the expression was not different from that of UC. If the ratio was more or less than 1, it indicates that the expression was different from that of UC. These values of each group (n = 6) were subjected to a non-parametric Kolmogorov–Smirnov test using IBM-SPSS, version 23 (p ≤ 0.05). An asterisk (*) in Figure 1 and Figure 2 indicates that the means are significantly different from the untreated control (p ≤ 0.05). In the second set of experiments, the results of differential gene expression after inoculating the tomato plants with M. javanica were subjected to analysis of variance (ANOVA) (p ≤ 0.05), using IBM-SPSS, version 23. The various bars in Figure 3 represent the expression of each defence gene (PR-1, PR-3, PR-5, ACO, CAT and JERF 3) in the root and leaf tissues of the tomato plants in untreated + uninoculated control (UC), inoculated untreated (IU) and treated + inoculated (T) groups relative to the housekeeping gene actin.
3. Results
3.1. Gene Expression Analysis
The PR-1 gene expression increased in leaf tissues of the tomato plants treated with ACS5075. The relative fold-change of PR-1 expression compared to that of the untreated control plants at 9 dpt and 15 dpt was 4-fold higher when compared to 3 dpt (Figure 1a). However, the relative expression of PR-1 in the root tissues was very low and below 1 indicating that it was downregulated compared to UC, but it increased in both the leaf and root tissues upon ACS3048 treatment (Figure 2a). With ACS3048 treatment, in the leaf tissues, at 9 and 15 dpt, PR-1 gene expression was 3-fold higher when compared to 3 dpt. In root tissues, however, the PR-1 gene expression was noted to be highest at 15 dpt when compared to all the other time points. At 15 dpt, the expression was 5-fold higher when compared to that at 3 dpt (Figure 2a).
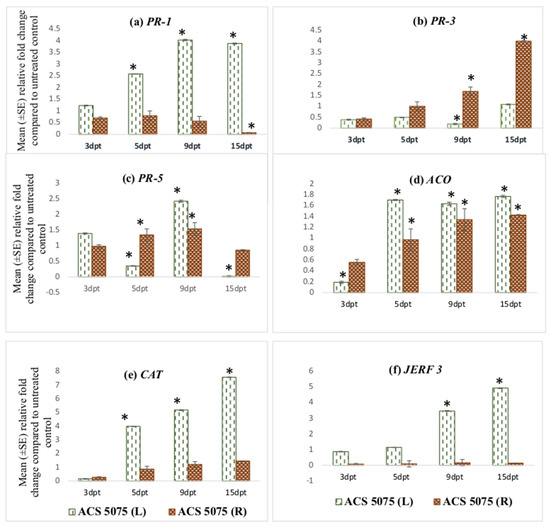
Figure 1.
Effect of ACS5075 treatment on expression levels of (a) PR-1, (b) PR-3, (c) PR-5, (d) ACO, (e) CAT and (f) JERF 3 genes in the leaf and root tissues of treated tomato plants compared to those in the untreated control (n = 6). (*) Symbol indicates a mean relative fold-change that is significantly different from that of the UC. Error bars indicate standard error reflecting variability within a sample (p-value ≤ 0.05). L refers to leaf tissues, and R refers to the root tissues, dpt refers to days post treatment.
Figure 1.
Effect of ACS5075 treatment on expression levels of (a) PR-1, (b) PR-3, (c) PR-5, (d) ACO, (e) CAT and (f) JERF 3 genes in the leaf and root tissues of treated tomato plants compared to those in the untreated control (n = 6). (*) Symbol indicates a mean relative fold-change that is significantly different from that of the UC. Error bars indicate standard error reflecting variability within a sample (p-value ≤ 0.05). L refers to leaf tissues, and R refers to the root tissues, dpt refers to days post treatment.
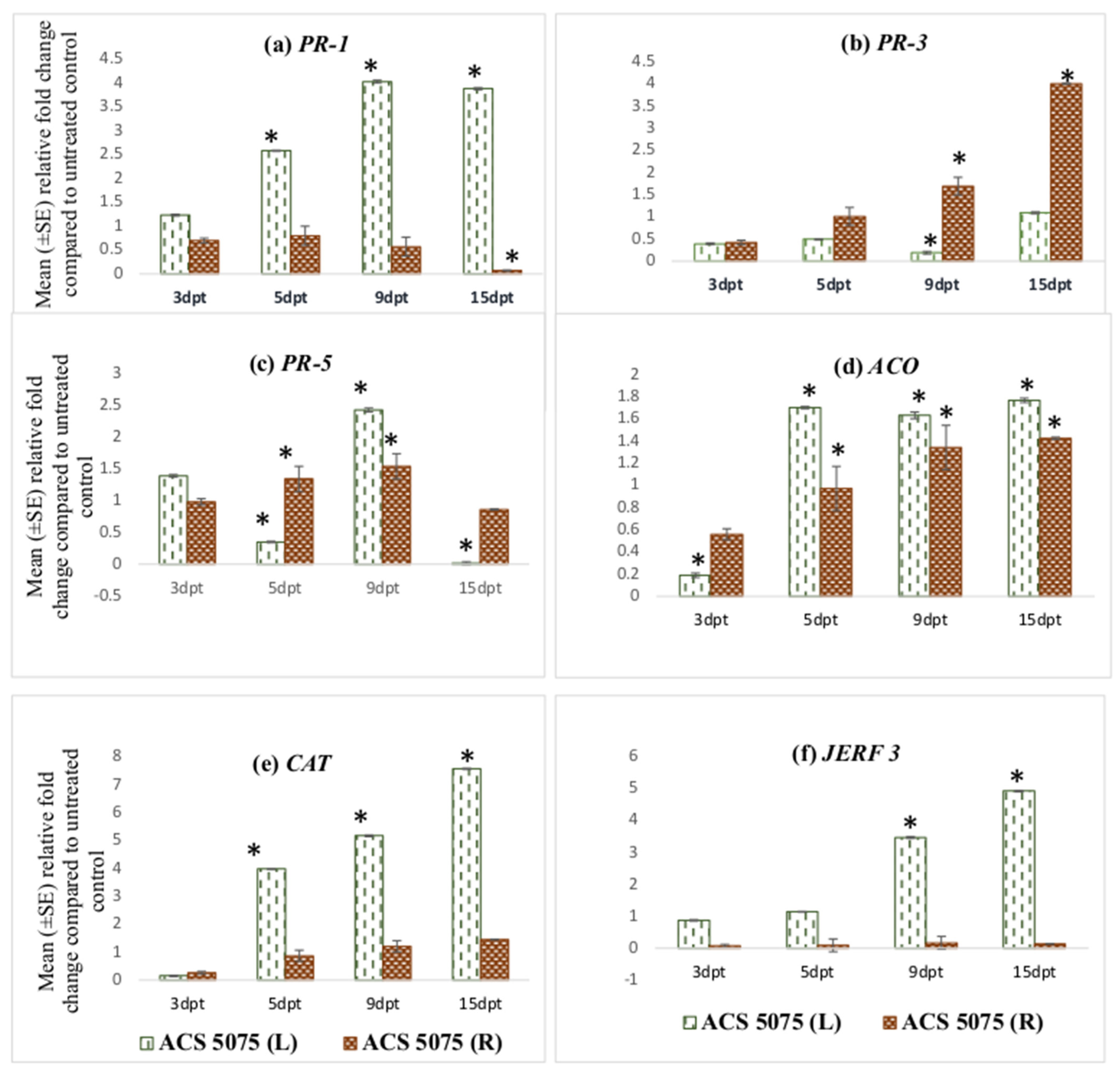
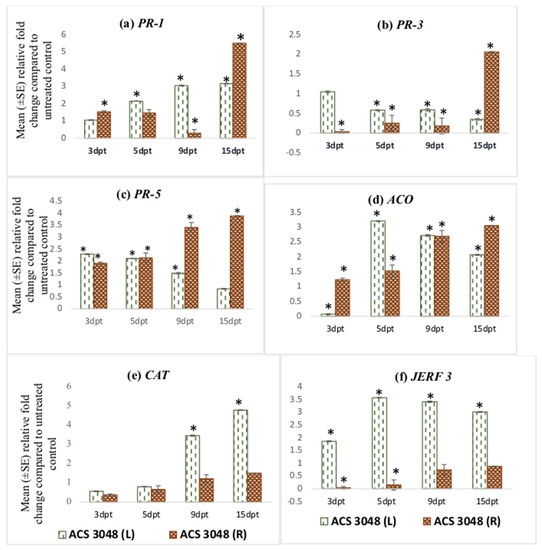
Figure 2.
Effect of ACS3048 treatment on expression levels of (a) PR-1, (b) PR-3, (c) PR-5, (d) ACO, (e) CAT and (f) JERF 3 genes in the leaf and root tissues of treated tomato plants compared to those in the untreated control (n = 6). (*) Symbol indicates a mean relative fold-change that is significantly different from that of the UC. Error bars indicate standard error reflecting variability within a sample (p-value ≤ 0.05). L refers to leaf tissues, and R refers to the root tissues, dpt refers to days post treatment.
Figure 2.
Effect of ACS3048 treatment on expression levels of (a) PR-1, (b) PR-3, (c) PR-5, (d) ACO, (e) CAT and (f) JERF 3 genes in the leaf and root tissues of treated tomato plants compared to those in the untreated control (n = 6). (*) Symbol indicates a mean relative fold-change that is significantly different from that of the UC. Error bars indicate standard error reflecting variability within a sample (p-value ≤ 0.05). L refers to leaf tissues, and R refers to the root tissues, dpt refers to days post treatment.
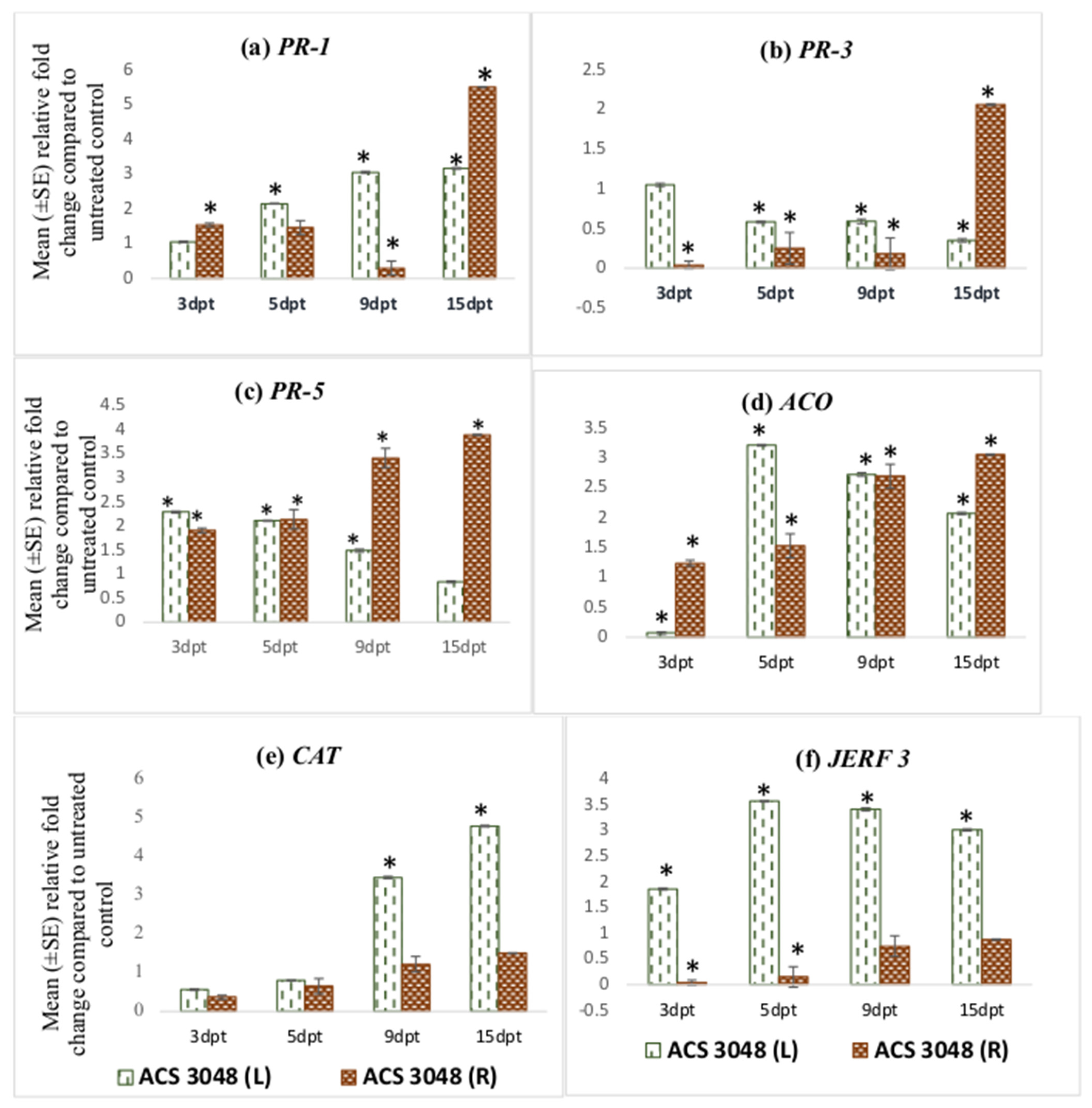
PR-3 gene expression was found to be high in the root tissues of the tomato plants treated with ACS5075. The highest expression was noted in the root tissues at 15 dpt, and this was 8-fold higher than that of the root tissues that were harvested at 3 dpt (Figure 1b). A similar trend of expression was also noted in roots treated with ACS3048 (Figure 2b), showing a 10-fold increase in PR-3 gene expression at 15 dpt when compared to that at 3 dpt. However, PR-3 gene expression in the leaf tissues of tomato plants treated with both products was downregulated, though it was not significantly different than that of the untreated control at most time points (Figure 1b and Figure 2b).
The PR-5 gene expression was found to be significantly higher than that of the UC in most of the leaf and root tissues treated with both products (Figure 1c and Figure 2c). Its expression was high in both leaf and root tissues at 9 dpt when compared to that at all other days. The PR-5 gene expression levels significantly dropped at 15 dpt in both leaf and root tissues in comparison to those at 3, 5, 9 dpt when treated with ACS5075 (Figure 1c). However, the treatment of plants with ACS3048 enhanced the PR-5 gene expression in the root tissues over time. The 15 dpt root tissues had the highest PR-5 gene expression, which was 2- and 1.4-fold higher compared to that at 3 and 9 dpt, respectively (Figure 2c).
ACS products increased ACO gene expression in both root and leaf tissues during the post-treatment days (Figure 1d and Figure 2d). Its expression was noted to be significantly high in ACS5075-treated leaf tissues at 5, 9 and 15 dpt, and it was 9-fold higher compared to that in the leaf tissues after 3 dpt (Figure 1d). A similar trend was also noted for the root tissues (Figure 1d) where the relative fold-change compared to untreated control tomato plants was 2-, 2.3- and 2.7-fold higher after 5, 9, and 15 dpt compared to 3 dpt. Treatment with ACS3048, showed a slightly different pattern of ACO gene expression in leaf tissues; its expression at 5, 9 and 15 dpt was significantly higher compared to that at 3 dpt, however, the expression levels tended to decrease from 5, 9 and 15 dpt. The ACO gene expression was found to be similar in root tissues of the tomato plants at 3 and 5 dpt. However, a 1.7-fold increase was noted in root tissue at 9 dpt; this increase was constant even at 15 dpt (Figure 2d).
The CAT gene was noted to be highly expressed in leaf tissues of the tomato plants treated with ACS5075 at 15 dpt when compared to that at 5 dpt (2-fold) and 9 dpt (1.6-fold) (Figure 1e). However, the CAT gene expression was not significantly different from that in the UC in the ACS5075-treated root tissues. Similarly, with ACS3048, leaf tissues recorded the highest CAT gene expression at 15 dpt compared to 3 dpt (25-fold), 5 dpt (10-fold) and 9 dpt (1.3-fold) (Figure 2e).
The JERF 3 gene expression under the ACS5075 treatment was significantly different from that in the UC only in the leaf tissues at 9 and 15 dpt. Its expression in root tissue was significantly lower than that of the UC (Figure 1f). ACS3048 treatment had a significant impact on JERF 3 gene expression in the treated leaf tissues at all time points examined. Its expression was highest at 5 dpt with a 3.5-fold change compared to that in the UC, followed by 9 dpt with a 3.4-fold change and 15 dpt had a 3-fold change compared to that in the UC (Figure 2f). The expression of the JERF 3 gene was noted to be significantly downregulated at 3 dpt and 5 dpt in tomato root tissues treated with ACS3048 (Figure 2f).
Figure 3 represents fold-changes in expression of each defence gene (PR-1, PR-3, PR-5, ACO, CAT and JERF 3) in the root and leaf tissues of the tomato plants in the untreated + uninoculated control (UC), inoculated untreated (IU) and treated + inoculated test groups (T), against the housekeeping gene actin. This set of experiments had two controls i.e., untreated + uninoculated control (UC) and inoculated untreated control (IU); therefore, the y-axis in these graphs (Figure 3) represents the relative expression values of each gene compared to actin. In these graphs (Figure 3), an asterisk (*) indicates that the mean fold-change is significantly different from that of UC.
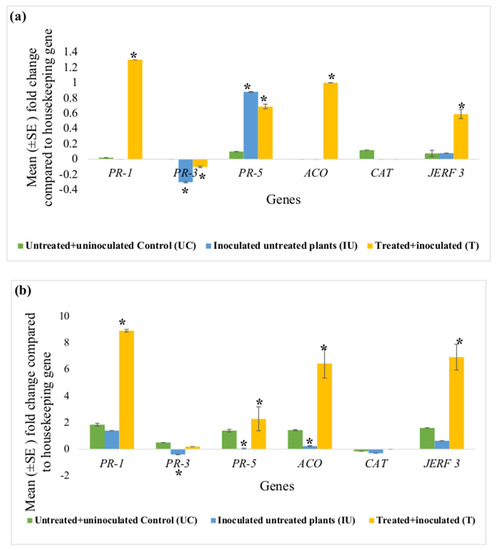
Figure 3.
Effect of concurrent ACS5075 treatment and M. javanica inoculation after 15 dpi on (a) leaf tissues and (b) root tissues. Error bars indicate standard error, reflecting variability within a sample (n = 6). (*) Symbol indicates a mean fold-change that is significantly different from that of untreated+ uninoculated control (UC).
Figure 3.
Effect of concurrent ACS5075 treatment and M. javanica inoculation after 15 dpi on (a) leaf tissues and (b) root tissues. Error bars indicate standard error, reflecting variability within a sample (n = 6). (*) Symbol indicates a mean fold-change that is significantly different from that of untreated+ uninoculated control (UC).
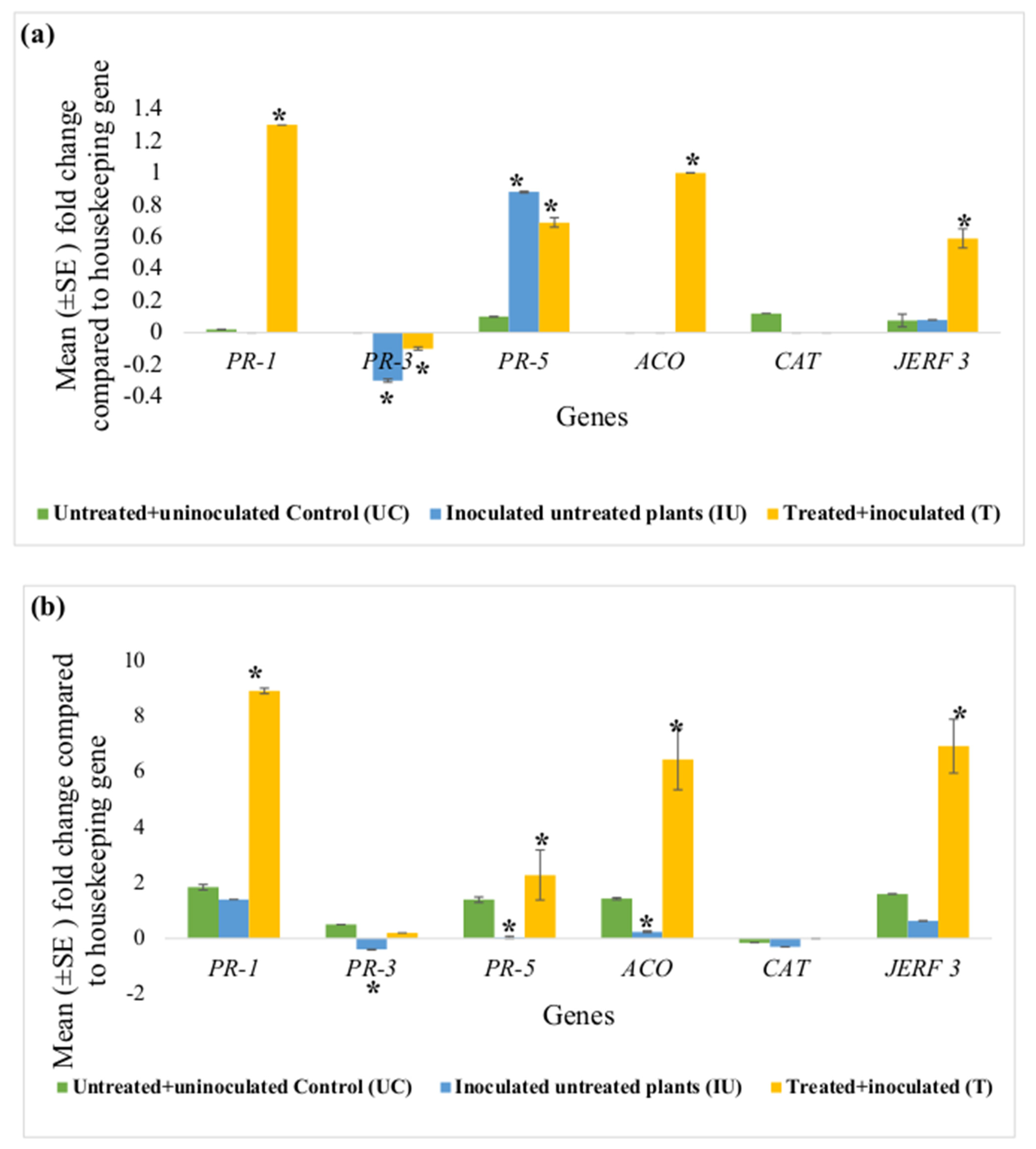
Comparing the UC and IU control plants, it was observed that in the leaf tissues, expression levels of none of the genes except for PR-3 and PR-5 were significantly different (Figure 3a). PR-3 expression levels were noted to be significantly downregulated in the leaf tissues of IU plants compared to those in UC plants. However, its expression was still downregulated in the leaf tissues even after the treatment with ACS5075. PR-5 gene expression levels significantly increased by 8.8-fold in the IU plants when compared to that in UC plants. This expression level did not change compared to that in IU plants in the leaf tissues after ACS5075 treatment. Upon treatment with ACS5075 (T), in the leaf tissues, PR-1, JERF 3 and ACO expression levels were significantly higher compared to those in both UC and IU plants (Figure 3a).
In the root tissue, most of the gene expression levels were reduced in the IU plants compared to those in the UC plants. However, with the treatment of ACS5075 (T), expression levels of PR-1, PR-5, ACO and JERF 3 in the root tissue were significantly increased when compared to those in IU and UC plants (Figure 3b). The expression of PR-1, PR-5, ACO and JERF 3 genes was 6.4-, 57-, 26.9- and 11-fold higher, respectively, in treated root tissues compared to that in the root tissues of the IU plants (Figure 3b).
Post-infection, in the ACS3048 treatment group, no significant fold-changes were noted for any of the genes analysed; therefore, the data are not presented.
3.2. Protein Analysis and Identification of Unique Proteins Using 2D Electrophoresis and N-Terminal Sequencing
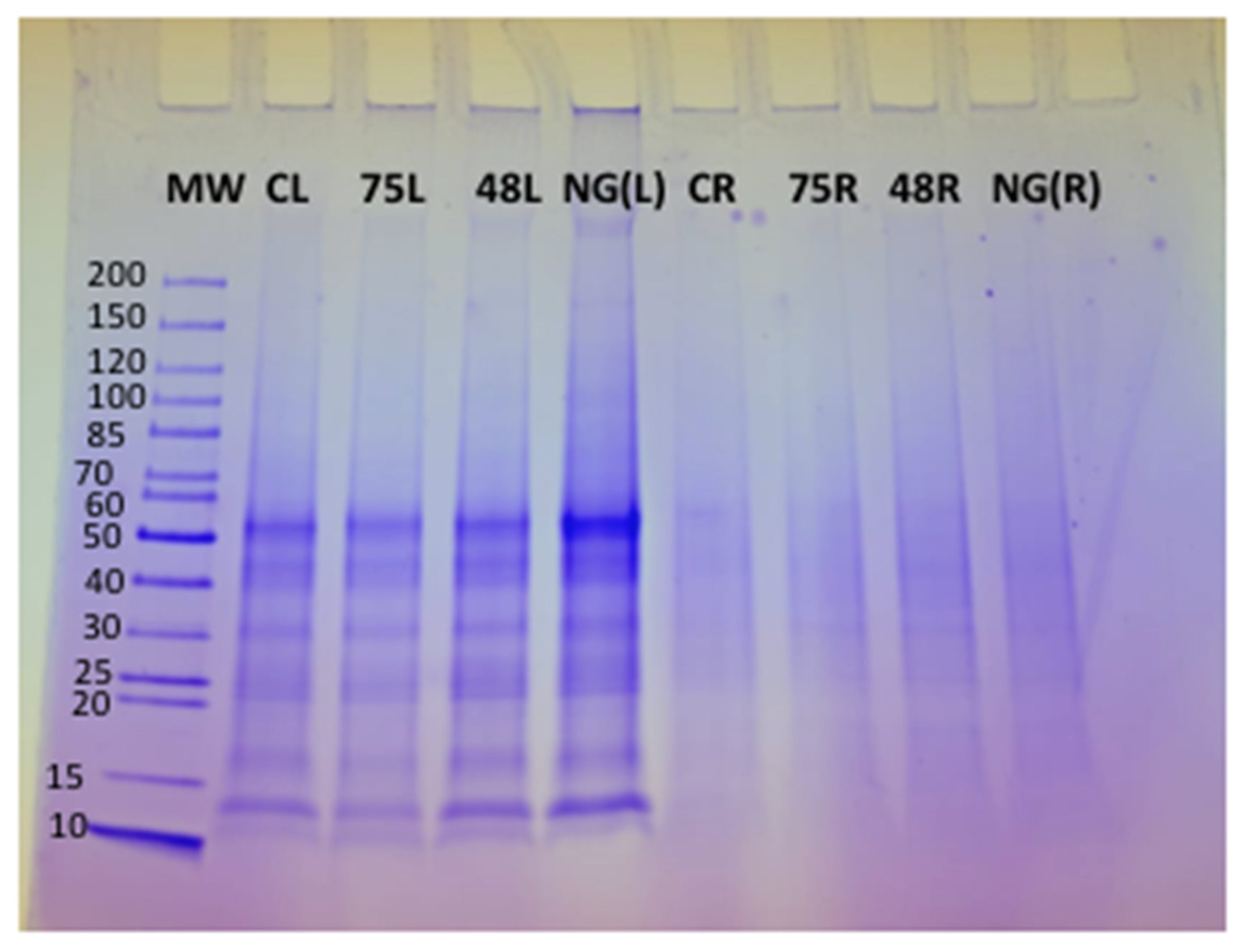
Tomato plants were harvested after 9 days of treatment with the respective ACS products. Nine-day treatment was chosen, as most of the gene expression increased significantly after 9 days of treatment with the ACS products. Approximately 5 mg of protein was loaded in each well of an SDS-PAGE gel to determine the quality of the protein extracted from both the roots and leaves of treated plants. The presence of clear protein bands on the SDS-PAGE gel indicated that good quality protein was extracted from the plant tissue (Figure 4). Protein was less abundant in the samples extracted from the roots, as the respective bands on the SDS-PAGE gel appeared quite faint and diffuse (Lane CR, 75R, 48R and NG(R)) (Figure 4). Protein extraction from the root samples was difficult, and even after repeated extractions, the protein quality was consistently low; hence, bands both on SDS-PAGE and 2D-gels were not clearly distinguishable (Lane CR, 75R, 48R, NG(R)).
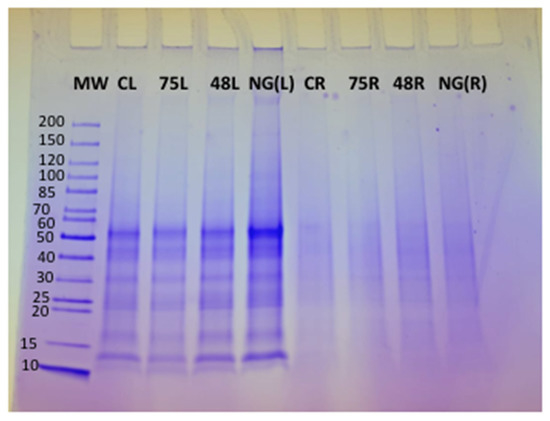
Figure 4.
SDS-PAGE gel of protein extracted from tomato plants treated with ACS products and NemguardTM. Lane 1: Pre-stained molecular weight protein ladder (10–200 KDa) (MW), Lane 2: untreated control leaf tissues (CL), Lane 3: ACS5075-treated leaf tissues (75L), Lane 4: ACS3048-treated leaf tissues (48L), Lane 5: NemguardTM treated leaf tissues NG(L), Lane 6: untreated control root tissues (CR), Lane 7: ACS5075-treated root tissues (75R), Lane 8: ACS3048-treated root tissues (48R) and Lane 9: NemguardTM-treated root tissues NG(R).
All the protein samples extracted from the leaf tissues were analysed with 2D-gel electrophoresis to identify unique protein spots (Figure 5). The gels were run 10–12 times to ensure the presence of these spots. Protein spots 1, 2 and 3 were present in all samples (Figure 5a–d).
A unique protein spot 4 was noted (Figure 5d) at 50 KDa in samples extracted from the plants treated with ACS3048, which was not observed with any other treatments, including untreated control samples.
Upon analysis of the peptide sequences from the N-terminal sequencing, which had been carried out on each of the excised spots, the following proteins were identified: protein spot 1 was identified as ‘disease resistance protein RPP13-like’, protein spot 2 as ‘phosphatidylinositol 4-phosphate 5-kinase 2’, spot 3 as ‘protein SABRE like’ and protein spot 4 as ‘uncharacterized protein LOC101250254’ (https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome) (accessed date: 20 April 2022). All the details related to the identified proteins are listed in Table 2.

Table 2.
List of specific proteins identified from the individual protein spots.
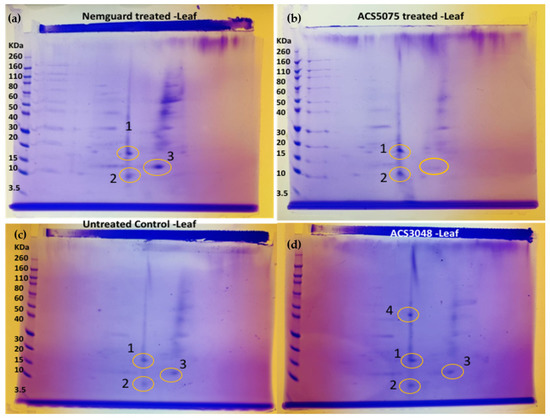
Figure 5.
2D-gel images of the protein extracted from leaf tissues of tomato plants treated with (a) commercial organic nematicide NemguardTM, (b) ACS5075, (c) untreated control and (d) ACS3048. The yellow circles indicate the protein spots. Numbers 1, 2, 3 and 4 indicate the protein spots.
Figure 5.
2D-gel images of the protein extracted from leaf tissues of tomato plants treated with (a) commercial organic nematicide NemguardTM, (b) ACS5075, (c) untreated control and (d) ACS3048. The yellow circles indicate the protein spots. Numbers 1, 2, 3 and 4 indicate the protein spots.
4. Discussion
The treatment of tomato plants with ACS products has been reported to reduce root-knot nematode infestation and to also enhance tomato plant growth [16,17]. In this study, efforts have been made to understand the mode of action of these products and to explore if the products make plants more resistant to RKN infection by priming their defence processes. In tomato, the JERF 3 gene (Acc. n. NM 001247533.2) encodes a trans-acting factor that responds to both ET and JA [12]. The ACC oxidase enzyme, which catalyses the final step of ET biosynthesis, is encoded by the ACO gene (Acc. n. XM 015225653.2) [22], while the catalase enzyme, encoded by the CAT gene (Acc. n. NM 001247257.2) [12], neutralises the harmful hydrogen peroxides produced by plants as a form of defence against pathogens and parasites.
In the current study tomato plants were treated with two ACS products, with and without RKN infection. Results indicate that treatment with both ACS products, ACS5075 and ACS3048, systemically primed ACO gene expression, as it was upregulated in both root and leaf tissues post-treatment and post-inoculation (Figure 1, Figure 2 and Figure 3). Similar results were reported in tomato plants treated with a mixture of beneficial biocontrol agents (BCA; [12]). In the primed state, plants have stronger and faster defence against pathogen attack due to their activated immune system. Upregulation of the ACO gene could enhance ET levels in the primed root and leaf tissues; ethylene is known to play an important role in plant defence against endoparasitic sedentary nematodes [23].
Apart from their contribution towards plant growth and development, plant hormones also play a very important role in enhancing plant responses to biotic and abiotic stresses [24,25,26]. Enhanced ET and ET signalling could increase hormone signalling crosstalk and therefore could also enhance the plant response to nematode infestation and subsequently induce resistance against RKN infection [23]. The ET signalling pathway is also reported to reduce attraction of soybean cyst nematodes (Heterodera glycines) towards Arabidopsis roots [23].
Among the PR genes, the PR-1 gene was found to be upregulated in both root and leaf tissues of tomato plants treated with ACS5075 post-infection with RKN and with ACS3048 post treatment without RKN infection (Figure 2 and Figure 3). Similar upregulation of the PR-1 gene was reported in wheat upon treatment with a fermentation-based elicitor, which further enhanced protection against powdery mildew in wheat [18]. PR-1 gene expression has been used as a useful molecular marker of SAR-mediated disease resistance [27]. The PR-1 family members have been reported to bind sterols [27] and are known to manifest antimicrobial activity towards sterol auxotrophs. The PR-1 gene is also reported to be induced upon treatment of tomato plants with salicylic acid and to therefore protect plants against biotrophic pathogens [12]. Higher PR-1 gene expression is also known to trigger resistance in tobacco cultivars primed with the fungicides strobilurin and pyraclostrobin against tobacco mosaic virus and Pseudomonas syringae pv. tabaci infection [28]. Enhanced PR-1 gene expression levels are known to enhance the of activity MPK3/6 proteins, which are known to play a very important role in regulating multiple defence pathways in plant fungal resistance [29]. Upregulation of the PR-1 gene is also reported to trigger immunity in tomato plants against P. syringae [30] and RKN [12] infection. Post-inoculation with M. javanica and treatment with ACS5075, in the root tissues, a positive correlation (r = 0.4 and 0.2) has been noted while correlating the expression of PR-1 and ACO genes, PR-5 and JERF 3 genes, respectively. This means with an increase in the expression of one gene, there is a positive increase in the fixed proportion of the expression of other genes. In the leaf tissues, a strong positive correlation (r = 0.8, 0.7, 0.6) was also noted while correlating ACO and JERF 3, JERF 3 and PR-1 and ACO and PR-1 genes, respectively. Overall, the expression of ACO and PR-1 genes was noted to increase in both root and leaf tissues thus playing an important role in enhancing plant defence against nematode infestation.
Changes in mRNA expression have biological meaning, most likely mediated by corresponding changes in protein levels, though this is not always the case. Protein extracts from the ACS-treated plants were examined to possibly confirm that the gene expression analysis translated to protein changes. Protein spot 1 was identified in all the tomato plants treated with ACS products and NemguardTM (Figure 5). Upon sequencing, this protein was identified to be ‘disease resistance protein RPP13-like’. This protein is known to have a very important function in conferring resistance to five isolates of Peronospora parasitica, which is a fungus reported to cause downy mildew in plants [31,32]. This protein belongs to a class of R- proteins, some of which (RPS2, RPM1 and RPS5) are known to confer resistance against bacteria [33], nematodes (Mi-1.2; [34]) and fungi (RPP8-Ler and I2; [35]). The presence of this protein could be one of the reasons for reduced M. javanica infestation in ACS-treated tomato plants. The occurrence of this ‘disease resistance protein RPP13-like’ protein could also be due to the higher expression of the PR-1 gene [29]. However, this protein spot was also observed even in the untreated control plants. Detailed future analysis is required to fully understand this observation.
Protein spot 2 (Figure 5) has been identified as ‘phosphatidylinositol 4-phosphate 5-kinase 2’. This protein belongs to a class of proteins that phosphorylate phosphatidylinositol 4-phosphate. These proteins have been previously reported to control membrane trafficking and to contribute towards the growth and development of Arabidopsis spp. [36]. They are also functionally linked to the regulation of numerous physiological processes in plants, such as membrane trafficking, clathrin-mediated endocytosis and the dynamics of the actin cytoskeleton [36,37,38] and may also be associated with the increased leaf biomass of the treated tomato plants, which was observed in a previous study [17]. Protein spot 3 (Figure 5) was identified as ‘protein SABRE-like’ (Table 2), and this protein was found in the plants treated with NemguardTM and ACS3048 (Figure 5). This protein is reported to enhance pollen tubes, root tip and root hair growth and cell expansion in Arabidopsis spp. and other eukaryotes [26]; indeed, an increase in root length was observed in potatoes treated with both ACS products [39]. This protein is known to counter balance the activity of ET to avoid abnormal cell expansion in Arabidopsis species [40]. Therefore, SABRE-protein activity is essential for the plant cells to attain a normal shape by actively contributing towards attaining ‘dynamic equilibrium’ [40].
Results indicate that treatment of tomato plants with both Alltech products, ACS5075 and ACS3048, primed ACO gene expression, as it was upregulated in both root and leaf tissues post-treatment and post-inoculation. In the primed state, plants have stronger and faster defence against pathogen attack due to their activated immune system [11]. Similarly with the PR genes, the PR-1 gene was upregulated in both root and leaf tissues of tomato plants treated with ACS5075 post-infection with RKN and with ACS3048 post-treatment without RKN infection. Upregulation of the PR-1 gene is known to trigger immunity in tomato plants facing pathogenic challenges [12]. These results show that the ACS products used in this study activated immune responses in the plants prior to challenge, and this response continued following challenge with RKN. This indicates the potential for these products as immune response-elicitors to help protect plants from biotic challenges. Although some protein differences were noted in this work, a more complete proteomic analysis is required to confirm the protein defence-response in the ACS-treated plants.
Author Contributions
Conceptualization, K.H. and T.K.-D.; methodology, A.P.; software, A.P. and A.S.; validation, A.P., K.H. and T.K.-D.; formal analysis, A.P.; investigation, A.P., K.H. and T.K.-D.; resources, K.H. and T.K.-D.; data curation, A.P., A.S. and K.Y.; writing—original draft preparation, A.P.; writing—review and editing, A.P., K.H. and T.K.-D.; visualization, A.P.; supervision, K.H. and T.K.-D.; project administration, T.K.-D.; funding acquisition, K.H. and T.K.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Alltech Bioscience Centre, Dunboyne, Co. Meath, Ireland, grant number PES1244. The article processing charges have been paid by South East Technological University under Open Access Journal APC Pilot Scheme.
Data Availability Statement
Data have been stored in the library repository of South East Technological University, Carlow, Ireland.
Acknowledgments
The authors acknowledge the support of Alltech Bioscience Centre and South east Technological University (SETU) for supporting this work. Thanks are due to Emmanuel Tzortzakakis from NAGREF, Plant Protection Institute, Heraklion, Crete, Greece for supplying Meloidogyne spp. populations for research. The authors would also like to thank Carloalberto Petti and Adriana Cunha Neves for all the advice and help that was provided to AP while performing the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Karuri, H.W.; Olago, D.; Neilson, R.; Mararo, E.; Villinger, J. A survey of root knot nematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Prot. 2017, 92, 114–121. [Google Scholar] [CrossRef]
- De Waele, D.; Elsen, A. Challenges in tropical plant nematology. Annu. Rev. Phytopathol. 2007, 45, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Abawi, G.S.; Widmer, T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000, 15, 37–47. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.X.; Song, B. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- Hajihassani, A. Chemical Nematicides in Georgia Vegetable Crops; Bulletin 1502; University of Georgia Extension: Athens, GA, USA, 2018. [Google Scholar]
- Ngala, B.M.; Woods, S.R.; Back, M.A. In vitro assessment of the effects of Brassica juncea and Raphanus sativus leaf and root extracts on the viability of Globodera pallida encysted eggs. Nematology 2015, 17, 543–556. [Google Scholar] [CrossRef]
- Lord, J.; Luca, L.; Atkinson, H.; Urwin, P. Biofumigation for control of pale potato cyst nematodes: Activity of brassica leaf extracts and green manures on Globodera pallida in vitro and in soil. J. Agric. Food Chem. 2011, 59, 7882–7890. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; da Barbosa, M.C.; Rosa, E. Suppressing potato cyst nematode, Globodera rostochiensis, with extracts of Brassicacea plants. Am. J. Potato Res. 2009, 86, 327–333. [Google Scholar] [CrossRef]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; Garcfa-Agustfn, P.; Gonzalez-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef] [PubMed]
- Chester, K.S. The Problem of Acquired Physiological Immunity in Plants. Q. Rev. Biol. 1933, 8, 275–324. [Google Scholar] [CrossRef]
- White, R.F. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 1979, 99, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, Z.; Escudero, N.; Beltrán-Anadón, D.; Saus, E.; Cunquero, M.; Andilla, J.; Loza-Alvarez, P.; Gabaldón, T.; Sorribas, F.J. Bacillus firmus Strain I-1582, a Nematode Antagonist by Itself and Through the Plant. Front. Plant Sci. 2020, 11, 796. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pulavarty, A.; Daly, R.; Horgan, K.; Kakouli-Duarte, T. Effect of Alltech® Crop Science products on root-knot nematode attraction and infestation in tomato plants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 815–824. [Google Scholar] [CrossRef]
- Twamley, T.; Gaffney, M.; Feechan, A. A Microbial Fermentation Mixture Primes for Resistance Against Powdery Mildew in Wheat. Front. Plant Sci. 2019, 10, 1241. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- LifeTechnologies ZOOM ® IPGRunner TM System. Available online: www.invitrogen.com/support (accessed on 20 January 2022).
- Brunelle, J.L.; Green, R. Coomassie Blue Staining; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 541. [Google Scholar]
- Oliveira, J.T.A.; Araujo-Filho, J.H.; Grangeiro, T.B.; Gondim, D.M.F.; Segalin, J.; Pinto, P.M.; Carlini, C.R.R.S.; Silva, F.D.A.; Lobo, M.D.P.; Costa, J.H.; et al. Enhanced synthesis of antioxidant enzymes, defense proteins and leghemoglobin in rhizobium-free cowpea roots after challenging with Meloydogine incognita. Proteomes 2014, 2, 527–549. [Google Scholar] [CrossRef]
- Hu, Y.; You, J.; Li, C.; Williamson, V.M.; Wang, C. Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci. Rep. 2017, 7, 41282. [Google Scholar] [CrossRef]
- Pulavarty, A.; Sarangi, B.K. Screening bamboo species for salt tolerance using growth parameters, physiological response and osmolytes accumulation as effective indicators. Chem. Ecol. 2018, 34, 340–354. [Google Scholar] [CrossRef]
- Pulavarty, A.; Kukde, S.; Shinde, V.M.; Sarangi, B.K. Morphological, physiological and biochemical adaptations of Eucalyptus citriodora seedlings under NaCl stress in hydroponic conditions. Acta Physiol. Plant. 2016, 38, 20. [Google Scholar] [CrossRef]
- Procissi, A.; Guyon, A.; Pierson, E.S.; Giritch, A.; Knuiman, B.; Grandjean, O.; Tonelli, C.; Derksen, J.; Pelletier, G.; Bonhomme, S. Kinky Pollen encodes a Sabre-like protein required for tip growth in Arabidopsis and conserved among eukaryotes. Plant J. 2003, 36, 894–904. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Herms, S.; Seehaus, K.; Koehle, H.; Conrath, U. A strobilurin fungicide enhances the resistance of tobacco against tobacco mosaic virus and Pseudomonas syringae pv tabaci. Plant Physiol. 2002, 130, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Beckers, G.J.M.; Jaskiewicz, M.; Liu, Y.; Underwood, W.R.; He, S.Y.; Zhang, S.; Conrath, U. Mitogen-Activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 2009, 21, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Yekondi, S.; Chen, P.W.; Tsai, C.H.; Yu, C.W.; Wu, K.; Zimmerli, L. Environmental history modulates Arabidopsis pattern-triggered immunity in a histone acetyltransferase1-dependent manner. Plant Cell 2014, 26, 2676–2688. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, H.; Li, L.; Hu, B.; Liu, H.; Liu, Z. Genome-wide Identification and Expression Analyses of RPP13-like Genes in Barley. Biochip J. 2018, 12, 102–113. [Google Scholar] [CrossRef]
- Bittner-Eddy, P.D.; Crute, I.R.; Holub, E.B.; Beynon, J.L. RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different a virulence determinants in Peronospora parasitica. Plant J. 2000, 21, 177–188. [Google Scholar] [CrossRef]
- Warren, R.F.; Henk, A.; Mowery, P.; Holub, E.; Innes, R.W. A mutation within the leucine-rich repeat domain of the arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 1998, 10, 1439–1452. [Google Scholar] [CrossRef]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Simons, G.; Groenendijk, J.; Wijbrandi, J.; Reijans, M.; Diergaarde, P.; Van Der Lee, T.; Bleeker, M.; Onstenk, J.; De Both, M.; Haring, M.; et al. Dissection of the Fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell 1998, 10, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Gerth, K.; Lin, F.; Daamen, F.; Menzel, W.; Heinrich, F.; Heilmann, M. Arabidopsis phosphatidylinositol 4-phosphate 5-kinase 2 contains a functional nuclear localization sequence and interacts with alpha-importins. Plant J. 2017, 92, 862–878. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Jia, W.J.; Chu, Y.J.; Xue, H.W. Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res. 2012, 22, 581–597. [Google Scholar] [CrossRef]
- Ischebeck, T.; Stenzel, I.; Hempel, F.; Jin, X.; Mosblech, A.; Heilmann, I. Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant J. 2011, 65, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Pulavarty, A.; Singh, A.; Smyth, D.; Mehta, J.P.; Horgan, K. Sustainable management of the potato cyst nematode, Globodera rostochiensis, with two microbial fermentation products. Front. Plant Sci. 2022, 13, 987059. [Google Scholar] [CrossRef]
- Aeschbacher, R.A.; Hauser, M.T.; Feldmann, K.A.; Benfey, P.N. The SABRE gene is required for normal cell expansion in Arabidopsis. Genes Dev. 1995, 9, 330–340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).