Comparative Genomic Analysis of Biofilm-Forming Polar Microbacterium sp. Strains PAMC22086 and PAMC21962 Isolated from Extreme Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Growth Curve Measurements
2.2. DNA Extraction and Genome Sequencing
2.3. Genome Annotation and Functional Prediction
2.4. Phylogenetic Analysis and Average Nucleotide Identity
2.5. Biofilm Formation
3. Results and Discussion
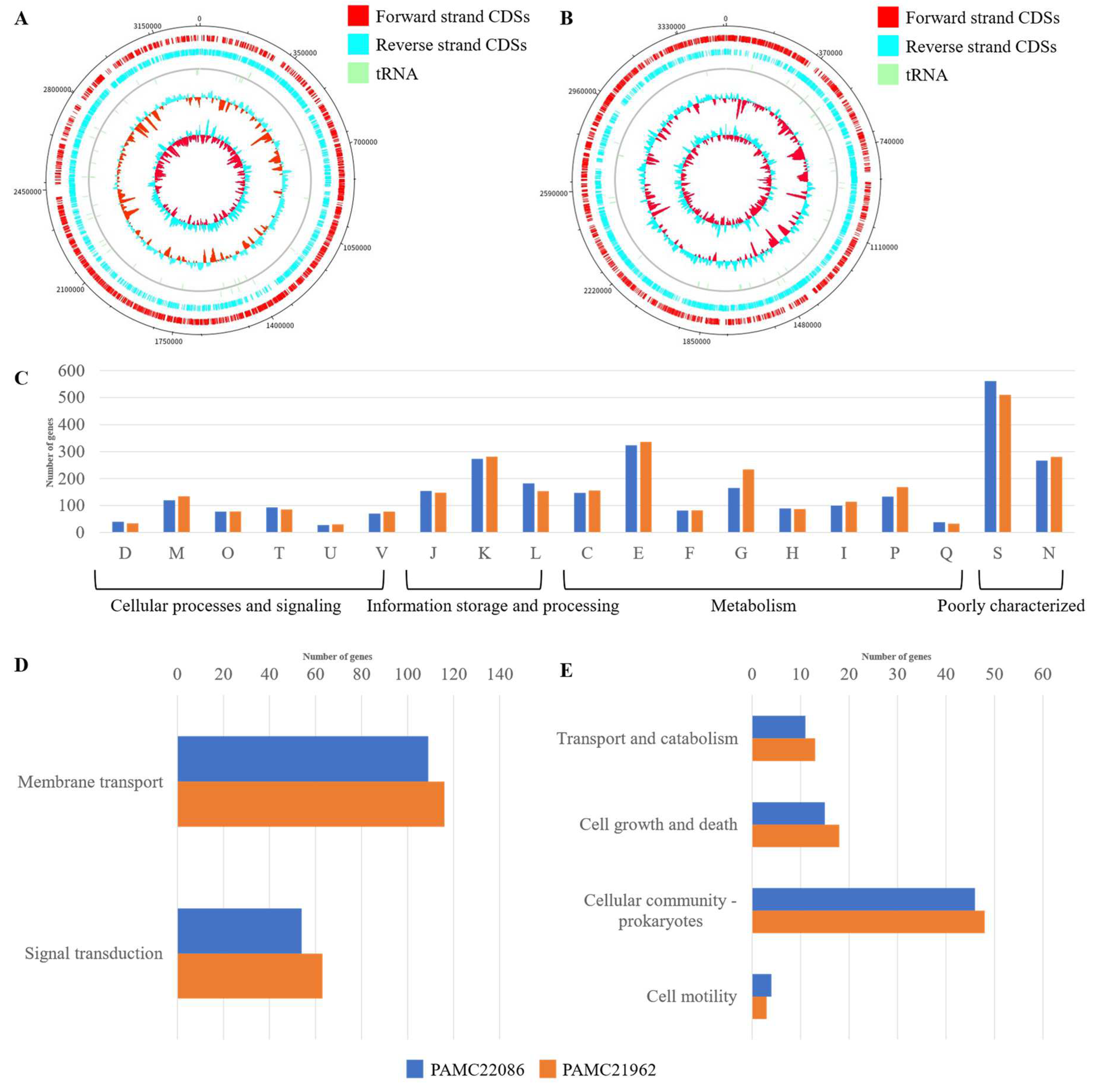
3.1. Overall Genome Features in PAMC22086 and PAMC21962
3.2. Phylogenetic Analyses and Average Nucleotide Identity
3.3. Identification of Gene Associated with Biofilm Formation
3.4. Differences in Biofilm-Related Genes between PAMC22086 and PAMC21962
3.5. Biofilm Formation Ability of PAMC22086 and PAMC21962
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J.; Kim, M.K.; Bui, T.P.N.; Kim, H.B.; Srinivasan, S.; Yang, D.C. Microbacterium ginsengiterrae sp. nov., a β-glucosidase-producing bacterium isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 2010, 60, 2808–2812. [Google Scholar] [CrossRef]
- Kalsi, N.; Drautz-Moses, D.I.; Uchida, A.; Purbojati, R.W.; Houghton, J.N.; Chénard, C.; Wong, A.; Kolundžija, S.; Clare, M.E.; Kushwaha, K.K.; et al. Complete genome sequence of Microbacterium sp. strain SGAir0570, isolated from tropical air collected in Singapore. Microbiol. Resour. Announc. 2019, 8, e00613-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spirina, E.V.; Kuleshov, K.V.; Yunusova, A.K.; Vishnivetskaya, T.A.; Rivkina, E.M. Draft genome sequence of Microbacterium sp. Gd 4-13, isolated from Gydanskiy Peninsula permafrost sediments of marine origin. Microbiol. Resour. Announc. 2019, 8, e00889-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordovez, V.; Schop, S.; Hordijk, K.; Dupré de Boulois, H.; Coppens, F.; Hanssen, I.; Raaijmakers, J.M.; Carrión, V.J. Priming of plant growth promotion by volatiles of root-associated Microbacterium spp. Appl. Environ. Microbiol. 2018, 84, e01865-18. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Yang, J.; Lu, S.; Pu, J.; Lai, X.H.; Jin, D.; Li, J.; Zhang, G.; Wang, X.; Zhang, S.; et al. Microbacterium wangchenii sp. nov., isolated from faeces of Tibetan gazelles (Procapra picticaudata) on the Qinghai-Tibet Plateau. Int. J. Syst. Evol. Microbiol. 2020, 70, 1307–1314. [Google Scholar] [CrossRef]
- Ouertani, R.; Ouertani, A.; Mahjoubi, M.; Bousselmi, Y.; Najjari, A.; Cherif, H.; Chamkhi, A.; Mosbah, A.; Khdhira, H.; Sghaier, H.; et al. New plant growth-promoting, chromium-detoxifying Microbacterium species isolated from a tannery wastewater: Performance and genomic insights. Front. Bioeng. Biotechnol. 2020, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Moreira, I.; Lopes, A.R.; Faria, C.; Spröer, C.; Schumann, P.; Nunes, O.C.; Manaia, C.M. Microbacterium invictum sp. nov., isolated from homemade compost. Int. J. Syst. Evol. Microbiol. 2009, 59, 2036–2041. [Google Scholar] [CrossRef] [Green Version]
- Mandakovic, D.; Cintolesi, Á.; Maldonado, J.; Mendoza, S.N.; Aïte, M.; Gaete, A.; Saitua, F.; Allende, M.; Cambiazo, V.; Siegel, A.; et al. Genome-scale metabolic models of Microbacterium species isolated from a high-altitude desert environment. Sci. Rep. 2020, 10, 5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.; Cho, H.; Kim, M.A.; Hamada, M.; Tamura, T.; Saitou, S.; Kim, S.K.; Kwon, S.W. Microbacterium protaetiae sp. nov., isolated from gut of larva of Protaetia brevitarsis seulensis. Int. J Syst. Evol. Microbiol. 2020, 70, 2226–2232. [Google Scholar] [CrossRef]
- Heineman, P.G. Orla-Jensen’s classification of lactic acid bacteria. J. Dairy Sci. 1920, 3, 143–155. [Google Scholar] [CrossRef]
- Collins, M.D.; Jones, D.; Kroppenstedt, R.M. Reclassification of Brevibacterium imperiale (Steinhaus) and “Corynebacterium laevaniformans” (Dias and Bhat) in a redefined genus Microbacterium (Orla-Jensen), as Microbacterium imperiale comb. nov. and Microbacterium laevaniformans nom. rev.; comb. nov. Syst. Appl. Microbiol. 1983, 4, 65–78. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hatano, K. Union of the genera Microbacterium Orla-Jensen and Aureobacterium Collins et al. in a redefined genus Microbacterium. Int. J. Syst. Bacteriol. 1998, 48, 739–747. [Google Scholar] [CrossRef]
- Krishnamurthi, S.; Bhattacharya, A.; Schumann, P.; Dastager, S.G.; Tang, S.K.; Li, W.J.; Chakrabarti, T. Microbacterium immunditiarum sp. nov., an actinobacterium isolated from landfill surface soil, and emended description of the genus Microbacterium. Int. J. Syst. Evol. Microbiol. 2012, 62, 2187–2193. [Google Scholar] [CrossRef]
- Fidalgo, C.; Riesco, R.; Henriques, I.; Trujillo, M.E.; Alves, A. Microbacterium diaminobutyricum sp. nov., isolated from Halimione portulacoides, which contains diaminobutyric acid in its cell wall, and emended description of the genus Microbacterium. Int. J. Syst. Evol. Microbiol. 2016, 66, 4492–4500. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J.D.; Sigamani, S.; Arul, D.; Nedunchelizan, K.; Pachiappan, P.; Ramamurthy, D. Molecular characterization and antioxidant assay of pigment producing bacteria, Sphingomonas paucimobilis and Microbacterium arborescens isolated from fresh water sediments. Nat. Prod. Res. 2020, 34, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zand, E.; Pfanner, H.; Domig, K.J.; Sinn, G.; Zunabovic-Pichler, M.; Jaeger, H. Biofilm-forming ability of Microbacterium lacticum and Staphylococcus capitis considering physicochemical and topographical surface properties. Foods 2021, 10, 611. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Sela, S.; Frank, S.; Belausov, E.; Pinto, R. A mutation in the luxS gene influences Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2006, 72, 5653–5658. [Google Scholar] [CrossRef] [Green Version]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Hu, F.; Li, Z.; Zhong, D.; Yuan, Y.; Zhang, J. Biofilm formation potential and chlorine resistance of typical bacteria isolated from drinking water distribution systems. RSC Adv. 2020, 10, 31295–31304. [Google Scholar] [CrossRef]
- Wagner, E.M.; Fischel, K.; Rammer, N.; Beer, C.; Palmetzhofer, A.L.; Conrady, B.; Roch, F.F.; Hanson, B.T.; Wagner, M.; Rychli, K. Bacteria of eleven different species isolated from biofilms in a meat processing environment have diverse biofilm forming abilities. Int. J. Food Microbiol. 2021, 349, 109232. [Google Scholar] [CrossRef]
- Chin, C.S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E.; et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.A.; Barrell, B.G.; Parkhill, J. ACT: The Artemis Comparison Tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [Green Version]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Magalhães, R.P.; Vieira, T.F.; Fernandes, H.S.; Melo, A.; Simões, M.; Sousa, S.F. The biofilms structural database. Trends Biotechnol. 2020, 38, 937–940. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez, R.L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Chen, F.; Liu, W.; Kong, W.; Wang, C.; Fang, X.; Ye, J. Adding nutrients to the biocontrol strain JK-SH007 promotes biofilm formation and improves resistance to stress. AMB Express 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Han, S.R.; Kim, K.H.; Ahn, D.H.; Park, H.; Oh, T.J. Complete genome sequence of carotenoid-producing Microbacterium sp. strain PAMC28756 isolated from an Antarctic lichen. J. Biotechnol. 2016, 226, 18–19. [Google Scholar] [CrossRef]
- Gupta, S.; Han, S.R.; Kim, B.; Lee, C.M.; Oh, T.J. Comparative analysis of genome-based CAZyme cassette in Antarctic Microbacterium sp. PAMC28756 with 31 other Microbacterium species. Genes Genom. 2022, 44, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; de Souza, Y.A.P.; Mansur, M.C.P.P.R.; Vermelho, A.B.; da Mota, F.F.; Rosado, A.S. Draft genome sequence of Microbacterium sp. strain LEMMJ01, isolated from Antarctic ornithogenic soil. Genome Announc. 2017, 5, e00672-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corretto, E.; Antonielli, L.; Sessitsch, A.; Höfer, C.; Puschenreiter, M.; Widhalm, S.; Swarnalakshmi, K.; Brader, G. Comparative genomics of Microbacterium species to reveal diversity, potential for secondary metabolites and heavy metal resistance. Front. Microbiol. 2020, 11, 1869. [Google Scholar] [CrossRef] [PubMed]
- Achour-Rokbani, A.; Cordi, A.; Poupin, P.; Bauda, P.; Billard, P. Characterization of the ars gene cluster from extremely arsenic-resistant Microbacterium sp. strain A33. Appl. Environ. Microbiol. 2010, 76, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, S.; Thrash, J.C.; Temperton, B. Implications of streamlining theory for microbial ecology. ISME J. 2014, 8, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Giles, S.K.; Stroeher, U.H.; Eijkelkamp, B.A.; Brown, M.H. Identification of genes essential for pellicle formation in Acinetobacter baumannii. BMC Microbiol. 2015, 15, 116. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Sun, S.; Ma, T.; Abdellah, Y.A.Y.; Wang, Y.; Mi, Y.; Yan, H.; Sun, G.; Hou, N.; Zhao, X.; et al. A novel estrone degradation gene cluster and catabolic mechanism in Microbacterium oxydans ML-6. Appl. Environ. Microbiol. 2023, 89, e0148922. [Google Scholar] [CrossRef]
- Xiong, W.; Peng, W.; Fu, Y.; Deng, Z.; Lin, S.; Liang, R. Identification of a 17β-estradiol-degrading Microbacterium hominis SJTG1 with high adaptability and characterization of the genes for estrogen degradation. J. Hazard Mater. 2023, 444, 130371. [Google Scholar] [CrossRef]
- Bernier, A.M.; Bernard, K. Draft genome sequences of Microbacterium hominis LCDC-84-0209T isolated from a human lung aspirate and Microbacterium laevaniformans LCDC 91-0039 isolated from a human blood culture. Genome Announc. 2016, 4, e00989-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shi, H.; Liang, Y.; Qin, L.; Zeng, H.; Song, X. Degradation characteristics and remediation ability of contaminated soils by using β-HCH degrading bacteria. Int. J. Environ. Res. Public Health 2023, 20, 2767. [Google Scholar] [CrossRef]
- Knoten, C.A.; Wells, G.; Coleman, J.P.; Pesci, E.C. A conserved suppressor mutation in a tryptophan auxotroph results in dysregulation of Pseudomonas quinolone signal synthesis. J. Bacteriol. 2014, 196, 2413–2422. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Liu, Q.; Kang, X.; Zhu, Z.; Yang, H.; Xi, X.; Zhang, X.; Du, Y.; Guo, M.; Tang, D.; et al. Glycogen metabolism impairment via single gene mutation in the glgBXCAP operon alters the survival rate of Escherichia coli under various environmental stresses. Front. Microbiol. 2020, 11, 588099. [Google Scholar] [CrossRef]
- Chellaiah, E.R.; Ravi, P.; Uthandakalaipandian, R. High fluoride resistance and virulence profile of environmental Pseudomonas isolated from water sources. Folia Microbiol. 2021, 66, 569–578. [Google Scholar] [CrossRef]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y.; et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS J. Pathol. Microbiol. Immunol. 2009, 117, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.H.; Jeong, S.G.; Park, A.; Jang, S.C.; Hong, S.G.; Lee, C.S. Effect of temperature on biofilm formation by Antarctic marine bacteria in a microfluidic device. Anal. Biochem. 2014, 446, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lenz, D.H.; Miller, M.B.; Zhu, J.; Kulkarni, R.V.; Bassler, B.L. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol. Microbiol. 2005, 58, 1186–1202. [Google Scholar] [CrossRef]
- Jackson, D.W.; Suzuki, K.; Oakford, L.; Simecka, J.W.; Hart, M.E.; Romeo, T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, E.L.; Brutinel, E.D.; Klem, E.R.; Fehr, A.R.; Yahr, T.L.; Wolfgang, M.C. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J. Bacteriol. 2010, 192, 2779–2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.J.; Li, X.H.; Kim, S.K.; Lee, J.H. Anthranilate acts as a signal to modulate biofilm formation, virulence, and antibiotic tolerance of Pseudomonas aeruginosa and surrounding bacteria. Microbiol. Spectr. 2022, 10, e0146321. [Google Scholar] [CrossRef] [PubMed]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [Green Version]
- Reimmann, C.; Beyeler, M.; Latifi, A.; Winteler, H.; Foglino, M.; Lazdunski, A.; Haas, D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 1997, 24, 309–319. [Google Scholar] [CrossRef]
- Lu, X.H.; An, S.Q.; Tang, D.J.; McCarthy, Y.; Tang, J.L.; Dow, J.M.; Ryan, R.P. RsmA regulates biofilm formation in Xanthomonas campestris through a regulatory network involving cyclic di-GMP and the Clp transcription factor. PLoS ONE. 2012, 7, e52646. [Google Scholar] [CrossRef]
- Fortuna, A.; Bähre, H.; Visca, P.; Rampioni, G.; Leoni, L. The two Pseudomonas aeruginosa DksA stringent response proteins are largely interchangeable at the whole transcriptome level and in the control of virulence-related traits. Environ. Microbiol. 2021, 23, 5487–5504. [Google Scholar] [CrossRef]
- Omadjela, O.; Narahari, A.; Strumillo, J.; Mélida, H.; Mazur, O.; Bulone, V.; Zimmer, J. BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 17856–17861. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Attila, C.; Wang, L.; Wood, T.K.; Valdes, J.J.; Bentley, W.E. Quorum sensing in Escherichia coli is signaled by AI-2/LsrR: Effects on small RNA and biofilm architecture. J. Bacteriol. 2007, 189, 6011–6020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Palaniyandi, S.; Herren, C.D.; Zhu, X.; Mukhopadhyay, S. Pleiotropic roles of uvrY on biofilm formation, motility and virulence in uropathogenic Escherichia coli CFT073. PLoS ONE 2013, 8, e55492. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.M.; Chiang, M.K.; Wang, M.; Ho, H.C.; Lu, M.C.; Lai, Y.C. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb. Pathog. 2014, 77, 89–99. [Google Scholar] [CrossRef]
- Chung, C.H.; Fen, S.Y.; Yu, S.C.; Wong, H.C. Influence of oxyR on growth, biofilm formation, and mobility of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 2015, 82, 788–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yu, C.; Chen, H.; Tian, F.; He, C. PXO_00987, a putative acetyltransferase, is required for flagellin glycosylation, and regulates flagellar motility, exopolysaccharide production, and biofilm formation in Xanthomonas oryzae pv. oryzae. Microb. Pathog. 2015, 85, 50–57. [Google Scholar] [CrossRef]
- Frees, D.; Chastanet, A.; Qazi, S.; Sørensen, K.; Hill, P.; Msadek, T.; Ingmer, H. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 2004, 54, 1445–1462. [Google Scholar] [CrossRef] [PubMed]
- Dees, M.W.; Brurberg, M.B.; Lysøe, E. Complete genome sequence of the biofilm-forming Microbacterium sp. strain BH-3-3-3, isolated from conventional field-grown lettuce (Lactuca sativa) in Norway. Genom. Data. 2016, 11, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Park, H.Y.; Park, W.; Kim, I.S.; Lee, S.T. Microbacterium xylanilyticum sp. nov., a xylan-degrading bacterium isolated from a biofilm. Int. J. Syst. Evol. Microbiol. 2005, 55, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Delgado-Baquerizo, M.; Hotaling, S.; Li, Y.; Sun, X.; Xu, Y.; Chu, H. Bacterial diversity and co-occurrence patterns differ across a worldwide spatial distribution of habitats in glacier ecosystems. Funct. Ecol. 2023, 37, 1520–1535. [Google Scholar] [CrossRef]
- Parkins, M.D.; Ceri, H.; Storey, D.G. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 2001, 40, 1215–1226. [Google Scholar] [CrossRef]
- Aarons, S.; Abbas, A.; Adams, C.; Fenton, A.; O’Gara, F. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 2000, 182, 3913–3919. [Google Scholar] [CrossRef] [Green Version]
- Zere, T.R.; Vakulskas, C.A.; Leng, Y.; Pannuri, A.; Potts, A.H.; Dias, R.; Tang, D.; Kolaczkowski, B.; Georgellis, D.; Ahmer, B.M.; et al. Genomic targets and features of BarA-UvrY (-SirA) signal transduction systems. PLoS ONE 2015, 10, e0145035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, A.L.; Payne, S.M. CsrA and Cra influence Shigella flexneri pathogenesis. Infect. Immun. 2010, 78, 4674–4682. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, D.; Zhu, J.; Liu, J.; Liu, W. The regulation of bacterial biofilm formation by cAMP-CRP: A mini-review. Front. Microbiol. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Son, J.H.; Kim, K.; Kim, H.J.; Kim, Y.J.; Shin, M.; Lee, J.C. Global regulator DksA modulates virulence of Acinetobacter baumannii. Virulence 2021, 12, 2750–2763. [Google Scholar] [CrossRef]
- Gennari, M.; Ghidini, V.; Caburlotto, G.; Lleo, M.M. Virulence genes and pathogenicity islands in environmental Vibrio strains nonpathogenic to humans. FEMS Microbiol. Ecol. 2012, 82, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Naor-Hoffmann, S.; Svetlitsky, D.; Sal-Man, N.; Orenstein, Y.; Ziv-Ukelson, M. Predicting the pathogenicity of bacterial genomes using widely spread protein families. BMC Bioinform. 2022, 23, 253. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Emergent heterogeneous microenvironments in biofilms: Substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| General Features | PAMC21962 | PAMC22086 |
|---|---|---|
| Genome Size (bp) | 3,047,328 | 3,256,707 |
| Contig (bp) | 3328 | 3330 |
| N50 length | 3,509,180 | 3,311,678 |
| GC% | 71 | 68.2 |
| CDSs | 3079 | 3070 |
| Number of proteins | 3205 | 3077 |
| Number of tRNA genes | 46 | 47 |
| Number of rRNA genes | 6 | 6 |
| Gene | Genes of PAMC21962 and PAMC22086 | Origin | Function | Reference |
|---|---|---|---|---|
| varA | PAMC21962.peg.1943 | Vibrio cholerae | varA controls the expression of numerous genes, most notably those required for virulence. | [56] |
| csrA | PAMC22086.peg.3288 | Escherichia coli | The csrA activates biofilm dispersal under various conditions. | [57] |
| cpdA | PAMC21962.peg.3315 PAMC22086.peg.2094 | Pseudomonas aeruginosa | The cpdA is required for cAMP homeostasis and regulation of virulence factors. | [58] |
| phnA | PAMC21962.peg.540 | Pseudomonas aeruginosa | The phnA and phnB act as a signal to modulate biofilm formation and virulence. | [59] |
| phnB | PAMC21962.peg.2458 PAMC22086.peg.645 | Pseudomonas aeruginosa | [60] | |
| rhlC | PAMC21962.peg.3145 PAMC22086.peg.1909 | Pseudomonas aeruginosa | The function of rhlC is to act as a ‘biofilm shield,’ significantly contributing to the increased tolerance of P. aeruginosa biofilms. | [54] |
| gacA | PAMC21962.peg.1943 | Pseudomonas aeruginosa | The gacA is a positive regulator of the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors, such as pyocyanin. | [61] |
| rsmA | PAMC22086.peg.3288 | Xanthomonas campestris | The rsmA regulates biofilm formation in Xanthomonas campestris through a regulatory network that involves cyclic di-GMP and the Clp transcription factor. | [62] |
| dksA | PAMC22086.peg.550 | Pseudomonas aeruginosa | The dksA regulates virulence gene expression. | [63] |
| bcsA | PAMC21962.peg.2698 | Rhodobacter sphaeroides | The bcsA is a cellulose synthase that encodes cellulose synthesis, an essential component of biofilms. | [64] |
| lsrR | PAMC21962.peg.2174 | Escherichia coli | The lsrR regulates the uptake of AI-2, which binds with lsrR to mediate biofilm architecture and formation by coordinating the interactions of genes related to biofilm formation. | [65] |
| uvrY | PAMC21962.peg.1943 | Escherichia coli | The uvrY expression of type 1 fimbriae, an important adhesin that facilitates adhesion to various abiotic surfaces. | [66] |
| glgC | PAMC21962.peg.285 PAMC22086.peg.2598 | Escherichia coli | The glgBXCAP operon influences physiological activities such as growth rate, glycogen accumulation and structure, biofilm formation, and environmental stress endurance. | [52] |
| glgP | PAMC21962.peg.158 | Escherichia coli | ||
| pgaC | PAMC21962.peg.2862 | Klebsiella pneumoniae | The pgaC regulates the production of Poly-N-acetylglucosamine, which plays a crucial role in biofilm formation. | [67] |
| oxyR | PAMC22086.peg.2252 | Vibrio parahaemolyticus | The oxyR participates in pathogenesis by oxidative stress defense mechanism and promoting biofilm formation. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Gurung, S.; Han, S.-R.; Lee, J.-H.; Oh, T.-J. Comparative Genomic Analysis of Biofilm-Forming Polar Microbacterium sp. Strains PAMC22086 and PAMC21962 Isolated from Extreme Habitats. Microorganisms 2023, 11, 1757. https://doi.org/10.3390/microorganisms11071757
Kim B, Gurung S, Han S-R, Lee J-H, Oh T-J. Comparative Genomic Analysis of Biofilm-Forming Polar Microbacterium sp. Strains PAMC22086 and PAMC21962 Isolated from Extreme Habitats. Microorganisms. 2023; 11(7):1757. https://doi.org/10.3390/microorganisms11071757
Chicago/Turabian StyleKim, Byeollee, Saru Gurung, So-Ra Han, Jun-Hyuck Lee, and Tae-Jin Oh. 2023. "Comparative Genomic Analysis of Biofilm-Forming Polar Microbacterium sp. Strains PAMC22086 and PAMC21962 Isolated from Extreme Habitats" Microorganisms 11, no. 7: 1757. https://doi.org/10.3390/microorganisms11071757
APA StyleKim, B., Gurung, S., Han, S.-R., Lee, J.-H., & Oh, T.-J. (2023). Comparative Genomic Analysis of Biofilm-Forming Polar Microbacterium sp. Strains PAMC22086 and PAMC21962 Isolated from Extreme Habitats. Microorganisms, 11(7), 1757. https://doi.org/10.3390/microorganisms11071757





