Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation and Preparation of the Probiotic Bacterium
2.2. Experimental Feed Preparation
2.3. Experimental Design, Animal Husbandry, and Ethics
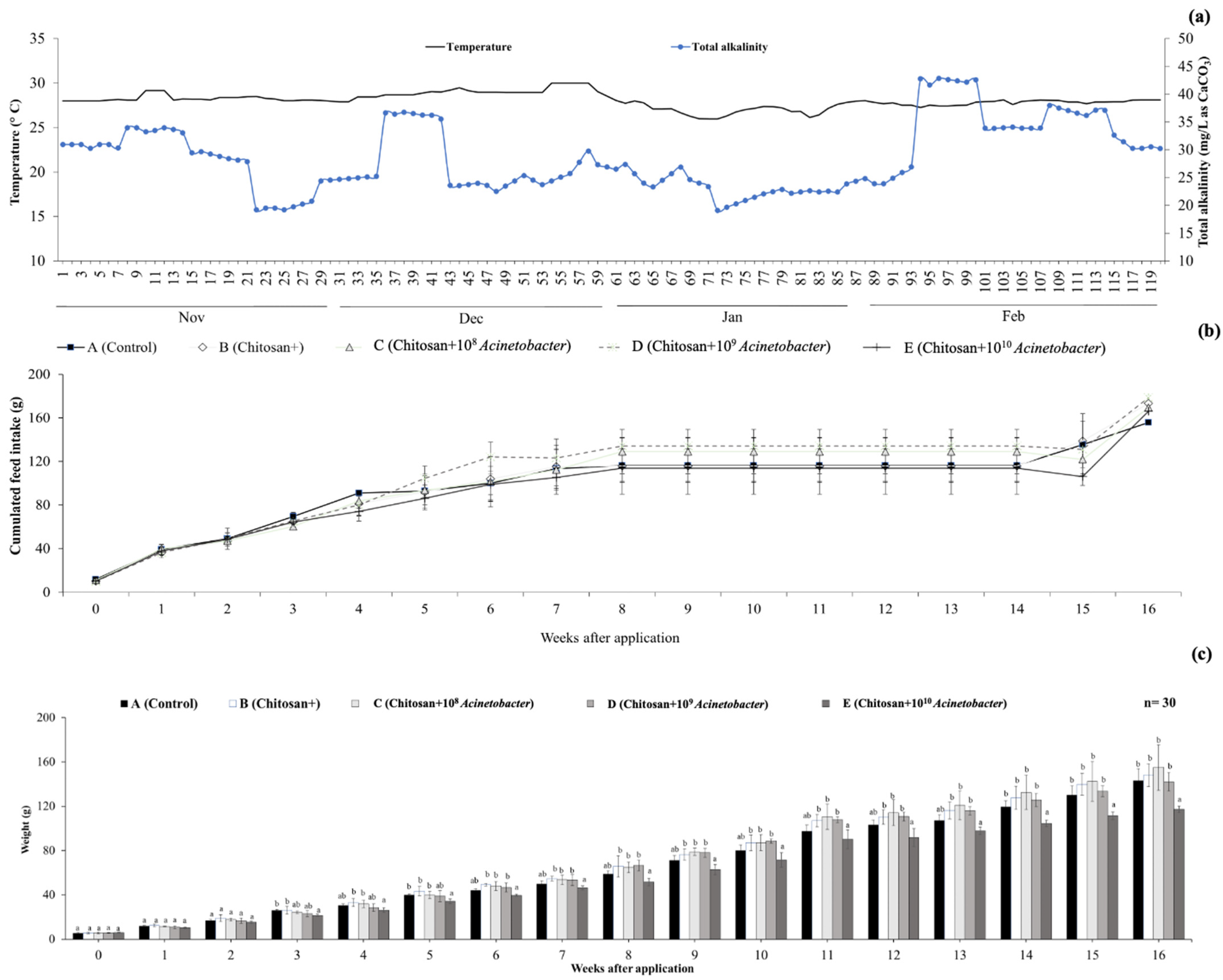
2.4. Water Quality Analysis and Assessment
2.5. Fish Growth Parameters
| WG (g) = Wt − Wi |
| TLG (cm) = TLt − TLi |
| ADG (g/individual/day) = (Wt − Wi)/t |
| SGR (%/day) = log (Wt) − log (Wi)/t ×100 |
| FCR = amount of total feed given/WG |
| Accumulated mortality (%) = 100 × (number of dead fish)/(total initial number of fish) |
2.6. Health Status, Serum Immune Parameters, and Immune-Related Gene Expression Analysis
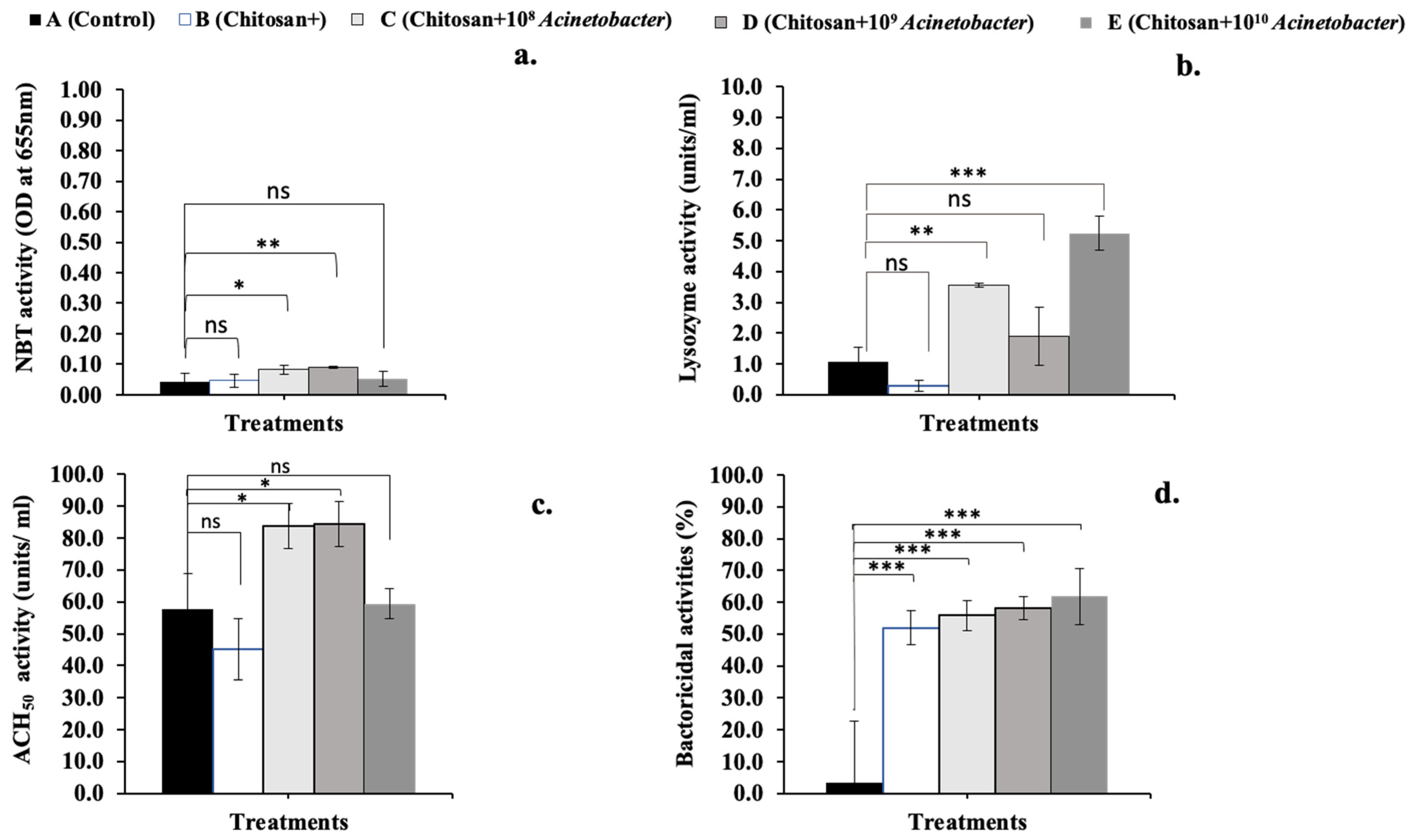
2.6.1. Serum Immune Parameters
Blood Collection
Respiratory Burst Analysis
Lysozyme Activity Assay
Hemolytic Activity by the Alternative Complement Pathway (ACH50) Assay
Bactericidal Activity Analysis
2.6.2. Hematological Analysis
Hematocrit
Total Blood Count and Differentiated Count
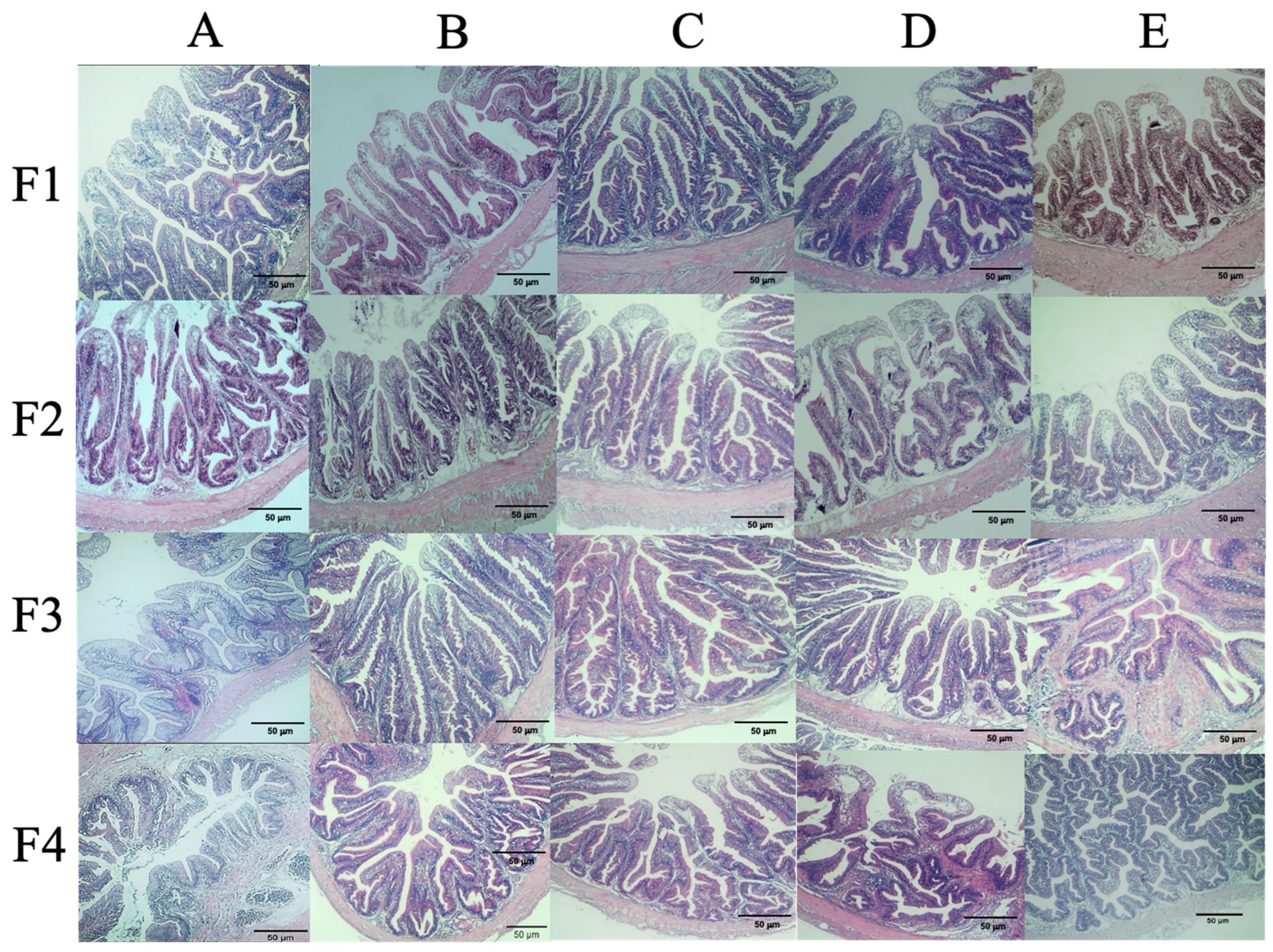
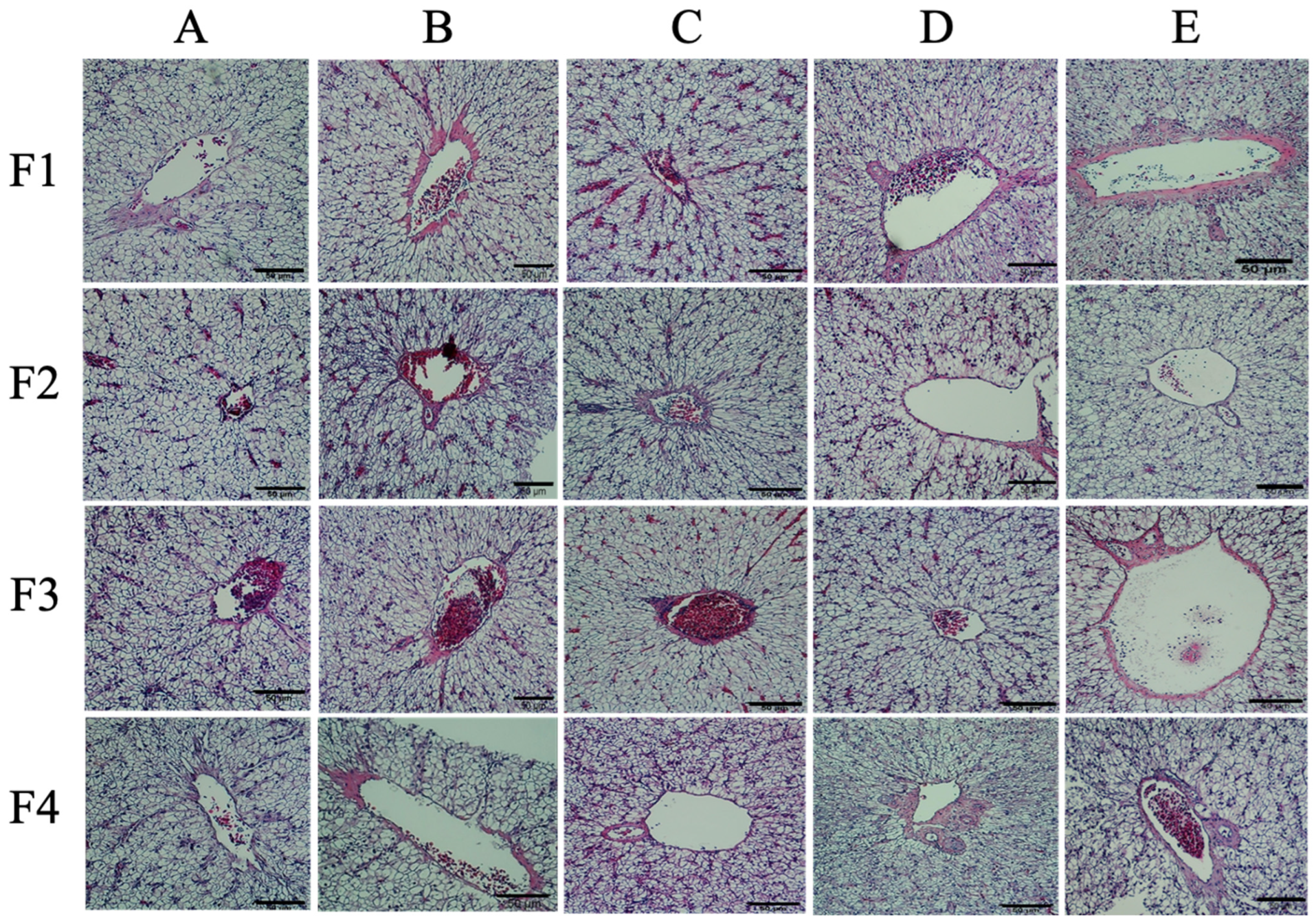
2.6.3. Histopathological Analyses
2.6.4. Effects of Synbiotics on the Expression of an Immune-Related Gene Using Quantitative Real-Time–PCR (qRT–PCR)
Total RNA Extraction of the Target Tissues
First-Strand cDNA Synthesis
Quantitative Real-Time PCR Analysis (qRT–PCR)
2.7. qPCR Analysis for Determination of the Probiotic Copy Numbers in the Intestine of the Sample Catfish
2.8. Effects of Prebiotics and Synbiotics on Disease Resistance against Aeromonas Hydrophila
2.8.1. Experimental Fish
2.8.2. Challenge Test
2.9. Data and Statistical Analysis
3. Results
3.1. Water Quality Analysis
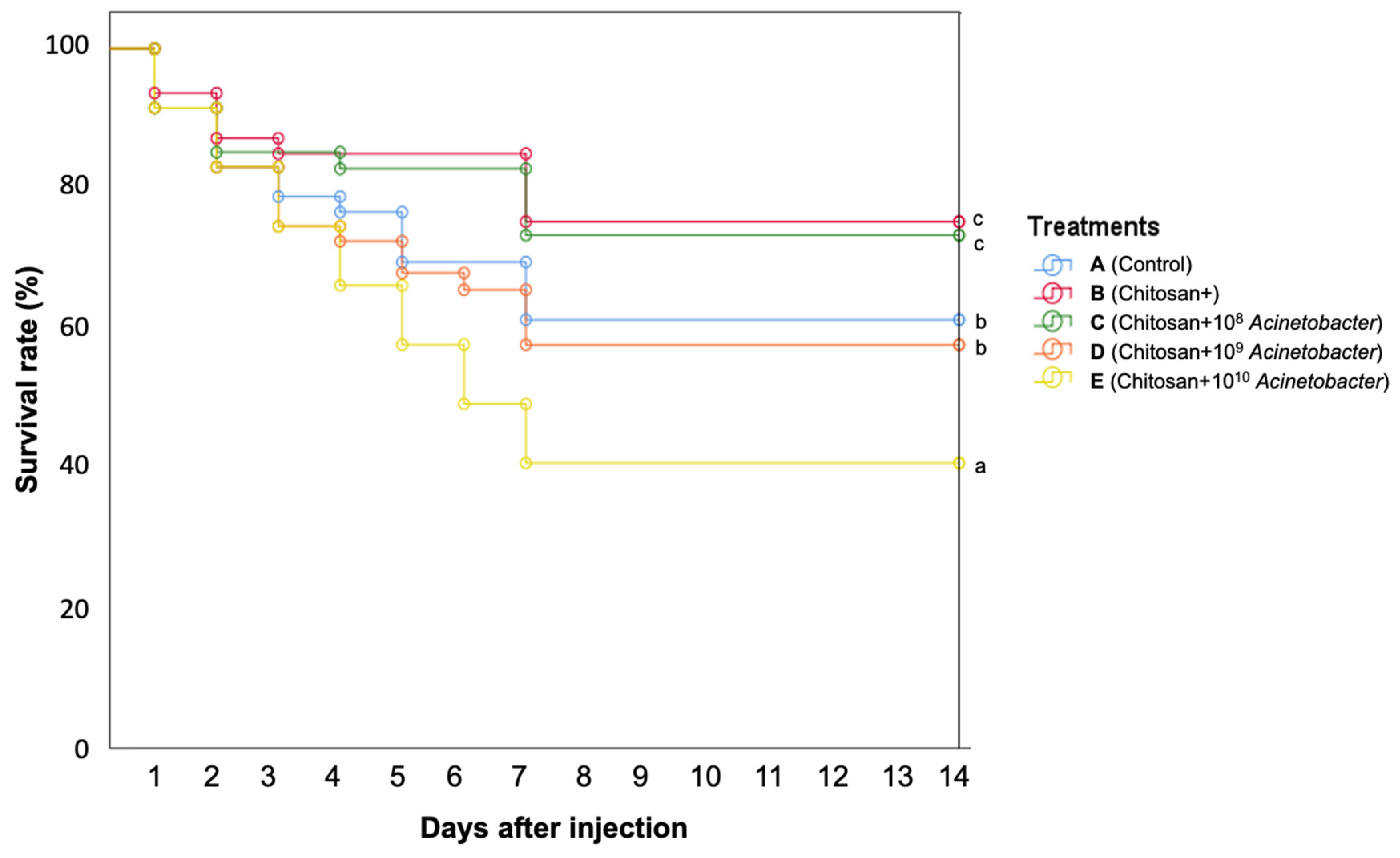
3.2. Growth Performance Analysis and Mortality
3.3. Hematological Analysis
3.4. Serum Immunological Analysis
3.5. Histological Analysis
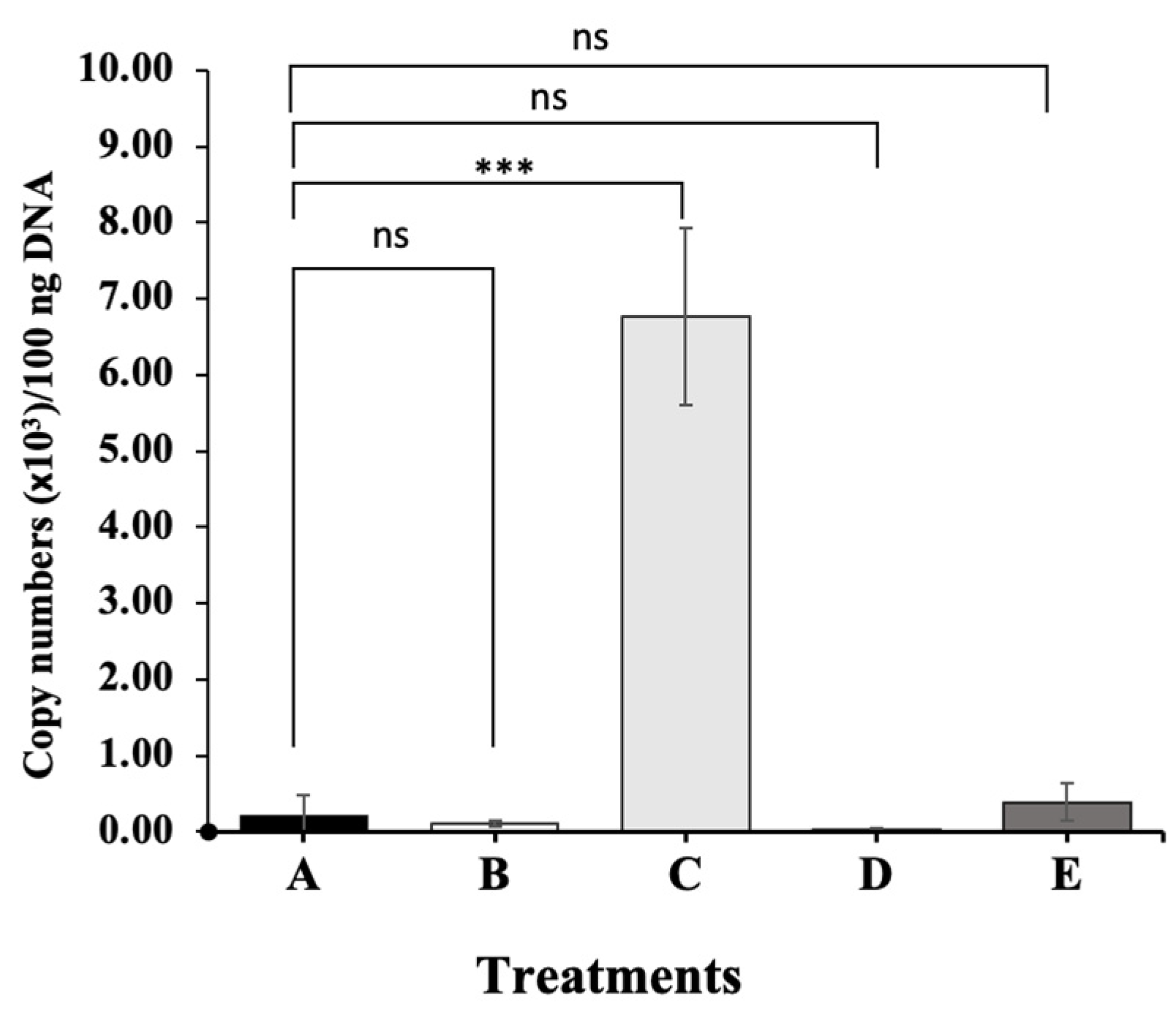
3.6. Quantification of the Copy Number of the Gyrase Subunit B Gene of Acinetobacter KU011TH in the Intestine of Experimental Catfish
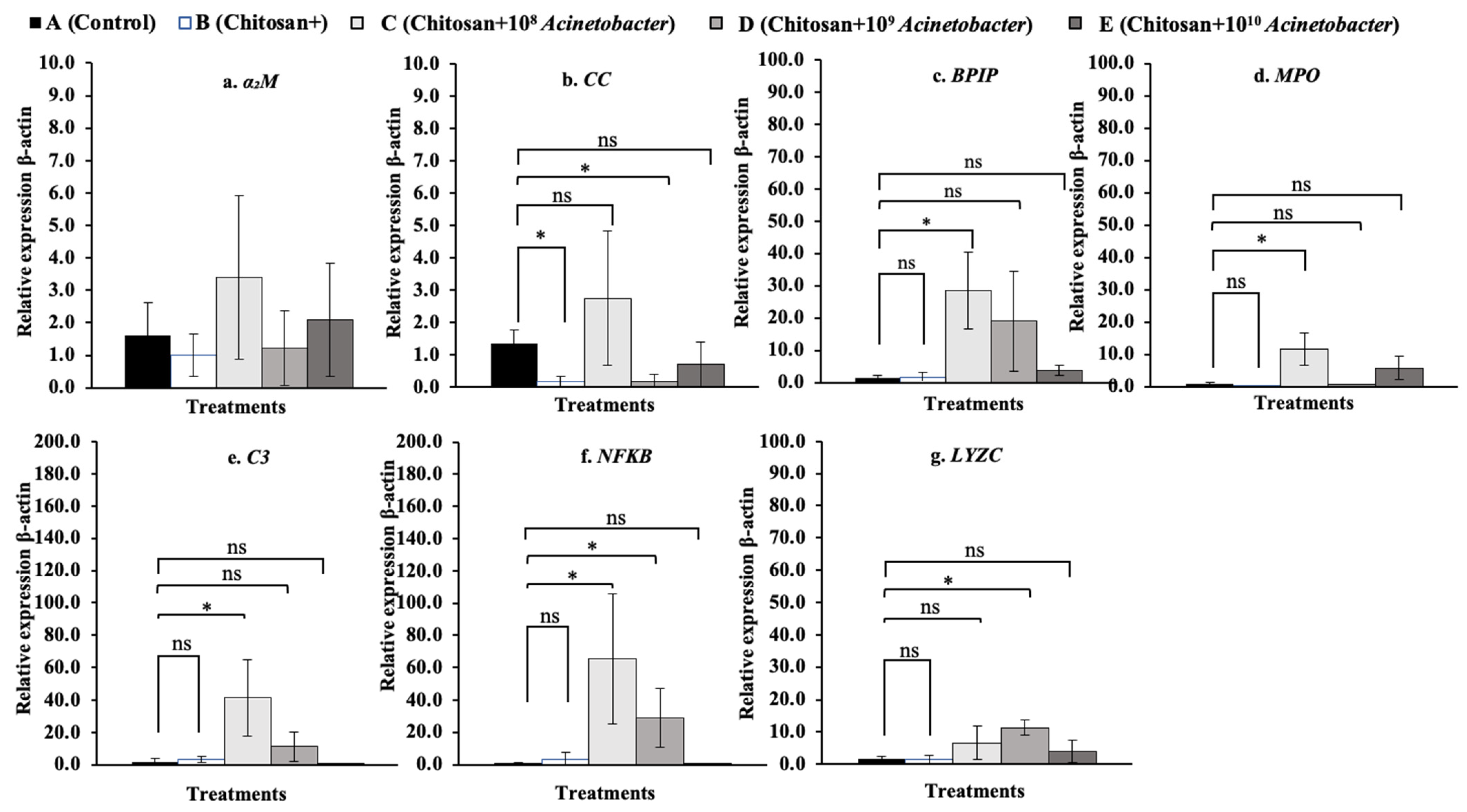
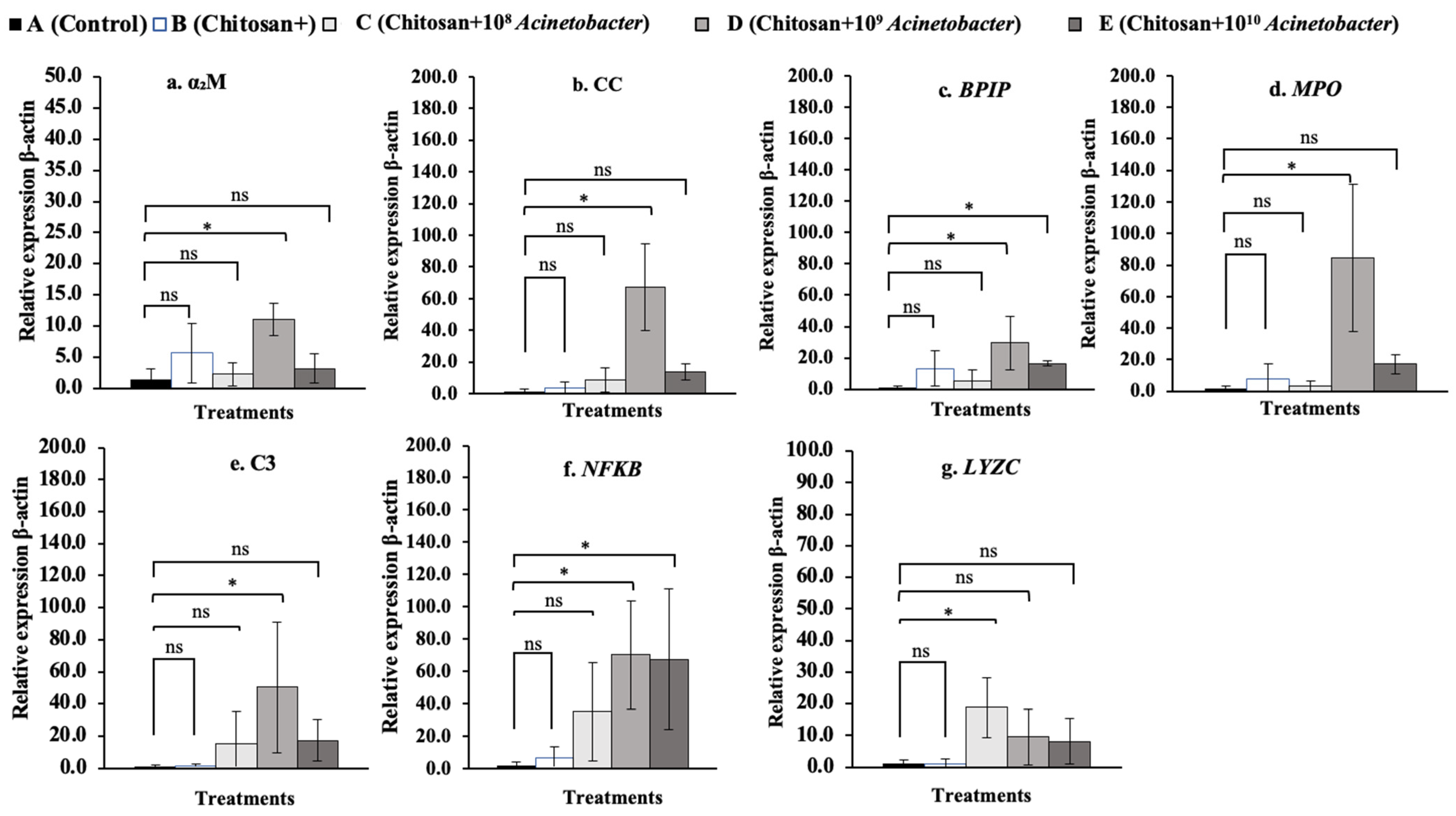
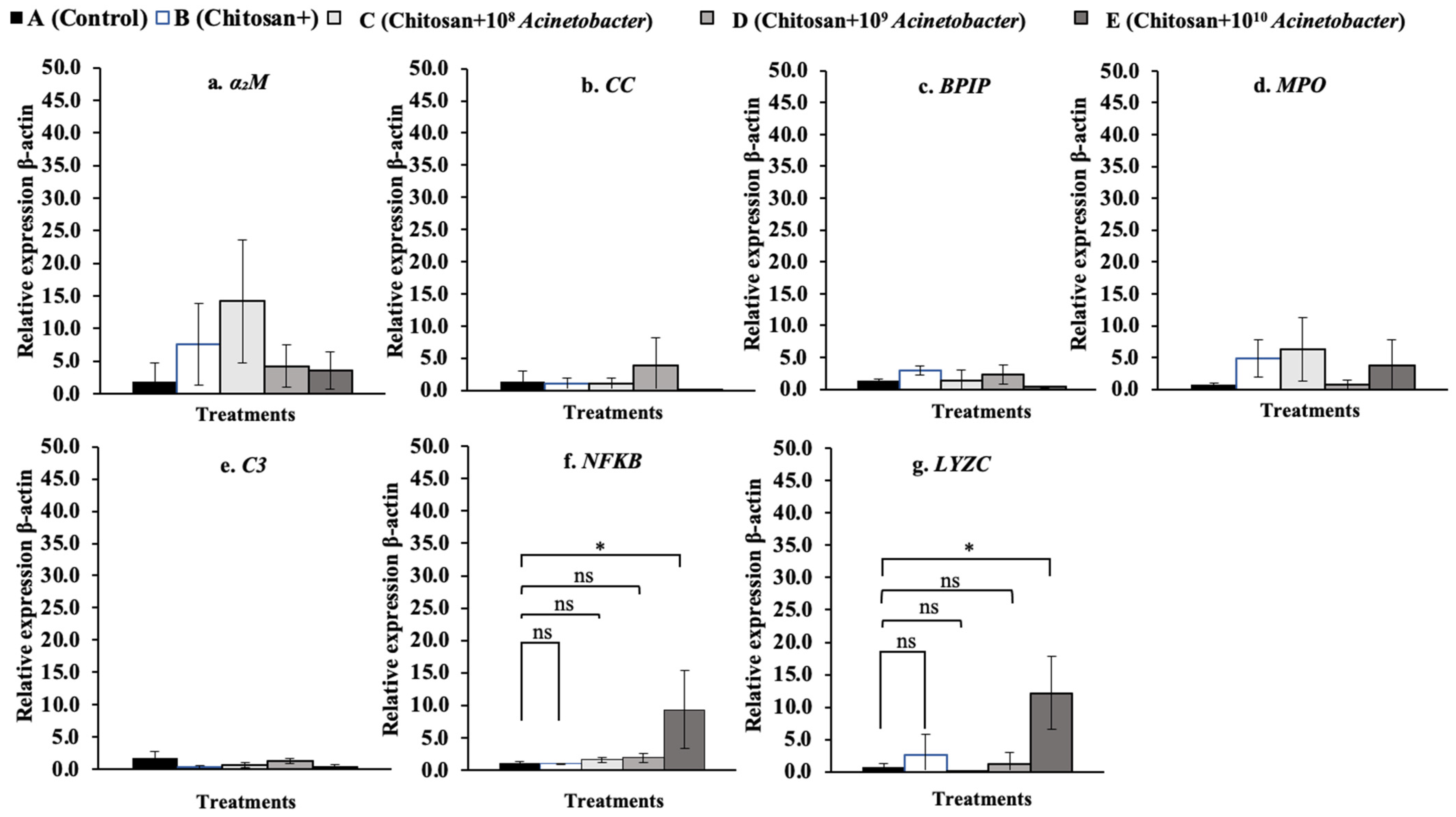
3.7. Immune-Related Gene Expression Analysis
3.8. Disease Resistance against A. hydrophila
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fisheries and Aquaculture Statistical Time Series, FishStatj Software, v2021.1.2, 2021. Available online: https://www.fao.org/fishery/en/topic/166235 (accessed on 31 December 2022).
- Whan-Air, W.; Thongprajukaew, K.; Salaeharae, T.; Yoonram, K. Identification of wild and farmed broadhead catfish (Clarias macrocephalus Günther, 1864) based on morphometry, digestive indexes and flesh quality. J. Oceanol. Limnol. 2018, 36, 1788–1797. [Google Scholar] [CrossRef]
- Dunham, R.A.; Elaswad, A. Catfish Biology and Farming. Annu. Rev. Anim. Biosci. 2018, 6, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Yong-Sulem, S.; Brummett, R.E.; Tabi, T.E.; Tchoumboué, J. Towards the maximum profitability of smallholder catfish nurseries: Predator defense and feeding-adapted stocking of Clarias gariepinus. Aquaculture 2007, 271, 371–376. [Google Scholar] [CrossRef]
- Senanan, W.; Kapuscinski, A.R.; Na-Nakorn, U.; Miller, L.M. Genetic impacts of hybrid catfish farming (Clarias macrocephalus × C. gariepinus) on native catfish populations in central Thailand. Aquaculture 2004, 235, 167–184. [Google Scholar] [CrossRef]
- FAO. Fisheries and Aquaculture Country Profiles: Thailand. 2022. Available online: https://www.fao.org/fishery/en/facp/tha?lang=en (accessed on 23 September 2022).
- Li, M.; Wang, Q.-L.; Lu, Y.; Chen, S.-L.; Li, Q.; Sha, Z.-X. Expression profiles of NODs in channel catfish (Ictalurus punctatus) after infection with Edwardsiella tarda, Aeromonas hydrophila, Streptococcus iniae and channel catfish hemorrhage reovirus. Fish Shellfish. Immunol. 2012, 33, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.B.G.; de Oliveira, W.F.; Marques, D.S.C.; Correia, M.T.D.S.; de Carvalho, E.V.M.M.; Coelho, L.C.B.B. The genus Aeromonas: A general approach. Microb. Pathog. 2019, 130, 81–94. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Probiotic Effects of a Novel Strain, Acinetobacter KU011TH, on the Growth Performance, Immune Responses, and Resistance against Aeromonas hydrophila of Bighead Catfish (Clarias macrocephalus Günther, 1864). Microorganisms 2019, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish. Immunol. 2005, 19, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Sun, C.; Li, S.; Hou, T.; Li, C. Therapeutic effect and immune mechanism of chitosan-gentamicin conjugate on Pacific white shrimp (Litopenaeus vannamei) infected with Vibrio parahaemolyticus. Carbohydr. Polym. 2021, 269, 118334. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Huang, J.; Huang, B.; Li, H. The exploitation of probiotics, prebiotics and synbiotics in aquaculture: Present study, limitations and future directions: A review. Aquac. Int. 2020, 28, 1017–1041. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Veter. World 2021, 14, 319–328. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Evaluation of existing guidelines for their adequacy for the food and feed risk assessment of microorganisms obtained through synthetic biology. EFSA J. 2022, 20, e07479. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; Davies, S.; Waines, P.; Emery, M.; Castex, M.; Gioacchini, G.; Carnevali, O.; Bickerdike, R.; Romero, J.; Merrifield, D. Dietary synbiotic application modulates Atlantic salmon (Salmo salar) intestinal microbial communities and intestinal immunity. Fish Shellfish. Immunol. 2013, 35, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological Approaches for Disease Control in Aquaculture: Advantages, Limitations and Challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef]
- Journals, I.; Omenwa, V.; Mbakwem-Aniebo, C.; Ibiene, A. Effects of Selected Probiotics on the Growth and Survival of Fry Fingerlings of Clarias gariepinus. J. Pharm. Biol. Sci. 2015, 10, 89–93. [Google Scholar] [CrossRef]
- Muhammad, F.; Ekawati, A.W.; Arifin, N.B.; Yuniarti, A.; Hariati, A.M. Effect of probiotics on survival rate and growth performance of Clarias gariepinus. Nat. Environ. Pollut. Technol. 2019, 18, 313–316. [Google Scholar]
- Abdul-Malik, A.; Aliyu-Paiko, M.; Adamu, K.M.; Aliyu-A, A.; Mohammed, J.N. Role of Prebiotic, Probiotic and Symbiotic Diets on Bacterial proliferation in Feed and Intestine of African (Clarias gariepinus) Catfish. J. Appl. Sci. Environ. Manag. 2023, 27, 87–93. [Google Scholar] [CrossRef]
- Naphanang, A.; Limsuwan, C.; Pungpang, S. Properties of Bacillus W120 in immunogenicity of hybrid catfish (Clarias macrocephalus × Clarias garieipinus). In Proceedings of the 51st Kasetsart University Annual Conference: Veterinary Medicine, Fisheries, Bangkok, Thailand, 5–7 February 2013; pp. 156–163. [Google Scholar]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Abu Hassim, H.; Yusoff, S.M.; Abu Bakar, S.N.; Srisapoome, P. Short- and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus). Fish Shellfish. Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef]
- Mateo-Estrada, V.; Graña-Miraglia, L.; López-Leal, G.; Castillo-Ramírez, S. Phylogenomics Reveals Clear Cases of Misclassification and Genus-Wide Phylogenetic Markers for Acinetobacter. Genome Biol. Evol. 2019, 11, 2531–2541. [Google Scholar] [CrossRef]
- Qin, J.; Feng, Y.; Lü, X.; Zong, Z. Characterization of Acinetobacter chengduensis sp. nov., isolated from hospital sewage and capable of acquisition of carbapenem resistance genes. Syst. Appl. Microbiol. 2020, 43, 126092. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Paria, P.; Das, A.; Bhowmick, S.; Sahoo, A.; Das, B. Molecular characterization and pathogenicity of a virulent Acinetobacter baumannii associated with mortality of farmed Indian Major Carp Labeo rohita (Hamilton 1822). Aquaculture 2017, 471, 157–162. [Google Scholar] [CrossRef]
- Cao, H.; Yu, L.; Ou, R.; Lu, L.; Yang, X.; Yang, Y. Acinetobacter johnsonii: An emerging pathogen for cultured blunt snout bream Megalobrama amblycephala. Isr. J. Aquac. Bamidgeh 2017, 69, 1–7. [Google Scholar]
- Kozińska, A.; Paździor, E.; Pękala, A.; Niemczuk, W. Acinetobacter johnsonii and Acinetobacter lwoffii—The emerging fish pathogens. Bull. Veter. Inst. Pulawy 2014, 58, 193–199. [Google Scholar] [CrossRef]
- Reddy, M.R.K.; Mastan, S.A. Emerging Acinetobacter schindleri in red eye infection of Pangasius sutchi. Afr. J. Biotechnol. 2013, 12, 6993–6996. [Google Scholar]
- Wanka, K.M.; Damerau, T.; Costas, B.; Krueger, A.; Schulz, C.; Wuertz, S. Isolation and characterization of native probiotics for fish farming. BMC Microbiol. 2018, 18, 119. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Kayansamruaj, P.; Srisapoome, P. Acinetobacter Strain KUO11TH, a Unique Organism Related to Acinetobacter pittii and Isolated from the Skin Mucus of Healthy Bighead Catfish and Its Efficacy Against Several Fish Pathogens. Microorganisms 2019, 7, 549. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Rohani, F.; Islam, S.M.; Hossain, K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C. Shahjahan Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish. Immunol. 2021, 120, 569–589. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Li, M.; Yang, B.; Liu, W.; Chu, Z.; Cui, B.; Chen, X. Lactobacillus rhamnosus Encapsulated in Alginate/Chitosan Microgels Manipulates the Gut Microbiome to Ameliorate Salt-Induced Hepatorenal Injury. Front. Nutr. 2022, 9, 872808. [Google Scholar] [CrossRef]
- Yumuk, H.; Alak, G.; Yanik, T. Effects of chitosan with vegetable oil on shelf life of brown trout (Salmo trutta fario) fillets fed on prebiotics. J. Food Saf. 2019, 39, e12684. [Google Scholar] [CrossRef]
- Chen, G.; Yin, B.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Supplementing chitosan oligosaccharide positively affects hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed dietary fish meal replacement with cottonseed protein concentrate: Effects on growth, gut microbiota, antioxidant function and immune response. Front. Mar. Sci. 2021, 18, 707627. [Google Scholar]
- Salam, M.A.; Rahman, M.A.; Paul, S.I.; Islam, F.; Barman, A.K.; Rahman, Z.; Shaha, D.C.; Rahman, M.M.; Islam, T. Dietary chitosan promotes the growth, biochemical composition, gut microbiota, hematological parameters and internal organ morphology of juvenile Barbonymus gonionotus. PLoS ONE 2021, 16, e0260192. [Google Scholar] [CrossRef]
- El-Naggar, M.; Salaah, S.; El-Shabaka, H.; El-Rahman, F.A.; Khalil, M.; Suloma, A. Efficacy of dietary chitosan and chitosan nanoparticles supplementation on health status of Nile tilapia, Oreochromis niloticus (L.). Aquac. Rep. 2021, 19, 100628. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Kreutz, L.C.; Barcellos, L.J.G.; de Faria Valle, S.; de Oliveira Silva, T.; Anziliero, D.; dos Santos, E.D.; Pivato, M.; Zanatta, R. Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish. Immunol. 2011, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Myrnes, B.; Seppola, M.; Johansen, A.; Øverbø, K.; Callewaert, L.; Vanderkelen, L.; Michiels, C.W.; Nilsen, I.W. Enzyme characterisation and gene expression profiling of Atlantic salmon chicken- and goose-type lysozymes. Dev. Comp. Immunol. 2013, 40, 11–19. [Google Scholar] [CrossRef]
- Sunyer, J.O.; Tort, L. Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are affected by the alternative complement pathway. Veter. Immunol. Immunopathol. 1995, 45, 333–345. [Google Scholar] [CrossRef]
- Azarin, H.; Aramli, M.S.; Imanpour, M.R.; Rajabpour, M. Effect of a Probiotic Containing Bacillus licheniformis and Bacillus subtilis and Ferroin Solution on Growth Performance, Body Composition and Haematological Parameters in Kutum (Rutilus frisii kutum) Fry. Probiotics Antimicrob. Proteins 2015, 7, 31–37. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African Catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac. Res. 2009, 40, 1642–1652. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yudiati, E.; Sedjati, S.; Susanto, A.; Azhar, N.; Alghazeer, R. Potency of Chitosan and Chitooligochitosan (COS) as Prebiotics for Streptococcus thermophillus and Lactobacillus bulgaricus Probiotics. J. Kelaut. Trop. 2021, 24, 25–33. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Ran, C.; He, S.; Yang, Y.; Zhou, Z. Effects of dietary scFOS and lactobacilli on survival, growth, and disease resistance of hybrid tilapia. Aquaculture 2017, 470, 50–55. [Google Scholar] [CrossRef]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Gewaily, M.S.; Abdo, S.E.; Moustafa, E.M.; AbdEl-Kader, M.F.; El-Razek, I.M.A.; El-Sharnouby, M.; Alkafafy, M.; Raza, S.H.A.; El Basuini, M.F.; Van Doan, H.; et al. Dietary Synbiotics Can Help Relieve the Impacts of Deltamethrin Toxicity of Nile Tilapia Reared at Low Temperatures. Animals 2021, 11, 1790. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, S.P.; Chakrabarti, R. Effect of temperature on digestive physiology, immune-modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture 2017, 479, 134–141. [Google Scholar] [CrossRef]
- Islam, J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquac. Soc. 2021, 53, 314–366. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Nguyen, T.M.I.; Konzer, L.; Zhao, H.; Wang, D.R.; Yang, F.; Schwarzbauer, J. Occurrence and origin of triazine herbicides in a tropical coastal area in China: A potential ecosystem threat. Estuar. Coast. Shelf Sci. 2020, 235, 106612. [Google Scholar] [CrossRef]
- Kojima, C.; Sasaki, H.; Tsuchiya, Y.; Goto, K. The influence of environmental temperature on appetite-related hormonal responses. J. Physiol. Anthr. 2015, 34, 22. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.-S.; Wang, L.; Song, K.; Lu, K.-L.; Zhang, C.-X.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Miegel, R.; Pain, S.; van Wettere, W.; Howarth, G.; Stone, D. Effect of water temperature on gut transit time, digestive enzyme activity and nutrient digestibility in yellowtail kingfish (Seriola lalandi). Aquaculture 2010, 308, 145–151. [Google Scholar] [CrossRef]
- Liang, H.; Xu, H.; Ge, X.; Zhu, J.; Ren, M.; Mi, H. Water temperature affects the protein requirements, growth performance, and nutritional metabolism of grass carp (Ctenopharyngodon idella) juveniles. Aquac. Rep. 2022, 25, 101267. [Google Scholar] [CrossRef]
- Ogunji, J.O.; Awoke, J. Effect of environmental regulated water temperature variations on survival, growth performance and haematology of African catfish, Clarias gariepinus. Our Nat. 2017, 15, 26–33. [Google Scholar] [CrossRef]
- Kasihmuddin, S.M.; Ghaffar, M.A.; Das, S.K. Rising Temperature Effects on Growth and Gastric Emptying Time of Freshwater African Catfish (Clarias gariepinus) Fingerlings. Animals 2021, 11, 3497. [Google Scholar] [CrossRef]
- Stone, N.; Shelton, J.L.; Haggard, B.E.; Thomforde, H.K. Interpretation of Water Analysis Reports for Fish Culture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013; p. 4606. [Google Scholar]
- Ahmad, T.; Singh, S.; Khangembam, B.; Sharma, J.; Chakrabarti, R. Food consumption and digestive enzyme activity of Clarias batrachus exposed to various temperatures. Aquac. Nutr. 2014, 20, 265–272. [Google Scholar] [CrossRef]
- Furuya, S.; Furuya, K. Roles of substance P and ATP in the subepithelial fibroblasts of rat intestinal villi. In International Review of Cell and Molecular Biology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Friedlander, M., Jarvik, J., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 133–189. [Google Scholar]
- Kuebutornye, F.K.A.; Wang, Z.W.; Lu, Y.S.; Abarike, E.D.; Sakyi, M.E.; Li, Y.; Xie, C.X.; Hlordzi, V. Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2020, 97, 83–95. [Google Scholar] [CrossRef]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic Bioactive Compounds Shape Fish Mucosal Immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef]
- Najafabad, M.K.; Imanpoor, M.R.; Taghizadeh, V.; Alishahi, A. Effect of dietary chitosan on growth performance, hematological parameters, intestinal histology and stress resistance of Caspian kutum (Rutilus frisii kutum Kamenskii, 1901) fingerlings. Fish Physiol. Biochem. 2016, 42, 1063–1071. [Google Scholar] [CrossRef]
- Wu, N.; Song, Y.-L.; Wang, B.; Zhang, X.-Y.; Zhang, X.-J.; Wang, Y.-L.; Cheng, Y.-Y.; Chen, D.-D.; Xia, X.-Q.; Lu, Y.-S.; et al. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fang, X.; Ma, N.; Lin, Q.; Huang, Z.; Liu, W.; Xu, M.; Chen, X.; Zhang, W.; Zhang, Y. Myeloperoxidase-deficient zebrafish show an augmented inflammatory response to challenge with Candida albicans. Fish Shellfish. Immunol. 2015, 44, 109–116. [Google Scholar] [CrossRef]
- Kewcharoen, W.; Srisapoome, P. Potential synbiotic effects of a Bacillus mixture and chitosan on growth, immune responses and VP(AHPND) resistance in Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Fish Shellfish. Immunol. 2022, 127, 715–729. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.; Cai, Y.; Shi, H.; Zhou, Y.; Zhang, D.; Guo, W.; Wang, S. Effects of five prebiotics on growth, antioxidant capacity, non-specific immunity, stress resistance, and disease resistance of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Animals 2023, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.A.; Salem, M.; Gaber, M.; Nour, A.M. Effect of chitosan supplemented diet on survival, growth, feed utilization, body composition and histology of sea bass (Dicentrarchus labrax). World J. Eng. Technol. 2015, 3, 38–47. [Google Scholar] [CrossRef]



| Treatments | |||||
|---|---|---|---|---|---|
| Growth Parameters | A (Control) | B | C | D | E |
| Initial length (IL; cm) | 8.87 ± 0.26 | 8.78 ±0.25 | 8.75 ± 0.20 | 9.03 ± 0.19 | 8.70 ± 0.10 |
| Final length (FL; cm) | 25.93 ± 0.61 | 26.28 ± 0.22 | 26.65 ± 0.72 | 26.28 ± 0.41 | 25.93 ± 0.50 |
| Initial weight (IW; g) | 5.49 ± 0.32 | 5.67 ± 0.48 | 5.72 ± 0.59 | 5.74 ± 0.31 | 5.90 ± 0.42 |
| Final weight (FW; g) | 150.63 ± 13.80 a | 165.28 ± 11.28 a | 156.80 ± 22.82 a | 155.03 ± 10.93 a | 128.67 ± 3.66 b |
| Length gain (LG; cm) | 17.06 ± 0.79 | 17.50 ± 0.34 | 17.91 ± 0.82 | 17.25 ± 0.50 | 17.23 ± 0.41 |
| Weight gain (WG; g) | 145.15 ± 18.01 | 159.61 ± 9.31 | 151.08 ± 27.84 | 149.29 ± 9.40 | 122.77 ± 6.27 |
| Average daily gain (ADG; g/individual/day) | 1.20 ± 0.14 | 1.31 ± 0.07 | 1.27 ± 0.19 | 1.23 ± 0.07 | 1.02 ± 0.05 |
| Specific growth rate (SGR; %/day) | 1.25 ± 0.05 a | 1.28 ± 0.06 a | 1.25 ± 0.04 a | 1.24 ± 0.03 a | 1.15 ± 0.03 b |
| Feed conversion ratio (FCR) | 1.81 ± 0.02 | 1.68 ± 0.30 | 1.82 ± 0.23 | 1.90 ± 0.11 | 1.98 ± 0.06 |
| Accumulative mortality (%) | 7.78 ± 5.09 | 7.78 ± 7.70 | 5.56 ± 6.94 | 6.67 ± 3.85 | 0.83 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Say, P.; Nimikul, S.; Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter. Microorganisms 2023, 11, 1807. https://doi.org/10.3390/microorganisms11071807
Say P, Nimikul S, Bunnoy A, Na-Nakorn U, Srisapoome P. Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter. Microorganisms. 2023; 11(7):1807. https://doi.org/10.3390/microorganisms11071807
Chicago/Turabian StyleSay, Pisey, Sukkrit Nimikul, Anurak Bunnoy, Uthairat Na-Nakorn, and Prapansak Srisapoome. 2023. "Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter" Microorganisms 11, no. 7: 1807. https://doi.org/10.3390/microorganisms11071807
APA StyleSay, P., Nimikul, S., Bunnoy, A., Na-Nakorn, U., & Srisapoome, P. (2023). Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter. Microorganisms, 11(7), 1807. https://doi.org/10.3390/microorganisms11071807









