Cytokine Responses during Mycobacterium tuberculosis H37Rv and Ascaris lumbricoides Costimulation Using Human THP-1 and Jurkat Cells, and a Pilot Human Tuberculosis and Helminth Coinfection Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Part 1: In Vitro Studies
2.1.1. Bacterial Cultures
2.1.2. Helminth (A. lumbricoides) Excretory-Secretory Protein Extracts
2.1.3. Cell Culture and Treatment
2.1.4. Real-Time-Quantitative PCR (RT-qPCR)
2.2. Part B: Human Ex-Vivo Experiment
2.3. Statistical Analysis
3. Results
3.1. Part 1: In Vitro Study
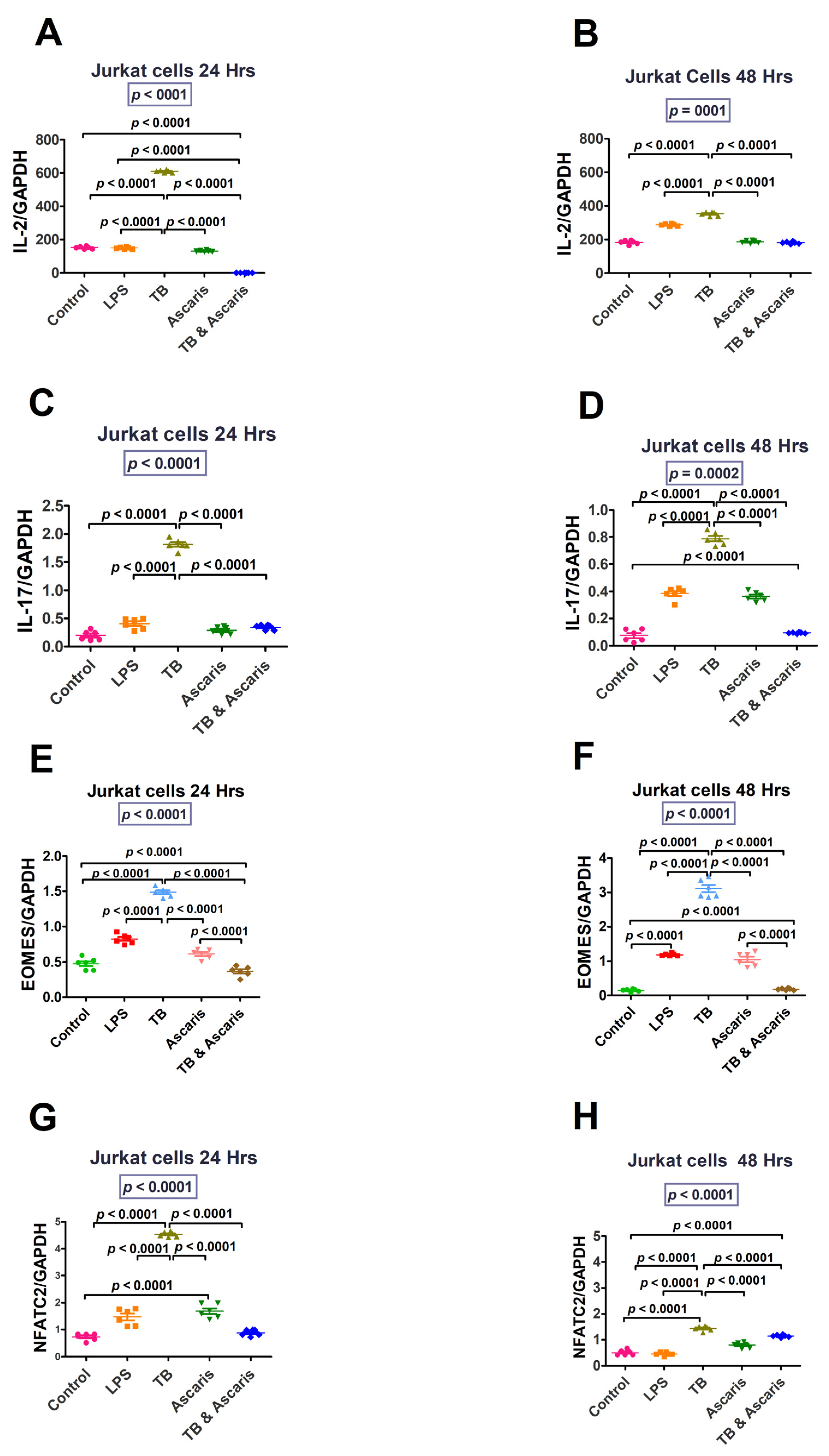
3.1.1. Th1/Pro-Inflammatory Immune Responses
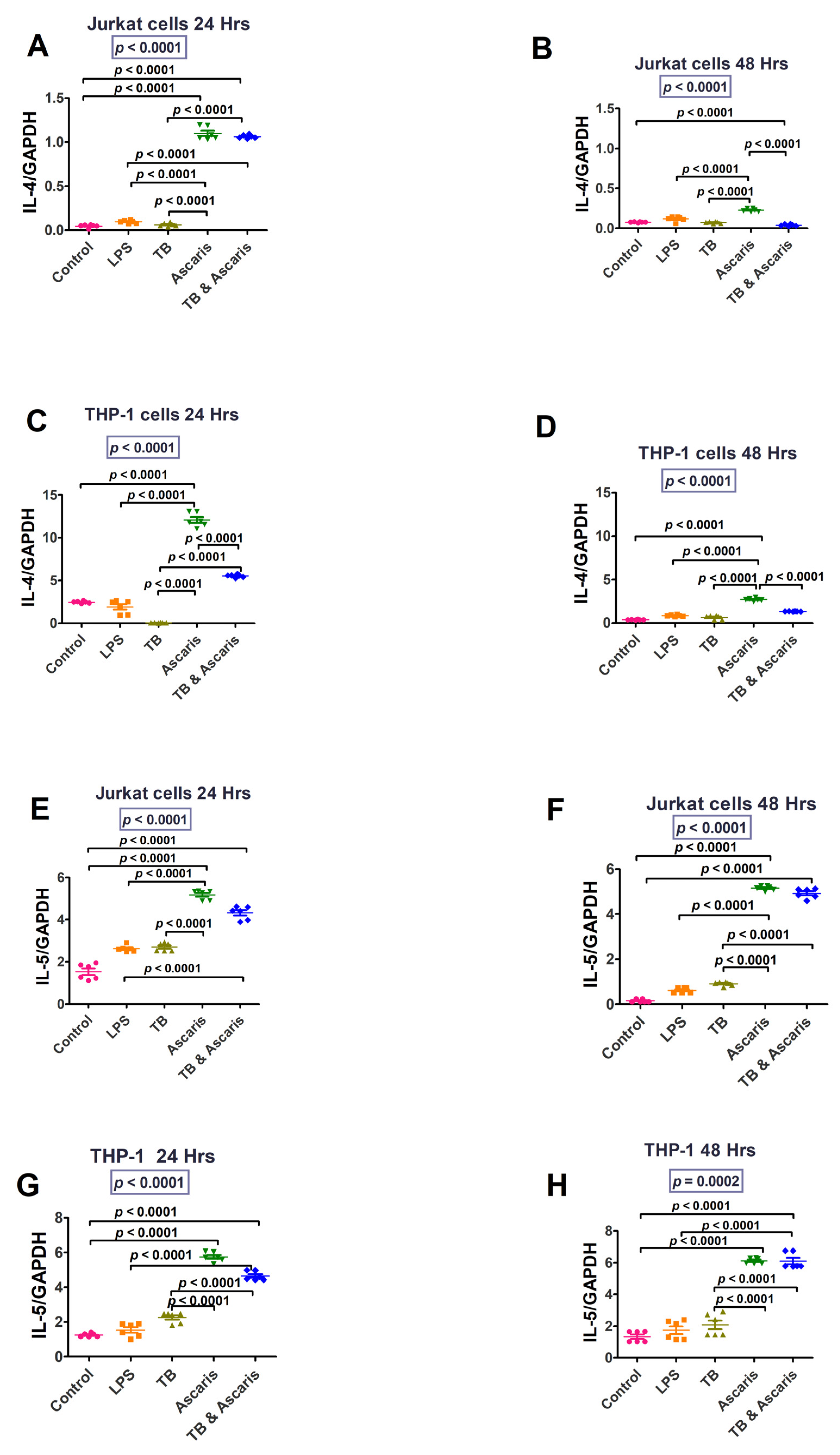
3.1.2. Th2/Anti-Inflammatory, Immune Responses
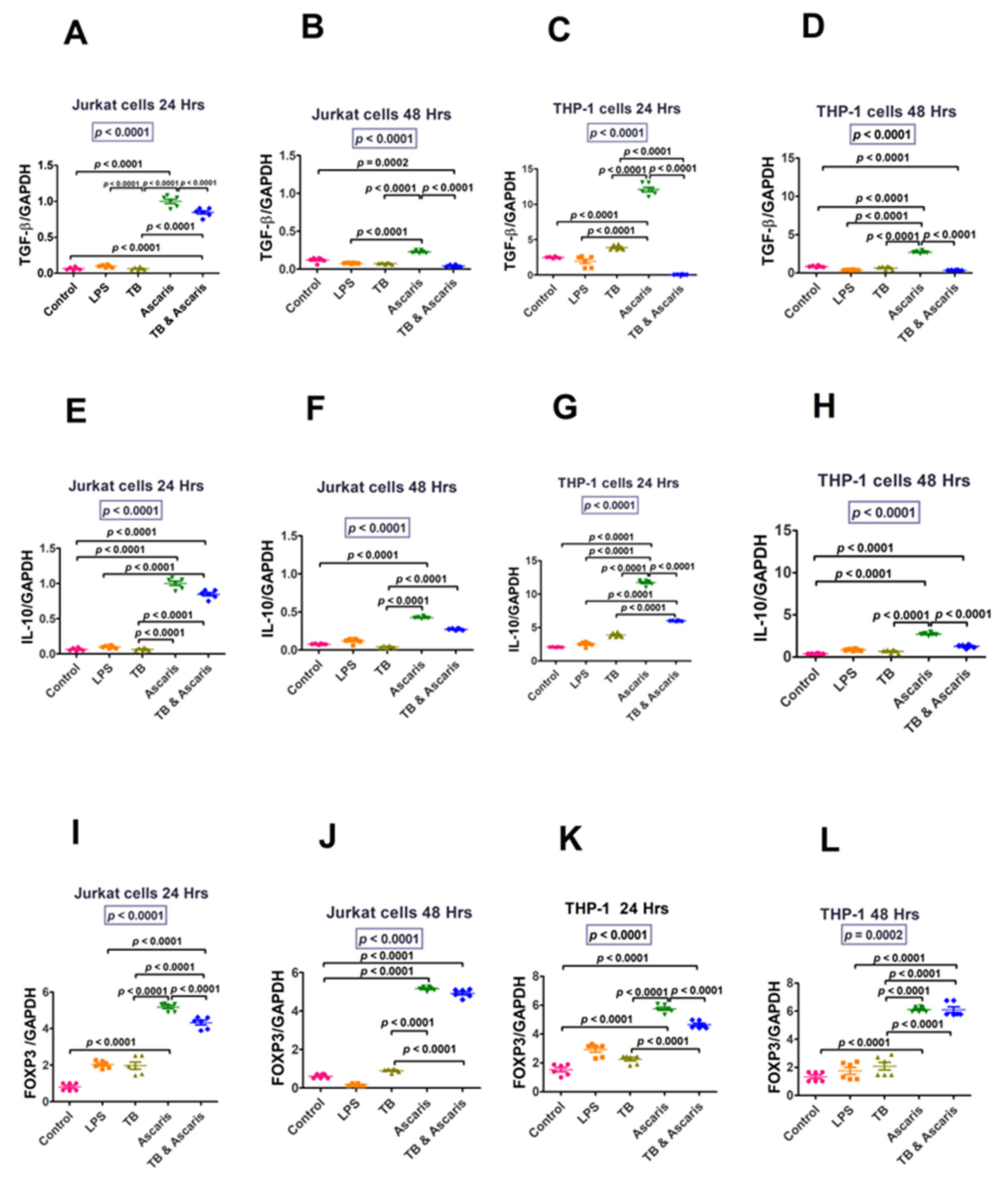
3.1.3. Regulatory Cytokines
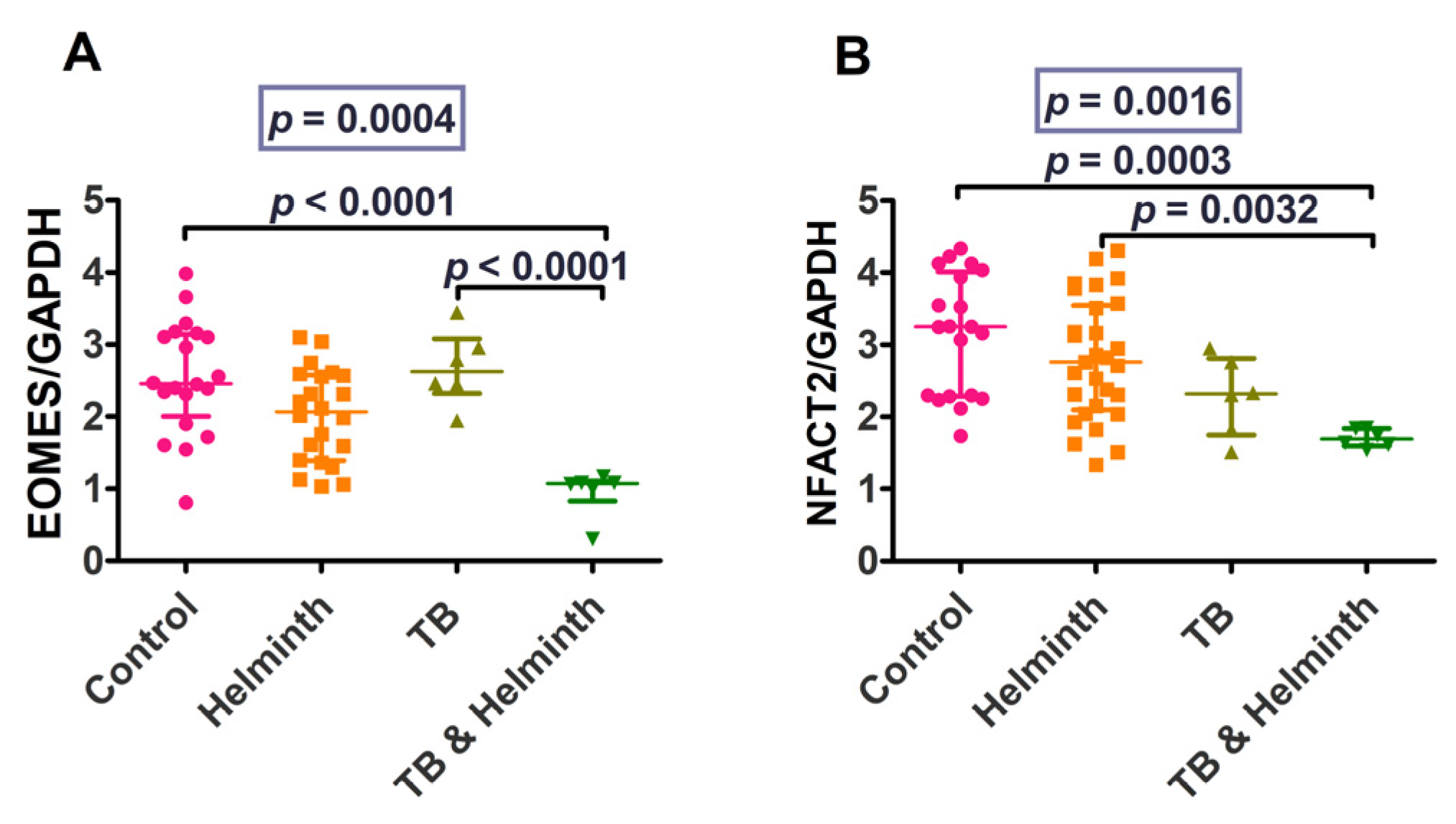
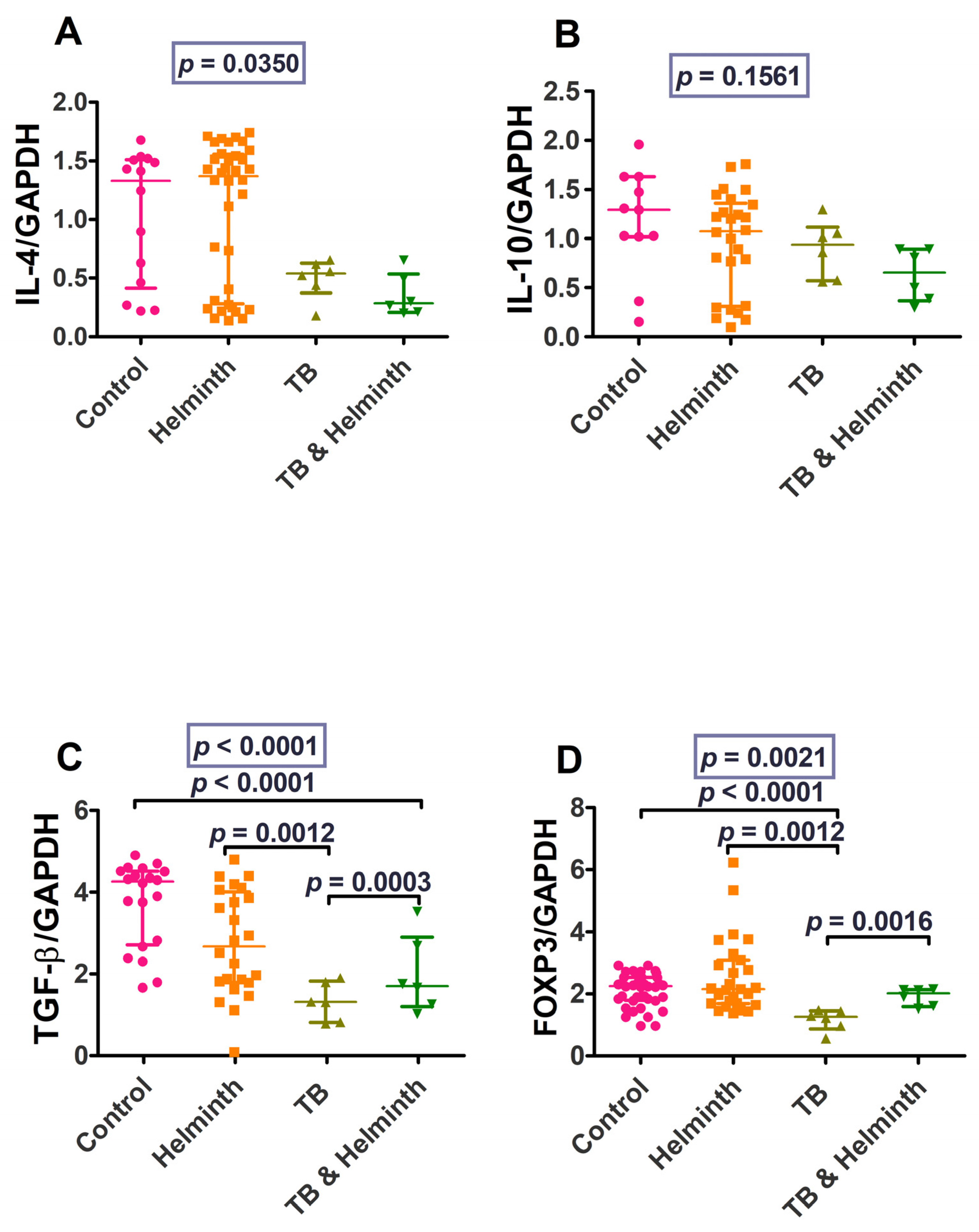
3.2. Part 2: Human Ex-Vivo Experiment Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Tuberculosis Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 16 February 2022).
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170. [Google Scholar] [CrossRef]
- CDC. Ascariasis. 2020. Available online: https://www.cdc.gov/parasites/ascariasis/index.html (accessed on 11 April 2022).
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019, 54, 1900655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Kathamuthu, G.R.; Moideen, K.; Banurekha, V.V.; Nair, D.; Fay, M.P.; Nutman, T.B.; Babu, S. Strongyloides stercoralis Coinfection Is Associated With Greater Disease Severity, Higher Bacterial Burden, and Elevated Plasma Matrix Metalloproteinases in Pulmonary Tuberculosis. J. Infect. Dis. 2020, 222, 1021–1026. [Google Scholar] [CrossRef]
- Santos, J.H.A.; Bührer-sékula, S.; Melo, G.C.; Cordeiro-Santos, M.; Pimentel, J.P.D.; Gomes-Silva, A.; Costa, A.G.; Saraceni, V.; Da-Cruz, A.M.; Lacerda, M.V.G.; et al. Ascaris lumbricoides coinfection reduces tissue damage by decreasing IL-6 levels without altering clinical evolution of pulmonary tuberculosis or Th1/Th2/Th17 cytokine profile. Rev. Soc. Bras. Med. Trop. 2019, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuradha, R.; Munisankar, S.; Bhootra, Y.; Dolla, C.; Kumaran, P.; Nutman, T.B.; Babu, S. Anthelmintic Therapy Modifies the Systemic and Mycobacterial Antigen-Stimulated Cytokine Profile in Helminth-Latent Mycobacterium tuberculosis Coinfection. Infect. Immun. 2017, 85, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bewket, G.; Kiflie, A.; Tajebe, F.; Abate, E.; Schö, T.; Blomgranid, R. Helminth species dependent effects on Th1 and Th17 cytokines in active tuberculosis patients and healthy community controls. PLoS Negl. Trop. Dis. 2022, 16, e0010721. [Google Scholar] [CrossRef]
- Abate, E.; Belayneh, M.; Idh, J.; Diro, E.; Elias, D.; Britton, S.; Aseffa, A.; Stendahl, O.; Schön, T. Asymptomatic Helminth Infection in Active Tuberculosis Is Associated with Increased Regulatory and Th-2 Responses and a Lower Sputum Smear Positivity. PLoS Negl. Trop. Dis. 2015, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Watanabe, C.; Ohtsuka, R. Impacts of dietary intake and helminth infection on diversity in growth among schoolchildren in rural South China: A four-year longitudinal study. Am. J. Hum. Biol. 2007, 19, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; Munisankar, S.; Bhootra, Y.; Kumar, N.P.; Dolla, C.; Kumaran, P.; Babu, S. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin. Vaccine Immunol. 2016, 23, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Palma, M.; Bleich, D.; Loke, P.; Gause, W.C. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014, 7, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Elias, D.; Akuffo, H.; Pawlowski, A.; Haile, M.; Schön, T.; Britton, S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 2005, 23, 1326–1334. [Google Scholar] [CrossRef]
- Elias, D.; Britton, S.; Aseffa, A.; Engers, H.; Akuffo, H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine 2008, 26, 3897–3902. [Google Scholar] [CrossRef]
- Silva, S.R.; Jacysyn, J.F.; Macedo, M.S.; Faquin-Mauro, E.L. Immunosuppressive components of Ascaris suum down-regulate expression of costimulatory molecules and function of antigen-presenting cells via an IL-10-mediated mechanism. Eur. J. Immunol. 2006, 36, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Leng, Q.; Weisman, Z.; Stein, M.; Galai, N.; Kalinkovich, A.; Bentwich, Z. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J. Clin. Invest. 2000, 106, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Resende Co, T.; Hirsch, C.S.; Toossi, Z.; Dietze, R.; Ribeiro-Rodrigues, R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin. Exp. Immunol. 2007, 147, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hasanain, A.F.; Zayed, A.A.; Mahdy, R.E.; Nafee, A.M.; Attia, R.A.; Mohamed, A.O. Hookworm infection among patients with pulmonary tuberculosis: Impact of co-infection on the therapeutic failure of pulmonary tuberculosis. Int. J. Mycobacteriol. 2015, 4, 318–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, M. What Does Tuberculin Reactivity after Bacille Calmette-Guérin Vaccination Tell Us? Clin. Infect. Dis. 2000, 31, S71–S74. [Google Scholar]
- Hesham, M.S.; Edariah, A.B.; Norhayati, M. Intestinal parasitic infections and micronutrient deficiency: A review. Med. J. Malaysia 2004, 59, 284–293. [Google Scholar] [PubMed]
- Aira, N.; Andersson, A.M.; Singh, S.K.; McKay, D.M.; Blomgran, R. Species dependent impact of helminth-derived antigens on human macrophages infected with Mycobacterium tuberculosis: Direct effect on the innate anti-mycobacterial response. PLoS Negl. Trop. Dis. 2017, 11, e0005390. [Google Scholar] [CrossRef] [Green Version]
- Erb, K.J.; Trujillo, C.; Fugate, M.; Moll, H. Infection with the helminth Nippostrongylus brasiliensis does not interfere with efficient elimination of Mycobacterium bovis BCG from the lungs of mice. Clin. Diagn. Lab. Immunol. 2002, 9, 727–730. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, N.; Kleynhans, L.; Thiart, L.; Van Helden, P.D.; Brombacher, F.; Horsnell, W.G.C.; Walzl, G. Acute helminth infection enhances early macrophage mediated control of mycobacterial infection. Mucosal Immunol. 2013, 6, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Potian, J.A.; Rafi, W.; Bhatt, K.; McBride, A.; Gause, W.C.; Salgame, P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 2011, 208, 1863–1874. [Google Scholar] [CrossRef]
- van Soelen, N.; Mandalakas, A.M.; Kirchner, H.L.; Walzl, G.; Grewal, H.M.; Jacobsen, M.; Hesseling, A.C. Effect of Ascaris Lumbricoides specific IgE on tuberculin skin test responses in children in a high-burden setting: A cross-sectional community-based study. BMC Infect. Dis. 2012, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Van der Zalm, M.M.; Van Soelen, N.; Mandalakas, A.M.; Jacobsen, M.; Detjen, A.K.; Marx, F.M.; Grewal, H.M.S.; Cotton, M.F.; Walzl, G.; Hesseling, A.C.; et al. The effect of deworming on tests of tuberculosis infection in children with recent tuberculosis exposure a randomized controlled trial. Pediatr. Infect. Dis. J. 2016, 35, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.M.; Singh, R.; Mkhize-Kwitshana, Z.L. Demographic profile of HIV and helminth-coinfected adults in KwaZulu-Natal, South Africa. S. Afr. J. Infect. Dis. 2023, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Herbst, S.; Schaible, U.E.; Schneider, B.E. Interferon Gamma Activated Macrophages Kill Mycobacteria by Nitric Oxide Induced Apoptosis. PLoS ONE 2011, 6, 19105. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pract. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, J.C.J.; Flynn, J.L.; Kirschner, D.E. Synergy between individual TNF-dependent functions determines granuloma performance for controlling Mycobacterium tuberculosis infection. J. Immunol. 2009, 182, 3706–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The Immune Response in Tuberculosis. Ann. Rev. Immunol. 2013, 31, 1. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Banurekha, V.V.; Nair, D.; Babu, S. Plasma Proinflammatory Cytokines Are Markers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. Open Forum Infect. Dis. 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, A.C.; van Daalen, K.R.; Crnko, S.; ten Broeke, T.; Bovenschen, N. Intracellular and Extracellular Roles of Granzyme K. Front. Immunol. 2021, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef]
- Rollings, C.M.; Sinclair, L.V.; Brady, H.J.M.; Cantrell, D.A.; Ross, S.H. Interleukin-2 shapes the cytotoxic T cell proteome and immune environment–sensing programs. Sci. Signal. 2018, 11, 526. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Lin, J.-X.; Leonard, W.J.; Hiscott, J.; Ware, C. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation This review comes from a themed issue on Cytokines Edited by. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Tan, Y.; Li, J.; Hu, P.; Guan, P.; Kuang, H.; Liang, Q.; Yu, Y.; Chen, Z.; Wang, Q.; et al. Combined IFN-γ and IL-2 release assay for detect active pulmonary tuberculosis: A prospective multicentre diagnostic study in China. J. Transl. Med. 2021, 19, 289. [Google Scholar] [CrossRef]
- Shimizu, K.; Sato, Y.; Kawamura, M.; Nakazato, H.; Watanabe, T.; Ohara, O.; Fujii, S. ichiro Eomes transcription factor is required for the development and differentiation of invariant NKT cells. Commun. Biol. 2019, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Torrado, E.; Cooper, A.M. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010, 21, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Verhagen, L.M.; Hermans, P.W.M.; Warris, A.; De Groot, R.; Maes, M.; Villalba, J.A.; Del Nogal, B.; Van Den Hof, S.; Mughini Gras, L.; Van Soolingen, D.; et al. Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis 2012, 92, 505–512. [Google Scholar] [CrossRef]
- Ustun, S.; Turgay, N.; Delibas, S.B.; Ertabaklar, H. Interleukin (IL) 5 levels and eosinophilia in patients with intestinal parasitic diseases. World J. Gastroenterol. 2004, 10, 86–96. [Google Scholar] [CrossRef]
- Walsh, K.P.; Brady, M.T.; Finlay, C.M.; Boon, L.; Mills, K.H.G. Infection with a Helminth Parasite Attenuates Autoimmunity through TGF-β-Mediated Suppression of Th17 and Th1 Responses. J. Immunol. 2009, 183, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Maizels, R.M.; Balic, A.; Gomez-Escobar, N.; Nair, M.; Taylor, M.D.; Allen, J.E. Helminth parasites-masters of regulation. Immunol. Rev. 2004, 201, 89–116. [Google Scholar] [CrossRef]




| Abbreviation | Definition |
|---|---|
| ATCC | American Type Culture Collection |
| A. lumbricoides | Ascaris lumbricoides |
| BCG | Bacille Calmette-Guerin |
| Eomes | Eomesodermin |
| ESP | Excretory-secretory protein |
| FoxP3 | Forkhead box P3 |
| GAPDH | Glyceraldehyde 3 diphosphate dehydrogenase |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| MHC | Major histocompatibility complex |
| Mtb | Mycobacterium tuberculosis |
| Nb | Nippostrongylus brasiliensis |
| NFATC2 | Nuclear factor of activated T-cells |
| OADC | Oleic acid albumin dextrose catalase enrichment |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SA | South Africa |
| TGF-β | Transforming growth factor-beta |
| Th1 | T-helper type 1 |
| Th2 | T-helper type 2 |
| TNF-α | Tumour necrosis factor-alpha |
| Treg | Regulatory T cells |
| Cytokine Gene | Thermofisher Catalogue Number |
|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (housekeeping gene) | Hs99999905_m1 |
| Interferon-gamma (INF-γ) | Hs00989291_m1 |
| Tumour necrosis factor-alpha (TNF-α) | Hs00174128_m1 |
| Granzyme B | Hs00188051_m1 |
| Perforin | Hs00169473_m1 |
| Interleukin-2 (IL-2) | Hs00174114_m1 |
| Interleukin-17 (IL-17) | Hs01056316_m1 |
| Nuclear factor of activated T-cells 2 (NFATC2) | Hs00905451_m1 |
| Eomesodermin (Eomes) | Hs00172872_m1 |
| Interleukin-4 (IL-4) | Hs00174122_m1 |
| Interleukin-5 (IL-5) | Hs99999031_m1 |
| Interleukin-10 (IL-10) | Hs00961622_m1 |
| Transforming growth factor beta (TGF-β) | Hs00234244_ml |
| Forkhead box P3 (FoxP3) | Hs01085834_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhengu, K.N.; Singh, R.; Naidoo, P.; Mpaka-Mbatha, M.N.; Nembe-Mafa, N.; Mkhize-Kwitshana, Z.L. Cytokine Responses during Mycobacterium tuberculosis H37Rv and Ascaris lumbricoides Costimulation Using Human THP-1 and Jurkat Cells, and a Pilot Human Tuberculosis and Helminth Coinfection Study. Microorganisms 2023, 11, 1846. https://doi.org/10.3390/microorganisms11071846
Bhengu KN, Singh R, Naidoo P, Mpaka-Mbatha MN, Nembe-Mafa N, Mkhize-Kwitshana ZL. Cytokine Responses during Mycobacterium tuberculosis H37Rv and Ascaris lumbricoides Costimulation Using Human THP-1 and Jurkat Cells, and a Pilot Human Tuberculosis and Helminth Coinfection Study. Microorganisms. 2023; 11(7):1846. https://doi.org/10.3390/microorganisms11071846
Chicago/Turabian StyleBhengu, Khethiwe N., Ravesh Singh, Pragalathan Naidoo, Miranda N. Mpaka-Mbatha, Nomzamo Nembe-Mafa, and Zilungile L. Mkhize-Kwitshana. 2023. "Cytokine Responses during Mycobacterium tuberculosis H37Rv and Ascaris lumbricoides Costimulation Using Human THP-1 and Jurkat Cells, and a Pilot Human Tuberculosis and Helminth Coinfection Study" Microorganisms 11, no. 7: 1846. https://doi.org/10.3390/microorganisms11071846






