Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products
Abstract
:1. Introduction
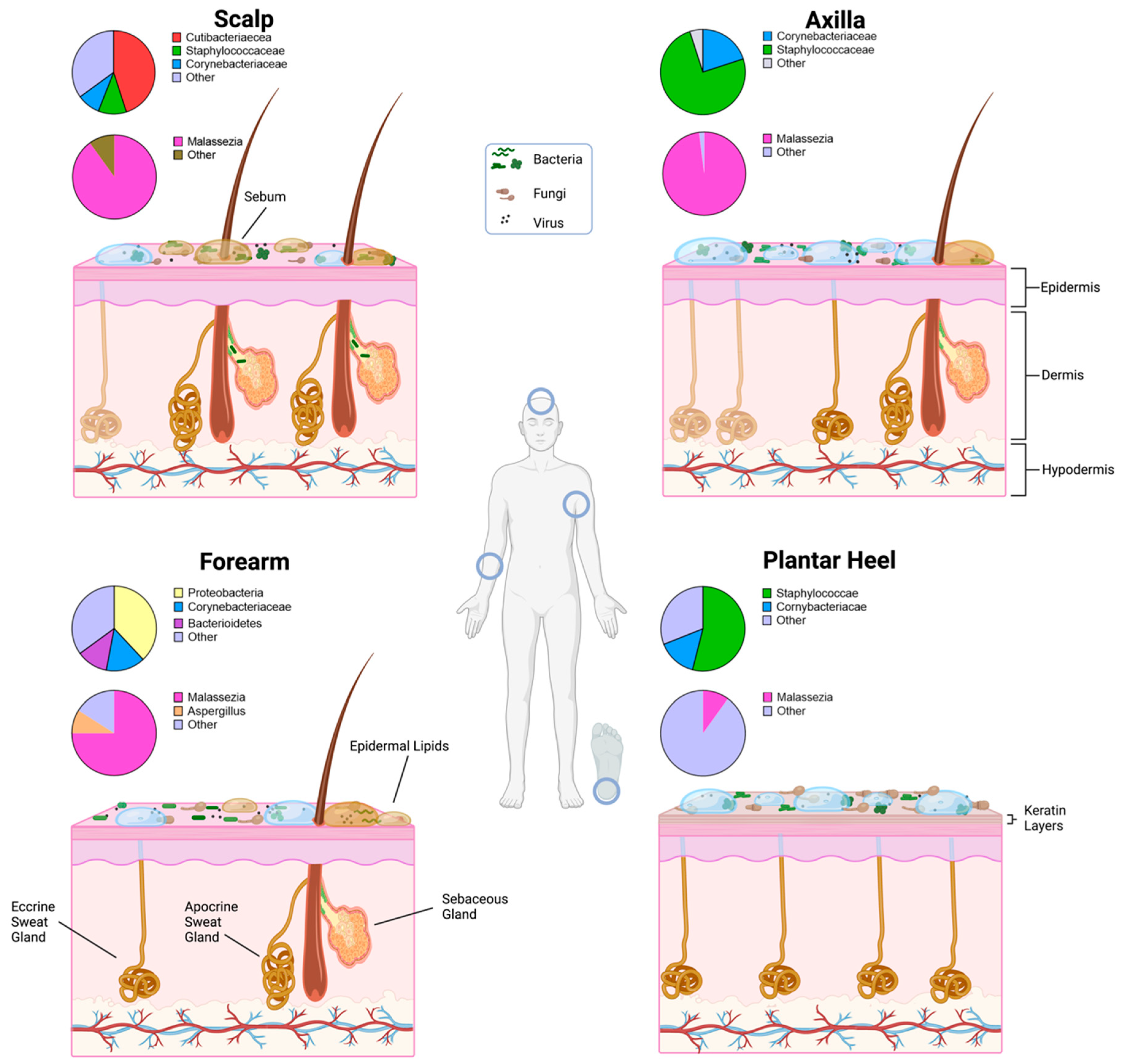
2. Skin Environment
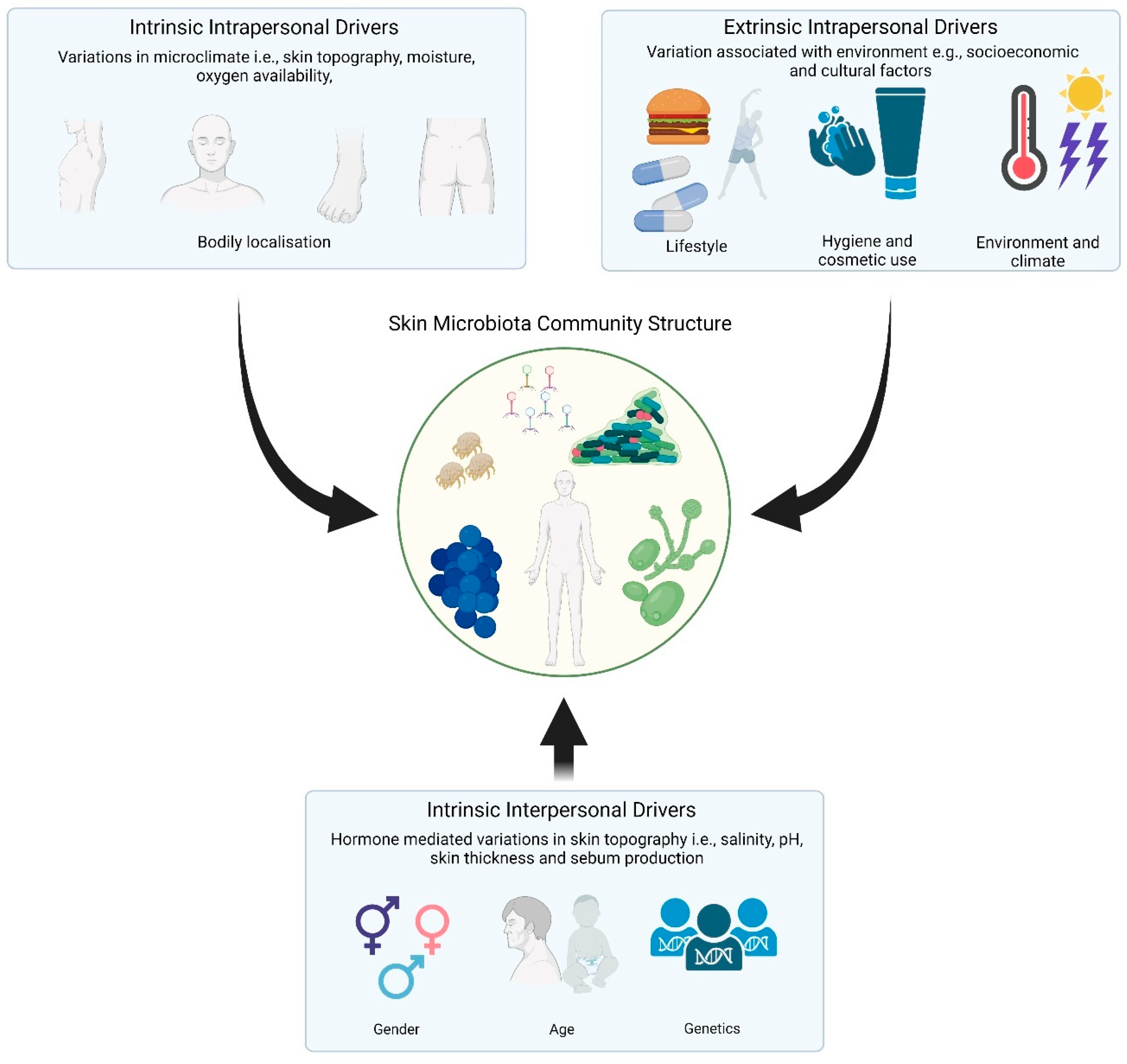
3. Drivers of Skin Microbiome Community Structure
4. Pathogenic Invasion and Community Disruption
5. Therapeutically Relevant Skin-Microbiome-Derived Compounds
5.1. Bacteriocins
5.2. Bacteriophages
5.3. Cutaneous Lipids
5.4. Biofilm Inhibitors
5.5. Quorum Sensing Modulators
5.6. Fungicidal Compounds
5.7. Skin Cancer Treatments
6. Skin Microbiome Applications in Personal Care Products
6.1. Acne Vulgaris
6.2. Atopic Dermatitis
6.3. Anti-Ageing
6.4. Skin Rejuvenation
6.5. Moisturisers
6.6. Cutaneous Hyperpigmentation
6.7. Rosacea
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in Exploring and Manipulating the Human Skin Microbiome. Microbiome 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef] [PubMed]
- Gorter, F.A.; Manhart, M.; Ackermann, M. Understanding the Evolution of Interspecies Interactions in Microbial Communities. Philos. Trans. R. Soc. B 2020, 375, 20190256. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug Discovery and Development: Role of Basic Biological Research. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 651. [Google Scholar] [CrossRef]
- Thompson, T. The Staggering Death Toll of Drug-Resistant Bacteria. Nature 2022. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage Therapy: From Biological Mechanisms to Future Directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial Psoriasin (S100A7) Protects Human Skin from Escherichia coli Infection. Nat. Immunol. 2004, 6, 57–64. [Google Scholar] [CrossRef]
- Moran, J.C.; Alorabi, J.A.; Horsburgh, M.J. Comparative Transcriptomics Reveals Discrete Survival Responses of S. aureus and S. epidermidis to Sapienic Acid. Front. Microbiol. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjana; Tiwari, S.K. Bacteriocin-Producing Probiotic Lactic Acid Bacteria in Controlling Dysbiosis of the Gut Microbiota. Front. Cell Infect. Microbiol. 2022, 12, 415. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Azcarate-Peril, M.A. Emerging Technologies for Gut Microbiome Research. Trends Microbiol. 2016, 24, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.H.; Segre, J.A. Skin Microbiome: Looking Back to Move Forward. J. Investig. Dermatol. 2012, 132, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrieri, A.P.; Haiminen, N.; Maudsley-Barton, S.; Gardiner, L.J.; Murphy, B.; Mayes, A.E.; Paterson, S.; Grimshaw, S.; Winn, M.; Shand, C.; et al. Explainable AI Reveals Changes in Skin Microbiome Composition Linked to Phenotypic Differences. Sci. Rep. 2021, 11, 4565. [Google Scholar] [CrossRef]
- Yu, J.; Ma, X.; Wang, X.; Cui, X.; Ding, K.; Wang, S.; Han, C. Application and Mechanism of Probiotics in Skin Care: A Review. J. Cosmet. Dermatol. 2022, 21, 886–894. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464/ (accessed on 19 June 2023).
- Kim, J.Y.; Dao, H. Physiology, Integument; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554386/ (accessed on 19 June 2023).
- Meisel, J.S.; Sfyroera, G.; Bartow-McKenney, C.; Gimblet, C.; Bugayev, J.; Horwinski, J.; Kim, B.; Brestoff, J.R.; Tyldsley, A.S.; Zheng, Q.; et al. Commensal Microbiota Modulate Gene Expression in the Skin. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Fischer, H.; Fumicz, J.; Rossiter, H.; Napirei, M.; Buchberger, M.; Tschachler, E.; Eckhart, L. Holocrine Secretion of Sebum Is a Unique DNase2-Dependent Mode of Programmed Cell Death. J. Investig. Dermatol. 2017, 137, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Atsugi, T.; Yokouchi, M.; Hirano, T.; Hirabayashi, A.; Nagai, T.; Ohyama, M.; Abe, T.; Kaneko, M.; Zouboulis, C.C.; Amagai, M.; et al. Holocrine Secretion Occurs Outside the Tight Junction Barrier in Multicellular Glands: Lessons from Claudin-1-Deficient Mice. J. Investig. Dermatol. 2020, 140, 298–308.e5. [Google Scholar] [CrossRef] [PubMed]
- Freinkel, R.K. The Origin of Free Fatty Acids in Sebum. I. Role of Coagulase Negative Staphylococci. J. Investig. Dermatol. 1968, 50, 186–188. [Google Scholar] [CrossRef] [Green Version]
- Lacey, R.W. Loss of the Antibacterial Action of Skin After Topical Neomycin. Br. J. Dermatol. 1969, 81, 435–439. [Google Scholar] [CrossRef]
- Marples, R.R.; Kligman, A.M.; Lantis, L.R.; Downing, D.T. The Role of the Aerobic Microflora in the Genesis of Fatty Acids in Human Surface Lipids. J. Investig. Dermatol. 1970, 55, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinley, K.J.; Webster, G.F.; Ruggieri, M.R.; Leyden, J.J. Regional Variations in Density of Cutaneous Propionibacteria: Correlation of Propionibacterium acnes Populations with Sebaceous Secretion. J. Clin. Microbiol. 1980, 12, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.; Wells, G.C. On the Biogenesis of the Free Fatty Acids in Human Skin Surface Fat. J. Investig. Dermatol. 1957, 29, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Shalita, A.R. Genesis of Free Fatty Acids. J. Investig. Dermatol. 1974, 62, 332–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.E.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. The Fatty Acids of Human Sebaceous Gland Phosphatidylcholine. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1978, 529, 380–386. [Google Scholar] [CrossRef]
- Takigawa, H.; Nakagawa, H.; Kuzukawa, M.; Mori, H.; Imokawa, G. Deficient Production of Hexadecenoic Acid in the Skin Is Associated in Part with the Vulnerability of Atopic Dermatitis Patients to Colonization by Staphylococcus aureus. Dermatology 2005, 211, 240–248. [Google Scholar] [CrossRef]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-Immunobiology Is Orchestrated by Sebum Lipids. Derm.-Endocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef] [Green Version]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An Update on the Role of the Sebaceous Gland in the Pathogenesis of Acne. Derm.-Endocrinol. 2011, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Vietri Rudan, M.; Watt, F.M. Mammalian Epidermis: A Compendium of Lipid Functionality. Front. Physiol. 2022, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Mark, H.; Harding, C.R. Amino Acid Composition, Including Key Derivatives of Eccrine Sweat: Potential Biomarkers of Certain Atopic Skin Conditions. Int. J. Cosmet. Sci. 2013, 35, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Murota, H.; Matsui, S.; Ono, E.; Kijima, A.; Kikuta, J.; Ishii, M.; Katayama, I. Sweat, the Driving Force behind Normal Skin: An Emerging Perspective on Functional Biology and Regulatory Mechanisms. J. Dermatol. Sci. 2015, 77, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Becker, J.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Perez-Perez, G.I.; Chen, Y.; Blaser, M.J. Quantitation of Major Human Cutaneous Bacterial and Fungal Populations. J. Clin. Microbiol. 2010, 48, 3575. [Google Scholar] [CrossRef] [Green Version]
- Howard, B.; Bascom, C.C.; Hu, P.; Binder, R.L.; Fadayel, G.; Huggins, T.G.; Jarrold, B.B.; Osborne, R.; Rocchetta, H.L.; Swift, D.; et al. Aging-Associated Changes in the Adult Human Skin Microbiome and the Host Factors That Affect Skin Microbiome Composition. J. Investig. Dermatol. 2022, 142, 1934–1946.e21. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the Skin Microbiota in Health and Disease. Semin. Immunol. 2013, 25, 370. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Voisin, B.; Kim, D.Y.; Kennedy, E.A.; Jo, J.H.; Shih, H.Y.; Truong, A.; Doebel, T.; Sakamoto, K.; Cui, C.Y.; et al. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 2019, 176, 982. [Google Scholar] [CrossRef] [Green Version]
- Swaney, M.H.; Sandstrom, S.; Kalan, L.R. Cobamide Sharing Is Predicted in the Human Skin Microbiome. mSystems 2022, 7, e00677-22. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Bauza-kaszewska, J.; Kraszewska, Z.; Wiktorczyk-kapischke, N.; Grudlewska-buda, K.; Kwiecińska-piróg, J.; Wałecka-zacharska, E.; Radtke, L.; Gospodarek-komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, C.; Satyamoorthy, K.; Murali, T.S. Microbial Interplay in Skin and Chronic Wounds. Curr. Clin. Microbiol. Rep. 2022, 9, 21–31. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Park, M.D.; Otto, M. Host Response to Staphylococcus epidermidis Colonization and Infections. Front. Cell Infect. Microbiol. 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Tabaja, H.; Tai, D.B.G.; Beam, E.; Abdel, M.P.; Tande, A.J. Clinical Profile of Monomicrobial Corynebacterium Hip and Knee Periprosthetic Joint Infections. Open Forum Infect. Dis. 2022, 9, ofac193. [Google Scholar] [CrossRef]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Clebak, K.T.; Malone, M.A. Skin Infections. Prim. Care 2018, 45, 433–454. [Google Scholar] [CrossRef]
- Gunaydin, S.D.; Arikan-Akdagli, S.; Akova, M. Fungal Infections of the Skin and Soft Tissue. Curr. Opin. Infect. Dis. 2020, 33, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.A.; Abbade, L.P.F. Severe Bacterial Skin Infections. An. Bras. Dermatol. 2020, 95, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Harkins, C.P.; Schwardt, N.H.; Portillo, J.A.; Zimmerman, M.D.; Carter, C.L.; Hossen, M.A.; Peer, C.J.; Polley, E.C.; Dartois, V.; et al. Alterations of Human Skin Microbiome and Expansion of Antimicrobial Resistance after Systemic Antibiotics. Sci. Transl. Med. 2021, 13, eabd8077. [Google Scholar] [CrossRef]
- Wozniak, J.M.; Mills, R.H.; Olson, J.; Caldera, J.R.; Sepich-Poore, G.D.; Carrillo-Terrazas, M.; Tsai, C.M.; Vargas, F.; Knight, R.; Dorrestein, P.C.; et al. Mortality Risk Profiling of Staphylococcus aureus Bacteremia by Multi-Omic Serum Analysis Reveals Early Predictive and Pathogenic Signatures. Cell 2020, 182, 1311–1327.e14. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front. Microbiol. 2021, 12, 677. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Telhig, S.; Ben Said, L.; Zirah, S.; Fliss, I.; Rebuffat, S. Bacteriocins to Thwart Bacterial Resistance in Gram Negative Bacteria. Front. Microbiol. 2020, 11, 2807. [Google Scholar] [CrossRef]
- Acedo, J.Z.; Chiorean, S.; Vederas, J.C.; van Belkum, M.J. The Expanding Structural Variety among Bacteriocins from Gram-Positive Bacteria. FEMS Microbiol. Rev. 2018, 42, 805–828. [Google Scholar] [CrossRef]
- Grada, A.; Bunick, C.G. Spectrum of Antibiotic Activity and Its Relevance to the Microbiome. JAMA Netw. Open 2021, 4, e215357. [Google Scholar] [CrossRef]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-Associated Dysbiosis Affects the Ability of the Gut Microbiota to Control Intestinal Inflammation upon Fecal Microbiota Transplantation in Experimental Colitis Models. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Ross, R.P.; Hill, C. Bacteriocins and Bacteriophage; a Narrow-Minded Approach to Food and Gut Microbiology. FEMS Microbiol. Rev. 2017, 41, S129–S153. [Google Scholar] [CrossRef]
- Mirande, C.; Bizine, I.; Giannetti, A.; Picot, N.; van Belkum, A. Epidemiological Aspects of Healthcare-Associated Infections and Microbial Genomics. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 823–831. [Google Scholar] [CrossRef]
- Tozzo, P.; Delicati, A.; Caenazzo, L. Human Microbiome and Microbiota Identification for Preventing and Controlling Healthcare-Associated Infections: A Systematic Review. Front. Public Health 2022, 10, 989496. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Kim, S.H. Recent Biotechnological Trends in Lactic Acid Bacterial Fermentation for Food Processing Industries. Syst. Microbiol. Biomanuf. 2021, 2, 14–40. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Sarkar, T.; Pati, S.; Basu, D.; Abdul Kari, Z.; Wei, L.S.; Smaoui, S.; Wen Goh, K.; et al. Bacteriocin: A Natural Approach for Food Safety and Food Security. Front. Bioeng. Biotechnol. 2022, 10, 1978. [Google Scholar] [CrossRef]
- Gallo, G.; Renzone, G.; Palazzotto, E.; Monciardini, P.; Arena, S.; Faddetta, T.; Giardina, A.; Alduina, R.; Weber, T.; Sangiorgi, F.; et al. Elucidating the Molecular Physiology of Lantibiotic NAI-107 Production in Microbispora ATCC-PTA-5024. BMC Genom. 2016, 17, 42. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.N.; O’Connor, P.M.; Rea, M.C.; O’Sullivan, O.; Walsh, C.J.; Healy, B.; Mathur, H.; Field, D.; Hill, C.; Paul Ross, R. Nisin J, a Novel Natural Nisin Variant, Is Produced by Staphylococcus capitis Sourced from the Human Skin Microbiota. J. Bacteriol. 2020, 202, e00639-19. [Google Scholar] [CrossRef] [Green Version]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef]
- Janek, D.; Zipperer, A.; Kulik, A.; Krismer, B.; Peschel, A. High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors. PLoS Pathog. 2016, 12, e1005812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, C.C.; Zhang, W. Small Molecule Natural Products in Human Nasal/Oral Microbiota. J. Ind. Microbiol. Biotechnol. 2021, 48, 10. [Google Scholar] [CrossRef] [PubMed]
- Bitschar, K.; Sauer, B.; Focken, J.; Dehmer, H.; Moos, S.; Konnerth, M.; Schilling, N.A.; Grond, S.; Kalbacher, H.; Kurschus, F.C.; et al. Lugdunin Amplifies Innate Immune Responses in the Skin in Synergy with Host- and Microbiota-Derived Factors. Nat. Commun. 2019, 10, 2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, S.; Zipperer, A.; Wirtz, S.; Saur, J.; Konnerth, M.C.; Heilbronner, S.; Torres Salazar, B.O.; Grond, S.; Krismer, B.; Peschel, A. Secretion of and Self-Resistance to the Novel Fibupeptide Antimicrobial Lugdunin by Distinct Abc Transporters in Staphylococcus lugdunensis. Antimicrob. Agents Chemother. 2021, 65, e01734-20. [Google Scholar] [CrossRef] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular Aspects of Bacterial PH Sensing and Homeostasis. Nat. Rev. Microbiol. 2011, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Farha, M.A.; Verschoor, C.P.; Bowdish, D.; Brown, E.D. Collapsing the Proton Motive Force to Identify Synergistic Combinations against Staphylococcus aureus. Chem. Biol. 2013, 20, 1168–1178. [Google Scholar] [CrossRef] [Green Version]
- Shields, B.E.; Tschetter, A.J.; Wanat, K.A. Staphylococcus simulans: An Emerging Cutaneous Pathogen. JAAD Case Rep. 2016, 2, 428. [Google Scholar] [CrossRef]
- Power, N.; Calisti, G.; Price, F.; Watt, V.; Gamlin, W.; Dobson, L.E.; Ray, S.G. Staphylococcus simulans Endocarditis of Native Aortic and Mitral Valves. Case Report and Literature Review. Clin. Infect. Pract. 2020, 7–8, 100044. [Google Scholar] [CrossRef]
- Drobeniuc, A.; Traenkner, J.; Rebolledo, P.A.; Ghazaryan, V.; Rouphael, N. Staphylococcus simulans: A Rare Uropathogen. IDCases 2021, 25, e01202. [Google Scholar] [CrossRef]
- Jayakumar, J.; Kumar, V.A.; Biswas, L.; Biswas, R. Therapeutic Applications of Lysostaphin against Staphylococcus aureus. J. Appl. Microbiol. 2021, 131, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Brooks, S.A.; Eszterhas, S.; Heim, S.; Li, L.; Xiong, Y.Q.; Fang, Y.; Kirsch, J.R.; Verma, D.; Bailey-Kellogg, C.; et al. Globally Deimmunized Lysostaphin Evades Human Immune Surveillance and Enables Highly Efficacious Repeat Dosing. Sci. Adv. 2020, 6, eabb9011. [Google Scholar] [CrossRef] [PubMed]
- Blazanovic, K.; Zhao, H.; Choi, Y.; Li, W.; Salvat, R.S.; Osipovitch, D.C.; Fields, J.; Moise, L.; Berwin, B.L.; Fiering, S.N.; et al. Structure-Based Redesign of Lysostaphin Yields Potent Antistaphylococcal Enzymes That Evade Immune Cell Surveillance. Mol. Ther. Methods Clin. Dev. 2015, 2, 15021. [Google Scholar] [CrossRef]
- Tossavainen, H.; Raulinaitis, V.; Kauppinen, L.; Pentikäinen, U.; Maaheimo, H.; Permi, P. Structural and Functional Insights into Lysostaphin-Substrate Interaction. Front. Mol. Biosci. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V.; De Ruiz Holgado, A.P.; De Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 Inhibits Late Stages of HSV-1 and HSV-2 Replication in Vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Medina Amado, C.; Minahk, C.J.; Cilli, E.; Oliveira, R.G.; Dupuy, F.G. Bacteriocin Enterocin CRL35 Is a Modular Peptide That Induces Non-Bilayer States in Bacterial Model Membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183135. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Farizano, J.V.; Díaz Vergara, L.I.; Masias, E.; Baillo, A.A.; Torino, M.I.; Fadda, S.; Vanden Braber, N.L.; Montenegro, M.A.; Saavedra, L.; Minahk, C. Biotechnological Use of Dairy By-Products for the Production and Microencapsulation of the Food Preservative Enterocin CRL35. FEMS Microbiol. Lett. 2022, 369, fnac033. [Google Scholar] [CrossRef]
- Huang, F.; Teng, K.; Liu, Y.; Cao, Y.; Wang, T.; Ma, C.; Zhang, J.; Zhong, J. Bacteriocins: Potential for Human Health. Oxid. Med. Cell Longev. 2021, 2021, 5518825. [Google Scholar] [CrossRef]
- Quintana, V.M.; Torres, N.I.; Wachsman, M.B.; Sinko, P.J.; Castilla, V.; Chikindas, M. Anti-Herpes Simplex Virus Type 2 Activity of the Antimicrobial Peptide Subtilosin. J. Appl. Microbiol. 2014, 117, 1253. [Google Scholar] [CrossRef] [Green Version]
- Alajlani, M.M. Characterization of Subtilosin Gene in Wild Type Bacillus spp. and Possible Physiological Role. Sci. Rep. 2022, 12, 10521. [Google Scholar] [CrossRef]
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between Bacterial and Phage Communities in Natural Environments. Nat. Rev. Microbiol. 2021, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The Gut Virome: A New Microbiome Component in Health and Disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Nagata, N.; Kiguchi, Y.; Kojima, Y.; Miyoshi-Akiyama, T.; Kimura, M.; Ohsugi, M.; Ueki, K.; Oka, S.; Mizokami, M.; et al. Extensive Gut Virome Variation and Its Associations with Host and Environmental Factors in a Population-Level Cohort. Nat. Commun. 2022, 13, 5252. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keen, E.C. A Century of Phage Research: Bacteriophages and the Shaping of Modern Biology. Bioessays 2015, 37, 6. [Google Scholar] [CrossRef] [Green Version]
- Kasman, L.M.; Porter, L.D. Bacteriophages. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 280–283. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S. Understanding Bacteriophage Specificity in Natural Microbial Communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef] [Green Version]
- Kortright, K.E.; Chan, B.K.; Turner, P.E. High-Throughput Discovery of Phage Receptors Using Transposon Insertion Sequencing of Bacteria. Proc. Natl. Acad. Sci. USA 2020, 117, 18670–18679. [Google Scholar] [CrossRef]
- Dunne, M.; Prokhorov, N.S.; Loessner, M.J.; Leiman, P.G. Reprogramming Bacteriophage Host Range: Design Principles and Strategies for Engineering Receptor Binding Proteins This Review Comes from a Themed Issue on Nanobiotechnology-Phage Therapy. Curr. Opin. Biotechnol. 2021, 68, 272–281. [Google Scholar] [CrossRef]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic Engineering of Bacteriophages against Infectious Diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef]
- Yehl, K.; Lemire, S.; Yang, A.C.; Ando, H.; Mimee, M.; Torres, M.D.T.; de la Fuente-Nunez, C.; Lu, T.K. Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis. Cell 2019, 179, 459–469.e9. [Google Scholar] [CrossRef]
- Little, J.S.; Dedrick, R.M.; Freeman, K.G.; Cristinziano, M.; Smith, B.E.; Benson, C.A.; Jhaveri, T.A.; Baden, L.R.; Solomon, D.A.; Hatfull, G.F. Bacteriophage Treatment of Disseminated Cutaneous Mycobacterium chelonae Infection. Nat. Commun. 2022, 13, 2313. [Google Scholar] [CrossRef]
- Wang, J.; Meng, W.; Zhang, K.; Wang, J.; Lu, B.; Wang, R.; Jia, K. Topically Applied Bacteriophage to Control Multi-Drug Resistant Pseudomonas aeruginosa-Infected Wounds in a New Zealand Rabbit Model. Front. Microbiol. 2022, 13, 4171. [Google Scholar] [CrossRef]
- Golembo, M.; Puttagunta, S.; Rappo, U.; Weinstock, E.; Engelstein, R.; Gahali-Sass, I.; Moses, A.; Kario, E.; Ben-Dor Cohen, E.; Nicenboim, J.; et al. Development of a Topical Bacteriophage Gel Targeting Cutibacterium acnes for Acne Prone Skin and Results of a Phase 1 Cosmetic Randomized Clinical Trial. Ski. Health Dis. 2022, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.G.; Pitton, M.; Urholz, M.F.; Oberhaensli, S.; Bruggmann, R.; Leib, S.L.; Jakob, S.M.; Resch, G.; Que, Y.-A.; Cameron, D.R. Isolation and Characterization of Bacteriophages from the Human Skin Microbiome That Infect Staphylococcus epidermidis. FEMS Microbes 2022, 2, xtab003. [Google Scholar] [CrossRef] [PubMed]
- Shimamori, Y.; Mitsunaka, S.; Yamashita, H.; Suzuki, T.; Kitao, T.; Kubori, T.; Nagai, H.; Takeda, S.; Ando, H. Staphylococcal Phage in Combination with Staphylococcus epidermidis as a Potential Treatment for Staphylococcus aureus-Associated Atopic Dermatitis and Suppressor of Phage-Resistant Mutants. Viruses 2020, 13, 7. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Onsea, J.; Fintan Moriarty, T.; Pruidze, N.; Nadareishvili, L.; Dadiani, M.; Kutateladze, M.; Eliava, G. Bacteriophage Therapy for Human Musculoskeletal and Skin/Soft Tissue Infections. Clin. Microbiol. Infect. 2023, 29, 695–701. [Google Scholar] [CrossRef]
- Stacey, H.J.; De Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy: A Systematic Review of Clinical and Safety Trials. Antibiotics 2022, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Pina-Vaz, C.; Rodrigues, A.G. The Role of Phage Therapy in Burn Wound Infections Management: Advantages and Pitfalls. J. Burn. Care Res. 2022, 43, 336–342. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Verbanic, S.; Deacon, J.M.; Chen, I.A. The Chronic Wound Virome: Phage Diversity and Associations with Wounds and Healing Outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Khullar, L.; Harjai, K.; Chhibber, S. Therapeutic and Pro-Healing Potential of Advanced Wound Dressings Loaded with Bioactive Agents. Future Microbiol. 2023, 18, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Bacteriophages and the Microbiome in Dermatology: The Role of the Phageome and a Potential Therapeutic Strategy. Int. J. Mol. Sci. 2023, 24, 2695. [Google Scholar] [CrossRef]
- De Souza, C.M.; Tanir, T.; Orellana, M.; Escalante, A.; Koeris, M.S. Manufacturing Bacteriophages (Part 2 of 2): Formulation, Analytics and Quality Control Considerations. Pharmaceuticals 2021, 14, 895. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.L.; Jin, M. The Life Cycle Transitions of Temperate Phages: Regulating Factors and Potential Ecological Implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, Z.; Abedon, S.T. Diversity of Phage Infection Types and Associated Terminology: The Problem with ‘Lytic or Lysogenic’. FEMS Microbiol. Lett. 2016, 363, 47. [Google Scholar] [CrossRef] [Green Version]
- Venturini, C.; Fabijan, A.P.; Lubian, A.F.; Barbirz, S.; Iredell, J. Biological Foundations of Successful Bacteriophage Therapy. EMBO Mol. Med. 2022, 14, e12435. [Google Scholar] [CrossRef] [PubMed]
- Fillol-Salom, A.; Rostøl, J.T.; Ojiogu, A.D.; Chen, J.; Douce, G.; Humphrey, S.; Penadés, J.R. Bacteriophages Benefit from Mobilizing Pathogenicity Islands Encoding Immune Systems against Competitors. Cell 2022, 185, 3248–3262.e20. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; González, S.; Quiles-Puchalt, N.; Gutiérrez, D.; Penadés, J.R.; García, P.; Rodríguez, A. Lysogenization of Staphylococcus aureus RN450 by Phages Φ11 and Φ80α Leads to the Activation of the SigB Regulon. Sci. Rep. 2018, 8, 12662. [Google Scholar] [CrossRef]
- Guo, D.; Chen, J.; Zhao, X.; Luo, Y.; Jin, M.; Fan, F.; Park, C.; Yang, X.; Sun, C.; Yan, J.; et al. Genetic and Chemical Engineering of Phages for Controlling Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. Bacteriophage-Derived Endolysins Applied as Potent Biocontrol Agents to Enhance Food Safety. Microorganisms 2020, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Seité, S.; Aguilar, L.; Da Cruz, O.; Puech, J.; Frieling, J.; Demessant, A.L. Topical S. aureus-Targeting Endolysin Significantly Improves Symptoms and QoL in Individuals with Atopic Dermatitis. J. Drugs Dermatol. 2021, 20, 1323–1328. [Google Scholar] [CrossRef]
- Townsend, E.C.; Kalan, L.R. The Dynamic Balance of the Skin Microbiome across the Lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Bomar, L.; Brugger, S.D.; Yost, B.H.; Davies, S.S.; Lemon, K.P. Corynebacterium accolens Releases Antipneumococcal Free Fatty Acids from Human Nostril and Skin Surface Triacylglycerols. mBio 2016, 7, e01725-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhao, Q.; Zhong, Q.; Cheng, D.; Krutmann, J.; Wang, J.; Xia, J. Skin Microbiome, Metabolome and Skin Phenome, from the Perspectives of Skin as an Ecosystem. Phenomics 2022, 2, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, A.; Sabin, R.; Harding, C.; Watkinson, A.; Banks, J.; Ackerman, C. The Effect of Glycerol and Humidity on Desmosome Degradation in Stratum Corneum. Arch. Dermatol. Res. 1995, 287, 457–464. [Google Scholar] [CrossRef]
- Atrux-Tallau, N.; Romagny, C.; Padois, K.; Denis, A.; Haftek, M.; Falson, F.; Pirot, F.; Maibach, H.I. Effects of Glycerol on Human Skin Damaged by Acute Sodium Lauryl Sulphate Treatment. Arch. Dermatol. Res. 2010, 302, 435–441. [Google Scholar] [CrossRef]
- Salgaonkar, N.; Kadamkode, V.; Kumaran, S.; Mallemala, P.; Christy, E.; Appavoo, S.; Majumdar, A.; Mitra, R.; Dasgupta, A. Glycerol Fermentation by Skin Bacteria Generates Lactic Acid and Upregulates the Expression Levels of Genes Associated with the Skin Barrier Function. Exp. Dermatol. 2022, 31, 1364–1372. [Google Scholar] [CrossRef]
- Chen, Y.; Moran, J.C.; Campbell-Lee, S.; Horsburgh, M.J. Transcriptomic Responses and Survival Mechanisms of Staphylococci to the Antimicrobial Skin Lipid Sphingosine. Antimicrob. Agents Chemother. 2022, 66, e00569-21. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Hajime, M.; Tamura, E.; Hagihara, H.; Takigawa, H.; Araki, H.; Hajime, M.; Tamura, E.; Hagihara, H.; Takigawa, H. Human Sebaceous Cis-6-Hexadecenoic Acid: Possible Application of an Innate Antimicrobial Substance to Cosmetic Products for Mucous Membrane. J. Cosmet. Dermatol. Sci. Appl. 2017, 7, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial Biofilms and the Human Skin Microbiome. npj Biofilms Microbiomes 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenico, E.G.D.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the Cutaneous Microbiota Biofilms in the Pathogenesis of Atopic Dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, T.; Stevens, M.L.; Baatyrbek, A.K.; Alarcon, R.; He, H.; Kroner, J.W.; Spagna, D.; Grashel, B.; Sidler, E.; Martin, L.J.; et al. Biofilm Propensity of Staphylococcus aureus Skin Isolates Is Associated with Increased Atopic Dermatitis Severity and Barrier Dysfunction in the MPAACH Pediatric Cohort. Allergy 2021, 76, 302. [Google Scholar] [CrossRef]
- Kuehnast, T.; Cakar, F.; Weinhäupl, T.; Pilz, A.; Selak, S.; Schmidt, M.A.; Rüter, C.; Schild, S. Comparative Analyses of Biofilm Formation among Different Cutibacterium acnes Isolates. Int. J. Med. Microbiol. 2018, 308, 1027–1035. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, W.J.; Ren, Z.; Han, P. The Role of Staphylococcal Biofilm on the Surface of Implants in Orthopedic Infection. Microorganisms 2022, 10, 1909. [Google Scholar] [CrossRef]
- Garcia, D.; Mayfield, C.K.; Leong, J.; Deckey, D.G.; Zega, A.; Glasser, J.; Daniels, A.H.; Eberson, C.; Green, A.; Born, C. Early Adherence and Biofilm Formation of Cutibacterium acnes (Formerly Propionibacterium acnes) on Spinal Implant Materials. Spine J. 2020, 20, 981–987. [Google Scholar] [CrossRef]
- Nowrouzian, F.L.; Ljung, A.; Hesselmar, B.; Nilsson, S.; Adlerberth, I.; Wolda, A.E. Bacterial Carriage of Genes Encoding Fibronectin-Binding Proteins Is Associated with Long-Term Persistence of Staphylococcus aureus in the Nasal and Gut Microbiota of Infants. Appl. Environ. Microbiol. 2021, 87, 1–14. [Google Scholar] [CrossRef]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.I.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Degrades Specific Proteins Associated with Staphylococcus aureus Biofilm Formation and Host-Pathogen Interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Inhibits Staphylococcus aureus Biofilm Formation and Nasal Colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R. Antibiofilm Activity of Small Molecules Produced by Staphylococcus epidermidis against Staphylococcus aureus. Appl. Environ. Microbiol. 2020, 86, e00627-20. [Google Scholar] [CrossRef] [PubMed]
- Glatthardt, T.; Curityba de Mello Campos, J.; Chamon, R.C.; Freitas de Sá Coimbra, T.; de Almeida Rocha, G.; Figueira de Melo, M.A.; Parente, T.E.; Lobo, L.A.; Antunes, L.C.M.; dos Santos, K.R.N.; et al. Small Molecules Produced by Commensal Staphylococcus epidermidis Disrupt Formation of Biofilms by Staphylococcus aureus. Appl. Environ. Microbiol. 2020, 86, e02539-19. [Google Scholar] [CrossRef]
- Brooks, J.L.; Jefferson, K.K. Phase Variation of Poly-N-Acetylglucosamine Expression in Staphylococcus aureus. PLoS Pathog. 2014, 10, e1004292. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, B.J.; Kwon, A.R. The Grease Trap: Uncovering the Mechanism of the Hydrophobic Lid in Cutibacterium acnes Lipase. J. Lipid Res. 2020, 61, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.P.M.; Lood, R. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef]
- Huang, T.Y.; Jiang, Y.E.; Scott, D.A. Culturable Bacteria in the Entire Acne Lesion and Short-Chain Fatty Acid Metabolites of Cutibacterium acnes and Staphylococcus epidermidis Isolates. Biochem. Biophys. Res. Commun. 2022, 622, 45–49. [Google Scholar] [CrossRef]
- Sanford, J.A.; O’Neill, A.M.; Zouboulis, C.C.; Gallo, R.L. Short-Chain Fatty Acids from Cutibacterium acnes Activate Both a Canonical and Epigenetic Inflammatory Response in Human Sebocytes. J. Immunol. 2019, 202, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; O’Neill, A.M.; Williams, M.R.; Cau, L.; Nakatsuji, T.; Horswill, A.R.; Gallo, R.L. Short Chain Fatty Acids Produced by Cutibacterium acnes Inhibit Biofilm Formation by Staphylococcus epidermidis. Sci. Rep. 2020, 10, 21237. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019, 20, 4477. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The Role of Short-Chain Fatty Acids in Inflammatory Skin Diseases. Front. Microbiol. 2023, 13, 5417. [Google Scholar] [CrossRef] [PubMed]
- Scheuerl, T.; Hopkins, M.; Nowell, R.W.; Rivett, D.W.; Barraclough, T.G.; Bell, T. Bacterial Adaptation Is Constrained in Complex Communities. Nat. Commun. 2020, 11, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Abbamondi, G.R.; Tommonaro, G. Research Progress and Hopeful Strategies of Application of Quorum Sensing in Food, Agriculture and Nanomedicine. Microorganisms 2022, 10, 1192. [Google Scholar] [CrossRef]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum Sensing Pathways in Gram-Positive and -Negative Bacteria: Potential of Their Interruption in Abating Drug Resistance. J. Chem. 2019, 31, 161–187. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Chen, L.; Ku, L.; Li, M. Editorial: Frontiers in Bacterial Quorum Sensing Research. Front. Cell Infect. Microbiol. 2022, 12, 1293. [Google Scholar] [CrossRef]
- Grando, K.; Nicastro, L.K.; Tursi, S.A.; De Anda, J.; Lee, E.Y.; Wong, G.C.L.; Tükel, Ç. Phenol-Soluble Modulins from Staphylococcus aureus Biofilms Form Complexes with DNA to Drive Autoimmunity. Front. Cell Infect. Microbiol. 2022, 12, 517. [Google Scholar] [CrossRef]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between Viruses Guides Lysis–Lysogeny Decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and Promise of Bacterial Quorum Sensing Research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlet, C.P.; Brown, M.M.; Horswill, A.R. Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019, 27, 497. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.M.; Kwiecinski, J.M.; Cruz, L.M.; Shahbandi, A.; Todd, D.A.; Cech, N.B.; Horswill, A.R. Novel Peptide from Commensal Staphylococcus simulans Blocks Methicillin-Resistant Staphylococcus aureus Quorum Sensing and Protects Host Skin from Damage. Antimicrob. Agents Chemother. 2020, 64, e00172-20. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Williams, M.R.; Shahbandi, A.; Bunch, Z.L.; Lyon, L.M.; Nguyen, A.; Zaramela, L.S.; Todd, D.A.; Zengler, K.; Cech, N.B.; et al. The Ubiquitous Human Skin Commensal Staphylococcus hominis Protects against Opportunistic Pathogens. mBio 2022, 13, e00930-22. [Google Scholar] [CrossRef]
- Severn, M.M.; Cho, Y.S.K.; Manzer, H.S.; Bunch, Z.L.; Shahbandi, A.; Todd, D.A.; Cech, N.B.; Horswill, A.R. The Commensal Staphylococcus warneri Makes Peptide Inhibitors of MRSA Quorum Sensing That Protect Skin from Atopic or Necrotic Damage. J. Investig. Dermatol. 2022, 142, 3349–3352.e5. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Jayaraman, N.; Chatterji, D. Small-Molecule Inhibition of Bacterial Biofilm. ACS Omega 2020, 5, 3108–3115. [Google Scholar] [CrossRef]
- Balaban, N.; Goldkorn, T.; Gov, Y.; Hirshberg, M.; Koyfman, N.; Matthews, H.R.; Nhan, R.T.; Singh, B.; Uziel, O. Regulation of Staphylococcus aureus Pathogenesis via Target of RNAIII-Activating Protein (TRAP). J. Biol. Chem. 2001, 276, 2658–2667. [Google Scholar] [CrossRef] [Green Version]
- Ciulla, M.; Di Stefano, A.; Marinelli, L.; Cacciatore, I.; Di Biase, G. RNAIII Inhibiting Peptide (RIP) and Derivatives as Potential Tools for the Treatment of S. aureus Biofilm Infections. Curr. Top. Med. Chem. 2018, 18, 2068–2079. [Google Scholar] [CrossRef]
- Ye, H.; Wu, L.; Zhang, W.; Li, J.; Sun, X.; Chen, C.; Sun, Y.; Han, J. The Use of RNAIII Inhibitory Peptide in the Treatment of Elderly Patients with Staphylococcus aureus Infection and Construction of Fuzzy Mathematical Model for Curative Effect Evaluation. Results Phys. 2021, 25, 104323. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Ghiselli, R.; Goteri, G.; Scalise, A.; Orlando, F.; Silvestri, C.; Riva, A.; Saba, V.; Madanahally, K.D.; et al. RNAIII-Inhibiting Peptide Enhances Healing of Wounds Infected with Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2205–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimshaw, S.G.; Smith, A.M.; Arnold, D.S.; Xu, E.; Hoptroff, M.; Murphy, B. The Diversity and Abundance of Fungi and Bacteria on the Healthy and Dandruff Affected Human Scalp. PLoS ONE 2019, 14, e0225796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, C.; Schmid, B.; Buttafuoco, A.; Glatz, M.; Bosshard, P.P. In Vitro Efficacy of Antifungal Agents Alone and in Shampoo Formulation against Dandruff-Associated Malassezia spp. and Staphylococcus spp. Int. J. Cosmet. Sci. 2019, 41, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef] [PubMed]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE Pathogens in the Environment: Antibiotic Resistance Status, Community-Acquired Infection and Risk to Human Health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Ka-Wai Hui, E. Reasons for the Increase in Emerging and Re-Emerging Viral Infectious Diseases. Microbes Infect. 2006, 8, 905. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Saheb Kashaf, S.; Proctor, D.M.; Deming, C.; Saary, P.; Hölzer, M.; Mullikin, J.; Thomas, J.; Young, A.; Bouffard, G.; Barnabas, B.; et al. Integrating Cultivation and Metagenomics for a Multi-Kingdom View of Skin Microbiome Diversity and Functions. Nat. Microbiol. 2021, 7, 169–179. [Google Scholar] [CrossRef]
- Vijaya Chandra, S.H.; Srinivas, R.; Dawson, T.L.; Common, J.E. Cutaneous Malassezia: Commensal, Pathogen, or Protector? Front. Cell Infect. Microbiol. 2020, 10, 1. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Mexia, N.; Melliou, E.; Efstratiou, M.A.; Bassukas, I.D.; Velegraki, A. Antifungal Activity of Selected Malassezia Indolic Compounds Detected in Culture. Mycoses 2019, 62, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparber, F.; LeibundGut-Landmann, S. Host Responses to Malassezia spp. in the Mammalian Skin. Front. Immunol. 2017, 8, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparber, F.; De Gregorio, C.; Steckholzer, S.; Ferreira, F.M.; Dolowschiak, T.; Ruchti, F.; Kirchner, F.R.; Mertens, S.; Prinz, I.; Joller, N.; et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response That Coordinates Anti-Fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe 2019, 25, 389–403.e6. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal–Dendritic-Cell Interaction Specifies a Unique Protective Skin Immune Signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Götz, F.; Peschel, A.; Schaller, M.; Schittek, B. Skin Commensals Amplify the Innate Immune Response to Pathogens by Activation of Distinct Signaling Pathways. J. Investig. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida Albicans with Host Cells: Virulence Factors, Host Defense, Escape Strategies, and the Microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V. Candida Albicans Hyphae: From Growth Initiation to Invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. The Cervicovaginal Mucus Barrier. Int. J. Mol. Sci. 2020, 21, 8266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, S.; Wu, C.; Gu, F.; Yang, Y. Probiotics: Potential Novel Therapeutics Against Fungal Infections. Front. Cell Infect. Microbiol. 2021, 11, 793419. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int. J. Mol. Sci. 2022, 23, 1813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qureshi, A.A.; Fortner, R.T.; Hankinson, S.E.; Wei, Q.; Wang, L.E.; Eliassen, A.H.; Willett, W.C.; Hunter, D.J.; Han, J. Teenage Acne and Cancer Risk in US Women: A Prospective Cohort Study. Cancer 2015, 121, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, H.; He, J.; Yang, L.; Zhou, X.; Li, Z.; Zhou, H.; Zhao, H.; Li, Y. Atopic Dermatitis and Skin Cancer Risk: A Systematic Review. Dermatol. Ther. 2022, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Butrón-Bris, B.; Daudén, E.; Rodríguez-Jiménez, P. Psoriasis Therapy and Skin Cancer: A Review. Life 2021, 11, 1109. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, M.; Danby, F.W.; Han, J.; Qureshi, A.A. Personal History of Rosacea and Risk of Incident Cancer among Women in the US. Br. J. Cancer 2015, 113, 520. [Google Scholar] [CrossRef] [Green Version]
- Mercier-Parot, L.T.-D.H. Skeletal Malformations in Rats Produced by 6-Hydroxylaminopurine. Attempts at Prevention. C. R. Seances Soc. Biol. Fil. 1973, 167, 5–10. [Google Scholar]
- Stepchenkova, E.I.; Kozmin, S.G.; Alenin, V.V.; Pavlov, Y.I. Genome-Wide Screening for Genes Whose Deletions Confer Sensitivity to Mutagenic Purine Base Analogs in Yeast. BMC Genet. 2005, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A Commensal Strain of Staphylococcus epidermidis Protects against Skin Neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuji, T.; Fenical, W.; Gallo, R.L. Response to Comment on “A Commensal Strain of Staphylococcus epidermidis Protects against Skin Neoplasia” by Nakatsuji et Al. Sci. Adv. 2019, 5, 5611–5622. [Google Scholar] [CrossRef] [Green Version]
- Kozmin, S.G.; Rogozin, I.B.; Moore, E.A.; Abney, M.; Schaaper, R.M.; Pavlov, Y.I. Comment on “A Commensal Strain of Staphylococcus epidermidis Protects against Skin Neoplasia” by Nakatsuji et Al. Sci. Adv. 2019, 5, 3915–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Li, X.; Huang, W.; Rao, X.; Lai, Y. Pharmacological Properties of Indirubin and Its Derivatives. Biomed. Pharmacother. 2022, 151, 113112. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Molujin, A.M.; Abbasiliasi, S.; Nurdin, A.; Lee, P.C.; Gansau, J.A.; Jawan, R. Bacteriocins as Potential Therapeutic Approaches in the Treatment of Various Cancers: A Review of In Vitro Studies. Cancers 2022, 14, 4758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tiwari, S.K. Bacteriocins of Probiotics as Potent Anticancer Agents. In Probiotic Research in Therapeutics; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1, pp. 231–250. [Google Scholar] [CrossRef]
- Tran, N.B.V.; Truong, Q.M.; Nguyen, L.Q.A.; Nguyen, N.M.H.; Tran, Q.H.; Dinh, T.T.P.; Hua, V.S.; Nguyen, V.D.; Lambert, P.A.; Nguyen, T.T.H. Prevalence and Virulence of Commensal Pseudomonas aeruginosa Isolates from Healthy Individuals in Southern Vietnam (2018–2020). Biomedicines 2022, 11, 54. [Google Scholar] [CrossRef]
- Zgheib, H.; Drider, D.; Belguesmia, Y. Broadening and Enhancing Bacteriocins Activities by Association with Bioactive Substances. Int. J. Environ. Res. Public Health 2020, 17, 7835. [Google Scholar] [CrossRef]
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Murphy, B.; Grimshaw, S.; Hoptroff, M.; Paterson, S.; Arnold, D.; Cawley, A.; Adams, S.E.; Falciani, F.; Dadd, T.; Eccles, R.; et al. Alteration of Barrier Properties, Stratum Corneum Ceramides and Microbiome Composition in Response to Lotion Application on Cosmetic Dry Skin. Sci. Rep. 2022, 12, 5223. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P.P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062. [Google Scholar] [CrossRef] [Green Version]
- Roux, P.F.; Oddos, T.; Stamatas, G. Deciphering the Role of Skin Surface Microbiome in Skin Health: An Integrative Multiomics Approach Reveals Three Distinct Metabolite–Microbe Clusters. J. Investig. Dermatol. 2022, 142, 469–479.e5. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.K.C.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to Manage Acne vulgaris. Drugs Context 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Mavranezouli, I.; Welton, N.J.; Daly, C.H.; Wilcock, J.; Bromham, N.; Berg, L.; Xu, J.; Wood, D.; Ravenscroft, J.C.; Dworzynski, K.; et al. Cost-effectiveness of Topical Pharmacological, Oral Pharmacological, Physical and Combined Treatments for Acne vulgaris. Clin. Exp. Dermatol. 2022, 47, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, A.H.; Masood, S.; Schlessinger, J. Acne Vulgari; StatePearls Publishing: Treasure Island, FL USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459173/ (accessed on 19 June 2023).
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The Potential of Probiotics for Treating Acne vulgaris: A Review of Literature on Acne and Microbiota. Dermatol. Ther. 2020, 33, e13279. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial Activity of Enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the Causative Agent in Acne vulgaris, and Its Therapeutic Effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological Effect of a Probiotic on Skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar]
- Lebeer, S.; Oerlemans, E.F.M.; Claes, I.; Henkens, T.; Delanghe, L.; Wuyts, S.; Spacova, I.; van den Broek, M.F.L.; Tuyaerts, I.; Wittouck, S.; et al. Selective Targeting of Skin Pathobionts and Inflammation with Topically Applied Lactobacilli. Cell Rep. Med. 2022, 3, 100521. [Google Scholar] [CrossRef]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Wollenberg, A.; Thomsen, S.F.; Lacour, J.P.; Jaumont, X.; Lazarewicz, S. Targeting Immunoglobulin E in Atopic Dermatitis: A Review of the Existing Evidence. World Allergy Organ. J. 2021, 14, 100519. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerre, R.D.; Holm, J.B.; Palleja, A.; Sølberg, J.; Skov, L.; Johansen, J.D. Skin Dysbiosis in the Microbiome in Atopic Dermatitis Is Site-Specific and Involves Bacteria, Fungus and Virus. BMC Microbiol. 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.F.; Ong, R.Y.; Common, J.E. Skin Microbiome of Atopic Dermatitis. Allergol. Int. 2022, 71, 31–39. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics in Skin Conditions: A Systematic Review of Animal and Human Studies and Implications for Future Therapies. Exp. Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transpl. 2018, 27, 729. [Google Scholar] [CrossRef]
- Zhou, W.; Fleming, E.; Legendre, G.; Roux, L.; Latreille, J.; Gendronneau, G.; Forestier, S.; Oh, J. Skin Microbiome Attributes Associate with Biophysical Skin Aging. bioRxiv 2023. [Google Scholar] [CrossRef]
- Murphy, B.; Hoptroff, M.; Arnold, D.; Cawley, A.; Smith, E.; Adams, S.E.; Mitchell, A.; Horsburgh, M.J.; Hunt, J.; Dasgupta, B.; et al. Compositional Variations between Adult and Infant Skin Microbiome: An Update. Microorganisms 2023, 11, 1484. [Google Scholar] [CrossRef]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common Skin Bacteria Protect Their Host from Oxidative Stress through Secreted Antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, M.; Kim, M.; Park, C.; Yoon, Y.; Lim, D.H.; Yeo, H.; Kang, S.; Lee, Y.G.; Beak, N.I.; et al. Spermidine-Induced Recovery of Human Dermal Structure and Barrier Function by Skin Microbiome. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Carmona-Gutierrez, D.; Kepp, O.; Kroemer, G. Spermidine Delays Aging in Humans. Aging 2018, 10, 2209. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [Green Version]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A Physiological Autophagy Inducer Acting as an Anti-Aging Vitamin in Humans? Autophagy 2018, 15, 165–168. [Google Scholar] [CrossRef]
- Stødkilde, K.; Nielsen, J.T.; Petersen, S.V.; Paetzold, B.; Brüggemann, H.; Mulder, F.A.A.; Andersen, C.B.F. Solution Structure of the Cutibacterium acnes-Specific Protein RoxP and Insights Into Its Antioxidant Activity. Front. Cell Infect. Microbiol. 2022, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Allhorn, M.; Arve, S.; Brüggemann, H.; Lood, R. A Novel Enzyme with Antioxidant Capacity Produced by the Ubiquitous Skin Colonizer Propionibacterium acnes. Sci. Rep. 2016, 6, 36412. [Google Scholar] [CrossRef] [Green Version]
- Teichmann, P.; Both, A.; Wolz, C.; Hornef, M.W.; Rohde, H.; Yazdi, A.S.; Burian, M. The Staphylococcus epidermidis Transcriptional Profile During Carriage. Front. Microbiol. 2022, 13, 1537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus epidermidis Contributes to Skin Barrier Homeostasis by Generating Protective Ceramides. Cell Host Microbe 2022, 30, 301–313.e9. [Google Scholar] [CrossRef]
- Li, Q.; Fang, H.; Dang, E.; Wang, G. The Role of Ceramides in Skin Homeostasis and Inflammatory Skin Diseases. J. Dermatol. Sci. 2020, 97, 2–8. [Google Scholar] [CrossRef]
- Di Marzio, L.; Cinque, B.; De Simone, C.; Cifone, M.G. Effect of the Lactic Acid Bacterium Streptococcus thermophilus on Ceramide Levels in Human KeratinocytesIn Vitro and Stratum Corneum In Vivo. J. Investig. Dermatol. 1999, 113, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Di Marzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of Skin-Ceramide Levels in Aged Subjects Following a Short-Term Topical Application of Bacterial Sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Izawa, N.; Hanamizu, T.; Iizuka, R.; Sone, T.; Mizukoshi, H.; Kimura, K.; Chiba, K. Streptococcus thermophilus Produces Exopolysaccharides Including Hyaluronic Acid. J. Biosci. Bioeng. 2009, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Kalan, L.R. Two-for-One: Dual Host-Microbe Functions of S. epidermidis Sph. Cell Host Microbe 2022, 30, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.E.C.; van Smeden, J.; Al-Khakany, H.; Melnik, E.; van Dijk, R.; Absalah, S.; Vreeken, R.J.; Haenen, C.C.P.; Lavrijsen, A.P.M.; Overkleeft, H.S.; et al. Skin of Atopic Dermatitis Patients Shows Disturbed β-Glucocerebrosidase and Acid Sphingomyelinase Activity That Relates to Changes in Stratum Corneum Lipid Composition. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158673. [Google Scholar] [CrossRef]
- Delanghe, L.; Spacova, I.; Van Malderen, J.; Oerlemans, E.; Claes, I.; Lebeer, S. The Role of Lactobacilli in Inhibiting Skin Pathogens. Biochem. Soc. Trans. 2021, 49, 617–627. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. The Science behind Skin Care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lee, P.Y.; Chen, C.Y.; Huang, S.L.; Huang, B.W.; Dai, F.J.; Chau, C.F.; Chen, C.S.; Lin, Y.S. Moisture Retention of Glycerin Solutions with Various Concentrations: A Comparative Study. Sci. Rep. 2022, 12, 10232. [Google Scholar] [CrossRef]
- Iglesia, S.; Kononov, T.; Zahr, A.S. A Multi-functional Anti-aging Moisturizer Maintains a Diverse and Balanced Facial Skin Microbiome. J. Appl. Microbiol. 2022, 133, 1791–1799. [Google Scholar] [CrossRef]
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.T.; Lim, K.M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandan, N.; Rajkumar, J.R.; Shi, V.Y.; Lio, P.A. A New Era of Moisturizers. J. Cosmet. Dermatol. 2021, 20, 2425–2430. [Google Scholar] [CrossRef]
- Bamford, J.T.M.; Flores-Genuino, R.N.S.; Ray, S.; Bigby, M.; Morales-Sánchez, M.A.; Arkoncel, M.; Realubit-Serrano, M.A.C. Interventions for the Treatment of Pityriasis Versicolor. Cochrane Database Syst. Rev. 2018, 2018, CD011208. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Stathopoulou, K.; Bassukas, I.D.; Alexopoulos, E.C.; Velegraki, A.; Skaltsounis, A.L. AhR Ligands, Malassezin, and Indolo[3,2-b]Carbazole Are Selectively Produced by Malassezia furfur Strains Isolated from Seborrheic Dermatitis. J. Investig. Dermatol. 2008, 128, 1620–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bogaard, E.H.; Esser, C.; Perdew, G.H. The Aryl Hydrocarbon Receptor at the Forefront of Host-Microbe Interactions in the Skin: A Perspective on Current Knowledge Gaps and Directions for Future Research and Therapeutic Applications. Exp. Dermatol. 2021, 30, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.; Bhawan, J.; Howell, M.; Desai, S.; Coryell, E.; Einziger, M.; Simpson, A.; Yaroshinsky, A.; McCraw, T. Histopathological Changes Induced by Malassezin: A Novel Natural Microbiome Indole for Treatment of Facial Hyperpigmentation. J. Drugs Dermatol. 2022, 21, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kurniadi, I.; Wijaya, W.H.; Timotius, K.H. Malassezia Virulence Factors and Their Role in Dermatological Disorders. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E.; Bhawan, J.; Howell, M.D.; Desai, S.; Coryell, E.; Nashawati, R.; Einziger, M.; Simpson, A.M.; Yaroshinsky, A.; Mc Craw, T. A Novel Proof-of-Concept Study Assessing the Lightening Effects and Safety of Malassezin for Treatment of Facial Hyperpigmentation. J. Am. Acad. Dermatol. 2022, 87, 456–458. [Google Scholar] [CrossRef]
- Fortuna, M.C.; Garelli, V.; Pranteda, G.; Romaniello, F.; Cardone, M.; Carlesimo, M.; Rossi, A. A Case of Scalp Rosacea Treated with Low Dose Doxycycline and Probiotic Therapy and Literature Review on Therapeutic Options. Dermatol. Ther. 2016, 29, 249–251. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Larson, P.J.; Chong, D.; Fleming, E.; Oh, J. Challenges Developing a Human Model System for Skin Microbiome Research. J. Investig. Dermatol. 2021, 141, 228. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; McFarland, A.G.; Blaustein, R.A.; Rose, L.J.; Perry-Dow, K.A.; Moghadam, A.A.; Hayden, M.K.; Young, V.B.; Hartmann, E.M. An Improved Workflow for Accurate and Robust Healthcare Environmental Surveillance Using Metagenomics. Microbiome 2022, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shute, A.; Bihan, D.G.; Lewis, I.A.; Nasser, Y. Metabolomics: The Key to Unraveling the Role of the Microbiome in Visceral Pain Neurotransmission. Front. Neurosci. 2022, 16, 937. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholas-Haizelden, K.; Murphy, B.; Hoptroff, M.; Horsburgh, M.J. Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products. Microorganisms 2023, 11, 1899. https://doi.org/10.3390/microorganisms11081899
Nicholas-Haizelden K, Murphy B, Hoptroff M, Horsburgh MJ. Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products. Microorganisms. 2023; 11(8):1899. https://doi.org/10.3390/microorganisms11081899
Chicago/Turabian StyleNicholas-Haizelden, Keir, Barry Murphy, Michael Hoptroff, and Malcolm J. Horsburgh. 2023. "Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products" Microorganisms 11, no. 8: 1899. https://doi.org/10.3390/microorganisms11081899
APA StyleNicholas-Haizelden, K., Murphy, B., Hoptroff, M., & Horsburgh, M. J. (2023). Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products. Microorganisms, 11(8), 1899. https://doi.org/10.3390/microorganisms11081899





