Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies
Abstract
:1. Introduction
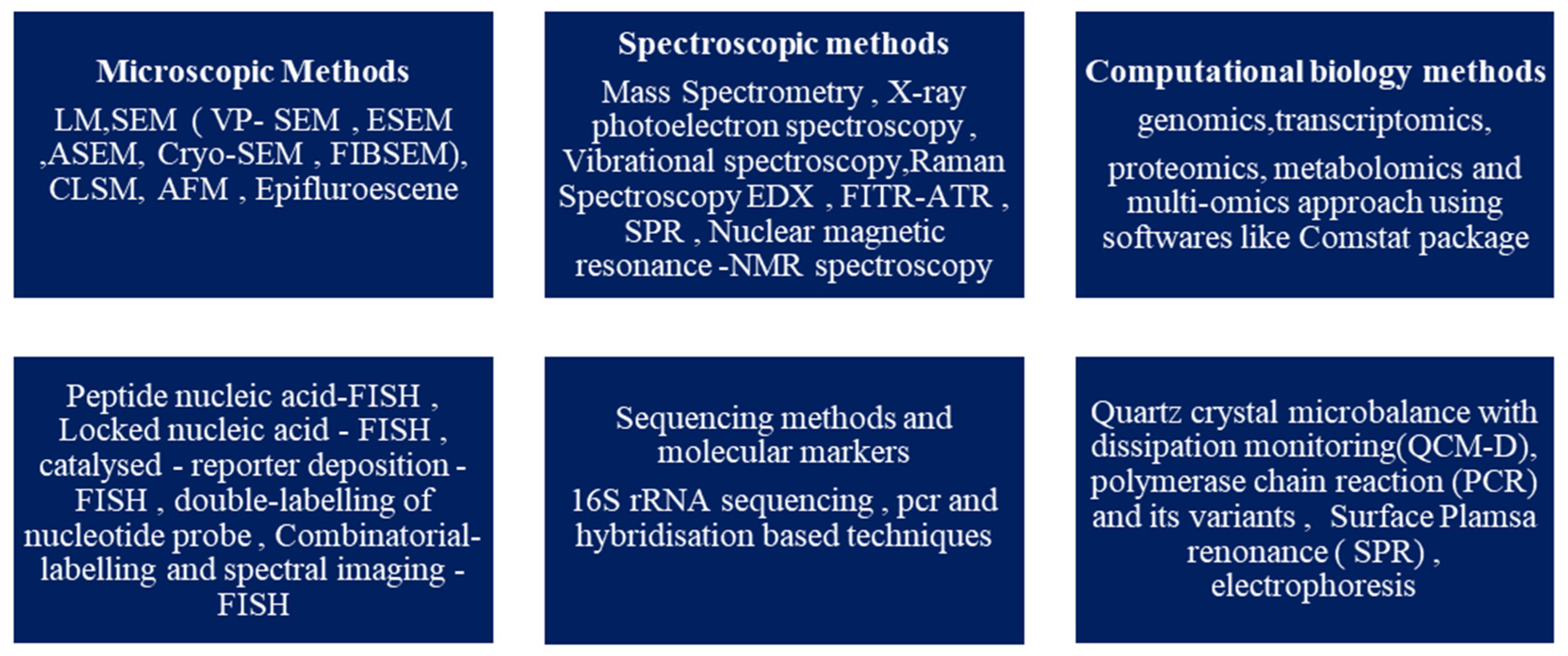
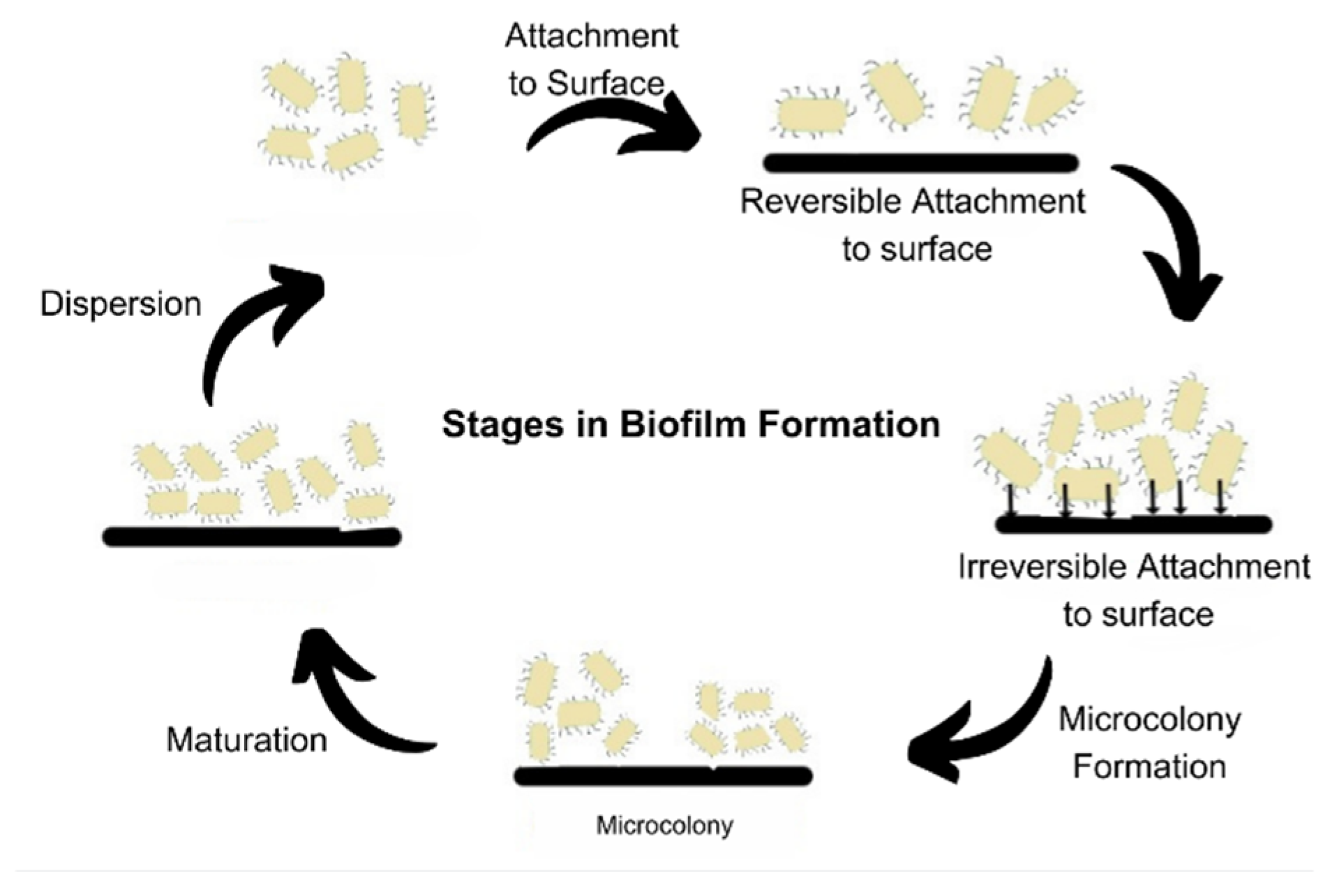
2. Biofilm and Its Development
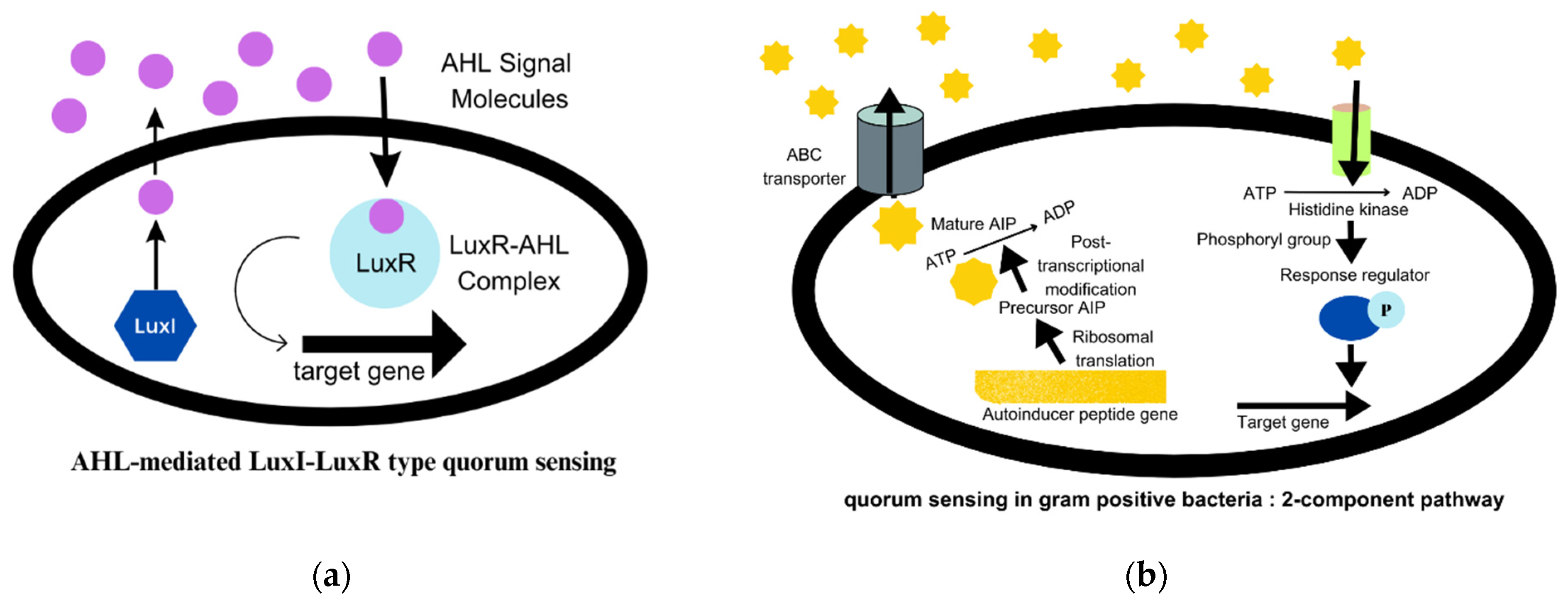
3. Biofilms and CRISPR-Cas System
4. Economic Importance of Biofilms
4.1. Biofilm in Environment
4.2. Biofilms in Health
4.2.1. Device-Related Biofilm Infection
Urinary Tract Infection (UTI)
Nosocomial Infections
Breast Implant Infection (BII)
Catheter-Related Bloodstream Infection
Periprosthetic Joint Infection (PJI)
Contact Lens Infections
Ventilator-Associated Pneumonia (VAP)
4.2.2. Tissue-Related Biofilm Infections
Dental Biofilms
Cystic Fibrosis (CF)
Infective Endocarditis (IE)
Chronic Wound Infections (CWI)
| Disease | Pathogens | Reference |
|---|---|---|
| Urinary tract infections | E. coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus (S.aureus, S. saprophyticus, S. epidermidis), Enterococci, Streptococci agalactiae, Corynebacterium urealyticum, Candida | [65] |
| Oral health problems (dental plaques, dental carries, and periodontitis) | Neisseria, Granulicatella, Streptococcus, Actinomyces, Veillonella | [116] |
| Nosocomial infections (healthcare-acquired infections) | Staphylococcus epidermidis, Candida albicans, Staphylococcus aureus, P. aeruginosa, Klebsiella pneumonia, Enterococcus faecalis, Proteus mirabilis | [67] |
| Sexually transmitted diseases (STDs) | Neisseria gonorrhoeae | [117] |
| Cystic fibrosis | Pseudomonas aeruginosa (infects adults), Staphylococcus aureus (infects children) | [118] |
| Infective endocarditis | Streptococci, Staphylococci, Enterococci | [109] |
5. Methods of Combating Biofilms
5.1. Phytoextracts
5.2. Nanoparticles against Biofilms
5.3. Antimicrobial Peptide (AMP)
5.4. Anti-Virulence Compounds from Plants
5.5. Phage Therapy
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamal, M.; Khan, A.W.; Andleeb, S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. J. Microbiol. Biotechnol. 2015, 4, 3. [Google Scholar]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on Sem and VP-SEM Pros and Cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Bramhachari, P.V. (Ed.) Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry; Springer: Singapore, 2019; ISBN 978-981-329-408-0. [Google Scholar]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Biofilms and Human Health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- National Board Inspection Code (NBIC). NBIC-Annual-Report-2022; National Board Inspection Code (NBIC): Taipei, Taiwan, 2022. [Google Scholar]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle– Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Cai, Y.-M. Non-Surface Attached Bacterial Aggregates: A Ubiquitous Third Lifestyle. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, N.; Singh, L.; Hashmi, M.Z.; Varma, A. Biofilms in Human Diseases: Treatment and Control; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-30757-8. [Google Scholar]
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio Tribo Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Rai, A.K.; Sharma, M.; Tripathi, M.; Prasad, R. Microbial Biofilms: Recent Advances and Progress in Environmental Bioremediation. Sci. Total Environ. 2022, 824, 153843. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Peng, C.; Peng, P.; Lin, Y.; Zhang, X.; Ren, H. Towards the Biofilm Characterization and Regulation in Biological Wastewater Treatment. Appl. Microbiol. Biotechnol. 2019, 103, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Ramkumar, N.; Andriotis, C.; Höltkemeier, T.; Yasmin, A.; Rochfort, S.; Wlodkowic, D.; Morrison, P.; Roddick, F.; Spangenberg, G.; et al. Applications of Microalgal Biofilms for Wastewater Treatment and Bioenergy Production. Biotechnol. Biofuels 2017, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Tolker-Nielsen, T. Biofilm Development. Microbiol. Spectr. 2018, 4, 10. [Google Scholar] [CrossRef]
- Bhatia, R.; Gulati, D.; Sethi, G. Biofilms and Nanoparticles: Applications in Agriculture. Folia Microbiol. 2021, 66, 159–170. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef]
- Coquant, G.; Grill, J.P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Abdul Hamid, N.W.; Nadarajah, K. Microbe Related Chemical Signalling and Its Application in Agriculture. Int. J. Mol. Sci. 2022, 23, 8998. [Google Scholar] [CrossRef]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of Plant Extracts and Phytochemicals against Resistant Salmonella Spp. in Biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Rajput, A.; Kaur, K.; Kumar, M. SigMol: Repertoire of Quorum Sensing Signaling Molecules in Prokaryotes. Nucleic Acids Res. 2016, 44, D634–D639. [Google Scholar] [CrossRef] [Green Version]
- Rumbaugh, K.P. How Well Are We Translating Biofilm Research from Bench-Side to Bedside? Biofilm 2020, 2, 100028. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Sun, X.; Wu, Y.; Gao, C.; Mortimer, M.; Holden, P.A.; Redmile-Gordon, M.; Huang, Q. Soil Biofilms: Microbial Interactions, Challenges, and Advanced Techniques for Ex-Situ Characterization. Soil Ecol. Lett. 2019, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Dufrêne, Y.F.; Boonaert, C.J.P.; Rouxhet, P.G. [28] Surface Analysis by X-ray Photoelectron Spectroscopy in Study of Bioadhesion and Biofilms. Methods Enzymol. 1999, 310, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, V.; Sc, B. Monitoring Early Stages of Bacterial Adhesion at Silica Surfaces through Image Analysis. Langmuir 2020, 36, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Nozhevnikova, A.N.; Botchkova, E.A.; Plakunov, V.K. Multi-Species Biofilms in Ecology, Medicine, and Biotechnology. Microbiology 2015, 84, 731–750. [Google Scholar] [CrossRef]
- Xu, Y.; Dhaouadi, Y.; Stoodley, P.; Ren, D. Sensing the Unreachable: Challenges and Opportunities in Biofilm Detection. Curr. Opin. Biotechnol. 2020, 64, 79–84. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent Advances in Nanotechnology for Eradicating Bacterial Biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef]
- Horvath, P.; Romero, D.A.; Coûté-Monvoisin, A.-C.; Richards, M.; Deveau, H.; Moineau, S.; Boyaval, P.; Fremaux, C.; Barrangou, R. Diversity, Activity, and Evolution of CRISPR Loci in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1401–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, M.; Dong, Y.; Cao, Q.; Zhao, D.; Ji, S.; Huang, H.; Jiang, M.; Liu, G.; Liu, Y. CRISPR Contributes to Adhesion, Invasion, and Biofilm Formation in Streptococcus agalactiae by Repressing Capsular Polysaccharide Production. Microbiol. Spectr. 2022, 10, e02113-21. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas Bacterial Immune System Cleaves Bacteriophage and Plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas Systems Target Endogenous Genes to Impact Bacterial Physiology and Alter Mammalian Immune Responses. Mol. Biomed. 2022, 3, 22. [Google Scholar] [CrossRef]
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation. Infect. Drug Resist. 2023, 16, 19–49. [Google Scholar] [CrossRef]
- Zuberi, A.; Misba, L.; Khan, A.U. CRISPR Interference (CRISPRi) Inhibition of LuxS Gene Expression in E. Coli: An Approach to Inhibit Biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 214. [Google Scholar] [CrossRef] [Green Version]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Yuan, L.; Hansen, M.F.; Røder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-Species Biofilms in the Food Industry: Current Knowledge and Novel Control Strategies. Crit. Rev. Food Sci. Nutr. 2020, 60, 2277–2293. [Google Scholar] [CrossRef]
- Mizan, M.d.F.R.; Jahid, I.K.; Ha, S.-D. Microbial Biofilms in Seafood: A Food-Hygiene Challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Y.; Zhang, X.; Gao, H.; Mou, L.; Wu, M.; Zhang, W.; Xin, F.; Jiang, M. Biofilm Application in the Microbial Biochemicals Production Process. Biotechnol. Adv. 2021, 48, 107724. [Google Scholar] [CrossRef]
- Ghosh, S.; Nag, M.; Lahiri, D.; Sarkar, T.; Pati, S.; Kari, Z.A.; Nirmal, N.P.; Edinur, H.A.; Ray, R.R. Engineered Biofilm: Innovative Nextgen Strategy for Quality Enhancement of Fermented Foods. Front. Nutr. 2022, 9, 808630. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.S.; Altaf, M.M.; Qais, F.A.; Ansari, F.A.; Rumbaugh, K.P. Biofilms: An Overview of Their Significance in Plant and Soil Health. In Biofilms in Plant and Soil Health; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–25. ISBN 978-1-119-24632-9. [Google Scholar]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial Biofilms in Nature: Unlocking Their Potential for Agricultural Applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally Important Microbial Biofilms: Present Status and Future Prospects. J. Basic Microbiol. 2017, 57, 548–573. [Google Scholar] [CrossRef]
- Urban Wastewater Scenario in India. Available online: https://aim.gov.in/images/Waste-Water-ver2_18102022.pdf (accessed on 1 September 2022).
- Li, L.; He, Z.; Liang, T.; Sheng, T.; Zhang, F.; Wu, D.; Ma, F. Colonization of Biofilm in Wastewater Treatment: A Review. Environ. Pollut. 2022, 293, 118514. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Chen, J.; Liu, H.; Liang, J.; Tang, J.; Wei, Y. Advances in Studies on Microbiota Involved in Nitrogen Removal Processes and Their Applications in Wastewater Treatment. Front. Microbiol. 2021, 12, 746293. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Wu, X.; Shu, Y.; Wang, C.; Tian, C.; Wu, H.; Xiao, B. Bioaugmentation Treatment of Nitrogen-Rich Wastewater with a Denitrifier with Biofilm-Formation and Nitrogen-Removal Capacities in a Sequencing Batch Biofilm Reactor. Bioresour. Technol. 2020, 303, 122905. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Z.; Cheng, X.; Xie, J.; Li, X.; Jiang, X.; Zhu, D. Treatment of Nitrogen-Rich Wastewater by Mixed Aeration Combined with Bioaugmentation in a Sequencing Batch Biofilm Reactor: Biofilm Formation and Nitrogen-Removal Capacity Analysis. J. Environ. Chem. Eng. 2023, 11, 109316. [Google Scholar] [CrossRef]
- Akao, P.K.; Singh, B.; Kaur, P.; Sor, A.; Avni, A.; Dhir, A.; Verma, S.; Kapoor, S.; Phutela, U.G.; Satpute, S.; et al. Coupled Microalgal–Bacterial Biofilm for Enhanced Wastewater Treatment without Energy Investment. J. Water Process Eng. 2021, 41, 102029. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic Significance of Biofilms: A Multidisciplinary and Cross-Sectoral Challenge. npj Biofilms Microbiomes 2022, 8, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Ahmed, W.; Metcalfe, S.; Smith, W.J.M.; Choi, P.M.; Jackson, G.; Cen, X.; Zheng, M.; Simpson, S.L.; Thomas, K.V.; et al. Impact of Sewer Biofilms on Fate of SARS-CoV-2 RNA and Wastewater Surveillance. Nat. Water 2023, 1, 272–280. [Google Scholar] [CrossRef]
- National Research Council (US) Board on Biology; National Research Council (US) Ocean Studies Board. Bacterial Biofilms and Biofouling: Translational Research in Marine Biotechnology. In Opportunities for Environmental Applications of Marine Biotechnology: Proceedings of the October 5–6, 1999, Workshop; National Academies Press (US): Cambridge, MA, USA, 2000. [Google Scholar]
- Ashraf, M.A.; Ullah, S.; Ahmad, I.; Qureshi, A.K.; Balkhair, K.S.; Abdur Rehman, M. Green Biocides, a Promising Technology: Current and Future Applications to Industry and Industrial Processes. J. Sci. Food Agric. 2014, 94, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. Biofouling and Me: My Stockholm Syndrome with Biofilms. Water Res. 2020, 173, 115576. [Google Scholar] [CrossRef]
- Von Borowski, R.G.; Zimmer, K.R.; Leonardi, B.F.; Trentin, D.S.; Silva, R.C.; de Barros, M.P.; Macedo, A.J.; Gnoatto, S.C.B.; Gosmann, G.; Zimmer, A.R. Red Pepper Capsicum Baccatum: Source of Antiadhesive and Antibiofilm Compounds against Nosocomial Bacteria. Ind. Crops Prod. 2019, 127, 148–157. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination Strategies to Enhance the Efficacy of Antimicrobial Peptides against Bacterial Biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the Effect of Anti-Biofilm Activity of Bioactive Compounds Extracted from Plants. J. Biosci. 2019, 44, 52. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef]
- Walsh, C.; Collyns, T. The Pathophysiology of Urinary Tract Infections. Surgery 2017, 35, 293–298. [Google Scholar] [CrossRef]
- Shah, C.; Baral, R.; Bartaula, B.; Shrestha, L.B. Virulence Factors of Uropathogenic Escherichia Coli (UPEC) and Correlation with Antimicrobial Resistance. BMC Microbiol. 2019, 19, 204. [Google Scholar] [CrossRef] [Green Version]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Hayward, C.; Ross, K.E.; Brown, M.H.; Whiley, H. Water as a Source of Antimicrobial Resistance and Healthcare-Associated Infections. Pathogens 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ibarburu, B.; Díaz-Navarro, M.; Ibarra, G.; Rivera, A.; Hafian, R.; Irigoyen, Ã.; Carrillo, R.; Pérez-Cano, R.; Muñoz, P.; García-Ruano, Á.; et al. Efficacy of Povidone Iodine against Microbial Biofilms in Breast Implants with Different Textures: Results From an in Vitro Study. Front. Microbiol. 2022, 13, 868347. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, M.; Jeong, T.; Vaienti, L.; De La Cruz, C.; Gimbel, M.L.; Nguyen, V.T.; Egro, F.M. The Role of Microorganisms in the Development of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Pathogens 2023, 12, 313. [Google Scholar] [CrossRef]
- Ngaage, L.M.; Elegbede, A.; Brao, K.; Chopra, K.; Gowda, A.U.; Nam, A.J.; Ernst, R.K.; Shirtliff, M.E.; Harro, J.; Rasko, Y.M. The Efficacy of Breast Implant Irrigant Solutions: A Comparative Analysis Using an In Vitro Model. Plast. Reconstr. Surg. 2020, 146, 301–308. [Google Scholar] [CrossRef]
- Bouza, E.; Guinea, J.; Guembe, M. The Role of Antifungals against Candida Biofilm in Catheter-Related Candidemia. Antibiotics 2014, 4, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raad, I.I.; Fang, X.; Keutgen, X.M.; Jiang, Y.; Sherertz, R.; Hachem, R. The Role of Chelators in Preventing Biofilm Formation and Catheter-Related Bloodstream Infections. Curr. Opin. Infect. Dis. 2008, 21, 385–392. [Google Scholar] [CrossRef]
- Zhang, L.; Gowardman, J.; Morrison, M.; Runnegar, N.; Rickard, C.M. Microbial Biofilms Associated with Intravascular Catheter-Related Bloodstream Infections in Adult Intensive Care Patients. Eur J. Clin. Microbiol. Infect Dis 2016, 35, 201–205. [Google Scholar] [CrossRef]
- Ryder, M.A. Catheter-Related Infections: It’s All About Biofilm. Top. Adv. Pract. Nurs. J. 2005, 5, 3. [Google Scholar]
- Wolcott, R. Biofilm and Catheter-Related Bloodstream Infections. Br. J. Nurs. 2021, 30, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Purrello, S.M.; Bonnet, E.; Novelli, A.; Tripodi, F.; Pascale, R.; Unal, S.; Milkovich, G. Central Venous Catheter-Related Biofilm Infections: An up-to-Date Focus on Meticillin-Resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2013, 1, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Bruenke, J.; Roschke, I.; Agarwal, S.; Riemann, T.; Greiner, A. Quantitative Comparison of the Antimicrobial Efficiency of Leaching versus Nonleaching Polymer Materials. Macromol. Biosci. 2016, 16, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; del Toro, M.D.; Guío, L.; et al. The Different Microbial Etiology of Prosthetic Joint Infections According to Route of Acquisition and Time after Prosthesis Implantation, Including the Role of Multidrug-Resistant Organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtlif, M.; Kathju, S.; Stoodley, P. Biofilms in Periprosthetic Orthopedic Infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [Green Version]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The Role of Microbial Biofilms in Prosthetic Joint Infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Robertson, D.M.; Parks, Q.M.; Young, R.L.; Kret, J.; Poch, K.R.; Malcolm, K.C.; Nichols, D.P.; Nichols, M.; Zhu, M.; Cavanagh, H.D.; et al. Disruption of Contact Lens–Associated Pseudomonas aeruginosa Biofilms Formed in the Presence of Neutrophils. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2844. [Google Scholar] [CrossRef] [Green Version]
- El-Ganiny, A.M.; Shaker, G.H.; Aboelazm, A.A.; El-Dash, H.A. Prevention of Bacterial Biofilm Formation on Soft Contact Lenses Using Natural Compounds. J. Ophthal. Inflamm. Infect. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Campolo, A.; Pifer, R.; Shannon, P.; Crary, M. Microbial Adherence to Contact Lenses and Pseudomonas aeruginosa as a Model Organism for Microbial Keratitis. Pathogens 2022, 11, 1383. [Google Scholar] [CrossRef]
- Dutta, D.; Stapleton, F.; Willcox, M. Ocular Surface Infection and Antimicrobials. Antibiotics 2022, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.-S. Contact Lenses Coated with Hybrid Multifunctional Ternary Nanocoatings (Phytomolecule-Coated ZnO Nanoparticles:Gallic Acid:Tobramycin) for the Treatment of Bacterial and Fungal Keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef]
- Khan, S.A.; Lee, C.-S. Recent Progress and Strategies to Develop Antimicrobial Contact Lenses and Lens Cases for Different Types of Microbial Keratitis. Acta Biomater. 2020, 113, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Bispo, P.; Haas, W.; Gilmore, M. Biofilms in Infections of the Eye. Pathogens 2015, 4, 111–136. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, J.R.; Dantas, L.R.; Tuon, F.F. Activity of Multipurpose Contact Lens Solutions against Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens and Candida albicans Biofilms. Ophthalmic Physiol. Opt. 2023, 00, 1–8. [Google Scholar] [CrossRef]
- Khazaal, S.S.; Al-Saryi, N.; Ibrahim, S.A. Immunomodulation by Acinetobacter baumannii of Endotracheal Tube Biofilm in Ventilator-Associated Pneumonia. Meta Gene 2020, 24, 100672. [Google Scholar] [CrossRef]
- Codru, I.R.; Sava, M.; Vintilă, B.I.; Bereanu, A.S.; Bîrluțiu, V. A Study on the Contributions of Sonication to the Identification of Bacteria Associated with Intubation Cannula Biofilm and the Risk of Ventilator-Associated Pneumonia. Medicina 2023, 59, 1058. [Google Scholar] [CrossRef]
- Farzi, N.; Oloomi, M.; Bahramali, G.; Siadat, S.D.; Bouzari, S. Antibacterial Properties and Efficacy of LL-37 Fragment GF-17D3 and Scolopendin A2 Peptides Against Resistant Clinical Strains of Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii In Vitro and In Vivo Model Studies. Probiotics Antimicro. Prot. 2023, 5, 1–19. [Google Scholar] [CrossRef]
- Baidya, S.; Sharma, S.; Mishra, S.K.; Kattel, H.P.; Parajuli, K.; Sherchand, J.B. Biofilm Formation by Pathogens Causing Ventilator-Associated Pneumonia at Intensive Care Units in a Tertiary Care Hospital: An Armor for Refuge. BioMed. Res. Int. 2021, 2021, 8817700. [Google Scholar] [CrossRef]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding Ventilator-Associated Pneumonia: Curcumin-Functionalized Endotracheal Tube and Photodynamic Action. Proc. Natl. Acad. Sci. USA 2020, 117, 22967–22973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, B.; Ni, D.; Sun, Y.; Wang, G.; Jiang, H. A Novel Antibacterial and Antifouling Nanocomposite Coated Endotracheal Tube to Prevent Ventilator-Associated Pneumonia. J. Nanobiotechnol. 2022, 20, 112. [Google Scholar] [CrossRef]
- Guimarães, R.; Milho, C.; Liberal, Â.; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida Spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Jalil, V.; Khan, M.; Haider, S.Z.; Shamim, S. Investigation of the Antibacterial, Anti-Biofilm, and Antioxidative Effect of Piper Betle Leaf Extract against Bacillus Gaemokensis MW067143 Isolated from Dental Caries, an In Vitro-In Silico Approach. Microorganisms 2022, 10, 2485. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Cunha Villar, C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a Risk Factor in Implant Treatment. Periodontol 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and Management of Pulmonary Infections in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Lerche, C.J.; Schwartz, F.; Theut, M.; Fosbøl, E.L.; Iversen, K.; Bundgaard, H.; Høiby, N.; Moser, C. Anti-Biofilm Approach in Infective Endocarditis Exposes New Treatment Strategies for Improved Outcome. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Chen, H.; Zhan, Y.; Zhang, K.; Gao, Y.; Chen, L.; Zhan, J.; Chen, Z.; Zeng, Z. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front. Med. 2022, 9, 774224. [Google Scholar] [CrossRef] [PubMed]
- Elgharably, H.; Hussain, S.T.; Shrestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Current Hypotheses in Cardiac Surgery: Biofilm in Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 56–59. [Google Scholar] [CrossRef]
- Gilbey, T.; Ho, J.; Cooley, L.A.; Petrovic Fabijan, A.; Iredell, J.R. Adjunctive Bacteriophage Therapy for Prosthetic Valve Endocarditis Due to Staphylococcus aureus. Med. J. Aust. 2019, 211, 142–143.e1. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.; Shai, S.; Shahane, A.; Kaushik, K.S. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’? Biomedicines 2019, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Mustoe, T. Understanding Chronic Wounds: A Unifying Hypothesis on Their Pathogenesis and Implications for Therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.; Herrera, D.; Herzberg, M.C.; et al. Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases. Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Su, X. Antibacterial Activity of 18β-Glycyrrhetinic Acid against Neisseria Gonorrhoeae in Vitro. Biochem. Biophys. Rep. 2023, 33, 101427. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Boudet, A.; Sorlin, P.; Menetrey, Q.; Chiron, R.; Lavigne, J.P.; Marchandin, H. Biofilm Formation by Staphylococcus aureus in the Specific Context of Cystic Fibrosis. Int. J. Mol. Sci. 2023, 24, 597. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Hashmi, Z.; Khan, N.; Ahmad, R.; Khan, W.H. Recent Strategies to Combat Multidrug Resistance. In Non-traditional Approaches to Combat Antimicrobial Drug Resistance; Wani, M.Y., Ahmad, A., Eds.; Springer Nature: Singapore, 2023; pp. 1–27. ISBN 978-981-19916-7-7. [Google Scholar]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm Exacerbates Antibiotic Resistance: Is This a Current Oversight in Antimicrobial Stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Stalder, T.; Cornwell, B.; Lacroix, J.; Kohler, B.; Dixon, S.; Yano, H.; Kerr, B.; Forney, L.J.; Top, E.M. Evolving Populations in Biofilms Contain More Persistent Plasmids. Mol. Biol. Evol. 2020, 37, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Thakur, A.; Sharma, S.; Kumar, M. ABiofilm: A Resource of Anti-Biofilm Agents and Their Potential Implications in Targeting Antibiotic Drug Resistance. Nucleic Acids Res. 2018, 46, D894–D900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olawuwo, O.S.; Famuyide, I.M.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Selected Medicinal Plant Leaf Extracts Against Pathogens Implicated in Poultry Diseases. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.R.; Pattnaik, S. Contribution of Phytoextracts in Challenging the Biofilms of Pathogenic Bacteria. Biocatal. Agric. Biotechnol. 2023, 48, 102642. [Google Scholar] [CrossRef]
- Meccatti, V.M.; Santos, L.F.; de Carvalho, L.S.; Souza, C.B.; Carvalho, C.A.T.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Antifungal Action of Herbal Plants’ Glycolic Extracts against Candida Species. Molecules 2023, 28, 2857. [Google Scholar] [CrossRef]
- Ortega-Lozano, A.J.; Hernández-Cruz, E.Y.; Gómez-Sierra, T.; Pedraza-Chaverri, J. Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections. Antibiotics 2023, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In Vitro Total Phenolics, Total Flavonoids, Antioxidant and Antibacterial Activities of Selected Medicinal Plants Using Different Solvent Systems. BMC Chem. 2022, 16, 64. [Google Scholar] [CrossRef]
- Dahiya, P.; Purkayastha, S. Phytochemical Screening and Antimicrobial Activity of Some Medicinal Plants Against Multi-Drug Resistant Bacteria from Clinical Isolates. Indian J. Pharm. Sci. 2012, 74, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Shukla, V.J. Antibacterial and Antifungal Activities from Leaf Extracts of Cassia Fistula l.: An Ethnomedicinal Plant. J. Adv. Pharm. Technol. Res. 2011, 2, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of Essential Oils to the Fight against Microbial Biofilms—A Review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Zeineldin, M.; Esmael, A.; Al-Hindi, R.R.; Alharbi, M.G.; Ashenafi Bekele, D.; Teklemariam, A.D. Beyond the Risk of Biofilms: An Up-and-Coming Battleground of Bacterial Life and Potential Antibiofilm Agents. Life 2023, 13, 503. [Google Scholar] [CrossRef]
- He, Z.; Xu, X.; Wang, C.; Li, Y.; Dong, B.; Li, S.; Zeng, J. Effect of Panax Quinquefolius Extract on Mycobacterium abscessus Biofilm Formation. Biofouling 2023, 39, 1–12. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. The Effects of Allium Sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria. Jundishapur J. Microbiol. 2015, 8, e18971. [Google Scholar] [CrossRef] [Green Version]
- Jena, S.; Sangeetha, D.; Ramya, P.; Pradhan, L.K. Antibacterial activity of leaf extracts of adhatoda vasica and solanum trilobatum against respiratory infection causing pathogens of clinical origin. Int. J. Life Sci. Pharma. Res. 2020, 51–55, ISSN 2250–048. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Shangguan, W.; Chen, S.; Zhong, Q. Antibiofilm Activities of the Cinnamon Extract against Vibrio parahaemolyticus and Escherichia coli. Arch. Microbiol. 2021, 203, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Nayeri Fasaei, B. Selected Plant Essential Oils Inhibit Biofilm Formation and LuxS- and Pfs-Mediated Quorum Sensing by Escherichia Coli O157:H7. Lett. Appl. Microbiol. 2022, 74, 916–923. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Mukherjee, I.; Ghosh, S.; Dey, S.; Dash, S.; Ray, R.R. In-Vitro Study of the Efficacy of Ethanolic Extract of Zingiber Officinale on Biofilm Formed by Pharyngeal Isolate. In Biotechnology and Biological Sciences; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-00-300161-4. [Google Scholar]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm Activity of Green Synthesized Silver Nanoparticles against Biofilm Associated Enterococcal Urinary Pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in Combating Biofilm: A Smart and Promising Therapeutic Strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Ikuma, K.; Madden, A.S.; Decho, A.W.; Lau, B.L.T. Deposition of Nanoparticles onto Polysaccharide-Coated Surfaces: Implications for Nanoparticle–Biofilm Interactions. Environ. Sci. Nano 2014, 1, 117–122. [Google Scholar] [CrossRef]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When Nanoparticles Meet Biofilms—Interactions Guiding the Environmental Fate and Accumulation of Nanoparticles. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. Biofilms: A Battle against Another Paradigm of Antibiotic Resistance. Med. Chem. Commun. 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a Delivery System for the Treatment of Biofilm-Mediated Infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Park, J.; Li, C.-H.; Chattopadhyay, A.N.; Nabawy, A.; Landis, R.F.; Gupta, A.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Polymeric Nanoparticles Active against Dual-Species Bacterial Biofilms. Molecules 2021, 26, 4958. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ramos, M.A.; Da Silva, P.; Spósito, L.; De Toledo, L.; Bonifácio, B.; Rodero, C.F.; Dos Santos, K.; Chorilli, M.; Bauab, T.M. Nanotechnology-Based Drug Delivery Systems for Control of Microbial Biofilms: A Review. IJN 2018, 13, 1179–1213. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Lange, A.; Grzenia, A.; Wierzbicki, M.; Strojny-Cieslak, B.; Kalińska, A.; Gołębiewski, M.; Radzikowski, D.; Sawosz, E.; Jaworski, S. Silver and Copper Nanoparticles Inhibit Biofilm Formation by Mastitis Pathogens. Animals 2021, 11, 1884. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, M.; Sitaram, S.; Kannaiyan, S.K.; Subbiahdoss, G. Antibacterial Efficacy of Iron-Oxide Nanoparticles against Biofilms on Different Biomaterial Surfaces. Int. J. Biomater. 2014, 2014, 716080. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.F.; Elimelech, M. Toxic Effects of Single-Walled Carbon Nanotubes in the Development of E. Coli Biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef]
- Liang, G.; Shi, H.; Qi, Y.; Li, J.; Jing, A.; Liu, Q.; Feng, W.; Li, G.; Gao, S. Specific Anti-Biofilm Activity of Carbon Quantum Dots by Destroying P. Gingivalis Biofilm Related Genes. Int. J. Nanomed. 2020, 15, 5473–5489. [Google Scholar] [CrossRef]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial Peptides and Nanotechnology, Recent Advances and Challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Pontes, J.T.C.; Borges, A.B.T.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef]
- Ronneau, S.; Hallez, R. Make and Break the Alarmone: Regulation of (p)PpGpp Synthetase/Hydrolase Enzymes in Bacteria. FEMS Microbiol. Rev. 2019, 43, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel Strategies Based on Antimicrobial Peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, J.W. Bacterial Pathogenesis. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Díaz-Nuñez, J.L.; García-Contreras, R.; Castillo-Juárez, I. The New Antibacterial Properties of the Plants: Quo Vadis Studies of Anti-Virulence Phytochemicals? Front. Microbiol. 2021, 12, 667126. [Google Scholar] [CrossRef]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel Strategies to Combat Bacterial Biofilms. Mol. Biotechnol 2021, 63, 569–586. [Google Scholar] [CrossRef]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Wang, N.; Zhang, T.; Feng, Y.; Zhou, X.; Zeng, D.; Meng, J.; Liu, L.; Jin, L.; Yang, S. Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. Int. J. Mol. Sci. 2023, 24, 2897. [Google Scholar] [CrossRef]
- Jovanović, M.; Morić, I.; Nikolić, B.; Pavić, A.; Svirčev, E.; Šenerović, L.; Mitić-Ćulafić, D. Anti-Virulence Potential and In Vivo Toxicity of Persicaria Maculosa and Bistorta Officinalis Extracts. Molecules 2020, 25, 1811. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.P.; Meneses, L.; Brandão, A.C.; Azeredo, J. An Overview of the Current State of Phage Therapy for the Treatment of Biofilm-Related Infections. Curr. Opin. Virol. 2022, 53, 101209. [Google Scholar] [CrossRef]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting Biofilms Using Phages and Their Enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Jacobs, A.C.; Dugan, J.; Duplessis, C.; Rouse, M.; Deshotel, M.; Simons, M.; Biswas, B.; Nikolich, M.; Stockelman, M.; Tyner, S.D.; et al. Practical Applications of Bacteriophage Therapy: Biofilms to Bedside. In Antibacterial Drug Discovery to Combat MDR: Natural Compounds, Nanotechnology and Novel Synthetic Sources; Ahmad, I., Ahmad, S., Rumbaugh, K.P., Eds.; Springer: Singapore, 2019; pp. 459–497. ISBN 9789811398711. [Google Scholar]
- Atshan, S.S.; Hamat, R.A.; Aljaberi, M.A.; Chen, J.-S.; Huang, S.-W.; Lin, C.-Y.; Mullins, B.J.; Kicic, A. Phage Therapy as an Alternative Treatment Modality for Resistant Staphylococcus aureus Infections. Antibiotics 2023, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Kutter, E.; Bryan, D.; Wheat, G.; Kuhl, S. Resolving Digital Staphylococcal Osteomyelitis Using Bacteriophage—A Case Report. Antibiotics 2018, 7, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Bacteria | Extract | MIC | Plant | Reference |
|---|---|---|---|---|
| Staphylococcus aureus | Methanol extract | 1.25 mg/mL | Allium sativum | [136] |
| Ethanol extract | 2.5 mg/mL | Allium sativum | ||
| Hexane extract | 5 mg/mL | Cinnamomum verum | [126] | |
| Dichloromethane extract | 20 mg/mL | Cinnamomum verum | ||
| Ethanol extract | 10 mg/mL | Cinnamomum verum | ||
| Clove oil | 0.5 mg/mL | Syzygium aromaticum | ||
| Aqueous extract | 0.5 mg/mL | Solanum trilobatum | [137] | |
| Bacillus cereus | Methanol extract | 0.156 mg/mL | Allium sativum | |
| Ethanol extract | 0.078 mg/mL | Allium sativum | ||
| Streptococcus pneumoniae | Methanol extract | 0.312 mg/mL | Allium sativum | |
| Ethanol extract | 0.312 mg/mL | Allium sativum | ||
| Pseudomonas aeruginosa | Methanol extract | 1.25 mg/mL | Allium sativum | |
| Ethanol extract | 0.625 mg/mL | Allium sativum | ||
| Essential oil | 12–19 mg/mL | Cinnamomum cassia | [126] | |
| Ethanol extract | 10 mg/mL | Cinnamomum verum | ||
| Dichloromethane extract | 20 mg/mL | Cinnamomum verum | ||
| Hexane extract | 10 mg/mL | Cinnamomum verum | ||
| Escherichia coli | Methanol extract | 0.625 mg/mL | Allium sativum | [136] |
| Ethanol extract | 0.156 mg/mL | Allium sativum | ||
| Essential oil | 26–35 mg/mL | Cinnamomum cassia | [126] | |
| Clove oil | 0.5 mg/mL | Syzygium aromaticum | ||
| Ethanol extract | 0.39 mg/mL | Syzygium aromaticum | ||
| Essential oil | 0.25 mg/mL | Cuminum cyminum | ||
| Ethanol extract | 6.25 mg/mL (inhibition rate of 48.18% at MIC and eradication rate of 46.16% at 8 MIC) | Cinnamon | [138] | |
| Klebsiella pneumoniae | Methanol extract | 0.312 mg/mL | Allium sativum | [136] |
| Ethanol extract | 0.156 mg/mL | Allium sativum | ||
| Hexane extract | 20 mg/mL | Cinnamomum vernum | [126] | |
| Dichloromethane extract | 20 mg/mL | Cinnamomum vernum | ||
| Ethanol extract | 20 mg/mL | Cinnamomum vernum | ||
| Essential oil | 27–32 mg/mL | Cinnamomum cassia | ||
| Ethanol extract | 0.78 mg/mL | Syzygiumaromaticum | ||
| Essential oil | 0.8–3.5 mg/mL | Cuminum cyminum | ||
| Aqueous extract leaves | 0.63 mg/mL | Solanum trilobatum | [137] | |
| Water, methanol, ethanol, and petroleum ether extract | 160 µg/ml | Adhatodavasica | ||
| Enterobacter spp. | Ethanol extract | 0.78 mg/mL | Syzygium aromaticum | [126] |
| Acinetobacter baumanii | Ethanol extract | 0.78 mg/mL | Syzygium aromaticum | |
| Citrobacter spp. | Ethanol extract | 039 mg/mL | Syzygium aromaticum5 | |
| Enterococcus faecalis | Essential oil | 0.125 mg/mL | Cuminum cyminum | |
| Ethanol extract | 0.125 mg/mL | Cuminium cyminum | ||
| Methanol extract | 9.63 mg/mL | Piper nigrum | ||
| Ethanol extract | 100 mg/mL | Salvia rosmarinus (Rosemary) | ||
| Proteus mirabilis | Methanol extract | 9.63 mg/mL | Piper nigrum | |
| Essential oil | 30–39 mg/mL | Cinnamomum cassia | ||
| Ethanol extract | 0.39 mg/mL | Syzygium aromaticum | ||
| Aqueous extract | 32 µg/ml | Piper betle | ||
| Enterohemorrhagic Escherichia coli O157:H7 | Essential oil | 3.12 µg/mL (Inhibition of biofilm was noticed at MIC/2 and MIC/4 concentrations) | Thymus daenensis | [139] |
| Essential oil | 6.25 µg/mL (Inhibition of biofilm was noticed at MIC/2 and MIC/4 concentrations) | Satureja hortensis | ||
| Vibrio parahaemolytics | Ethanol extract | 6.25 mg/mL (Inhibition rate of 75.46% at MIC and eradication rate of 93.26% at 32MIC) | Cinnamon | [138] |
| Bacillus paramycoides | Ethanolic extract | 0.2514 µg/mL | Zingiber officinale | [140] |
| Group | Type | Sub-Type | Characteristics | References |
|---|---|---|---|---|
| Organic | Liposomes | - | Advantages include target specificity, non-immunogenicity, low toxicity, biofilm matrix fusogenicity, adaptability for payloads, improvement of antimicrobial agent efficiency, and reduction of infection recurrence. | [147] |
| Polymeric NPs | - | They show a strong antimicrobial nature, adaptable nature, and potential to penetrate biofilms of two species. | [148] | |
| Dendrimers | Cationic Dendrimers | Multivalency, well-organised structure, and solubility in water. | [149] | |
| Cyclodextrins | - | They can easily solubilise drugs and are poorly soluble in water, and can hence act as efficient modes of drug delivery. | [150] | |
| Solid–Lipid NPs | - | They provide low toxicity and more control over the release of drugs and a low cost of production. | ||
| Inorganic | Metallic NPs | Gold | AuNPs and AgNPs disrupt bacterial membranes, interact with cytoplasmic contents, and induce oxidative stress by releasing ROS and disrupting the metabolic activities of the bacterial cell. | [151] |
| Silver | ||||
| Copper | It has an antimicrobial property and is often used in combination with other metallic nanoparticles, such as silver NPs. | [152] | ||
| Silica | It is biocompatible, has a large surface area, and allows targeted drug delivery. | [153] | ||
| Metal Oxides | Iron oxide | They are mainly used owing to their magnetic properties and high levels of biocompatibility. | [154] | |
| Copper oxide | ||||
| Fullerene | - | Surfaces coated with fullerene have been seen to have less surface area infested with biofilm, and the formed biofilm has comparatively less biomasses. | [155] | |
| Quantum Dots | They have a small size, excellent biocompatibility, and cell permeability. | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms 2023, 11, 1934. https://doi.org/10.3390/microorganisms11081934
Ali A, Zahra A, Kamthan M, Husain FM, Albalawi T, Zubair M, Alatawy R, Abid M, Noorani MS. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms. 2023; 11(8):1934. https://doi.org/10.3390/microorganisms11081934
Chicago/Turabian StyleAli, Asghar, Andaleeb Zahra, Mohan Kamthan, Fohad Mabood Husain, Thamer Albalawi, Mohammad Zubair, Roba Alatawy, Mohammad Abid, and Md Salik Noorani. 2023. "Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies" Microorganisms 11, no. 8: 1934. https://doi.org/10.3390/microorganisms11081934
APA StyleAli, A., Zahra, A., Kamthan, M., Husain, F. M., Albalawi, T., Zubair, M., Alatawy, R., Abid, M., & Noorani, M. S. (2023). Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms, 11(8), 1934. https://doi.org/10.3390/microorganisms11081934








