The Multiomics Response of Bacillus subtilis to Simultaneous Genetic and Environmental Perturbations
Abstract
1. Introduction
2. Methods
2.1. Strains and Growth Conditions
2.2. Construction of Plasmids and Transformation
2.3. Measurement of Growth and Sampling
2.4. Metabolomics Analysis
2.5. Transcriptional Analysis
2.6. Correlation between Transcriptome and Metabolome
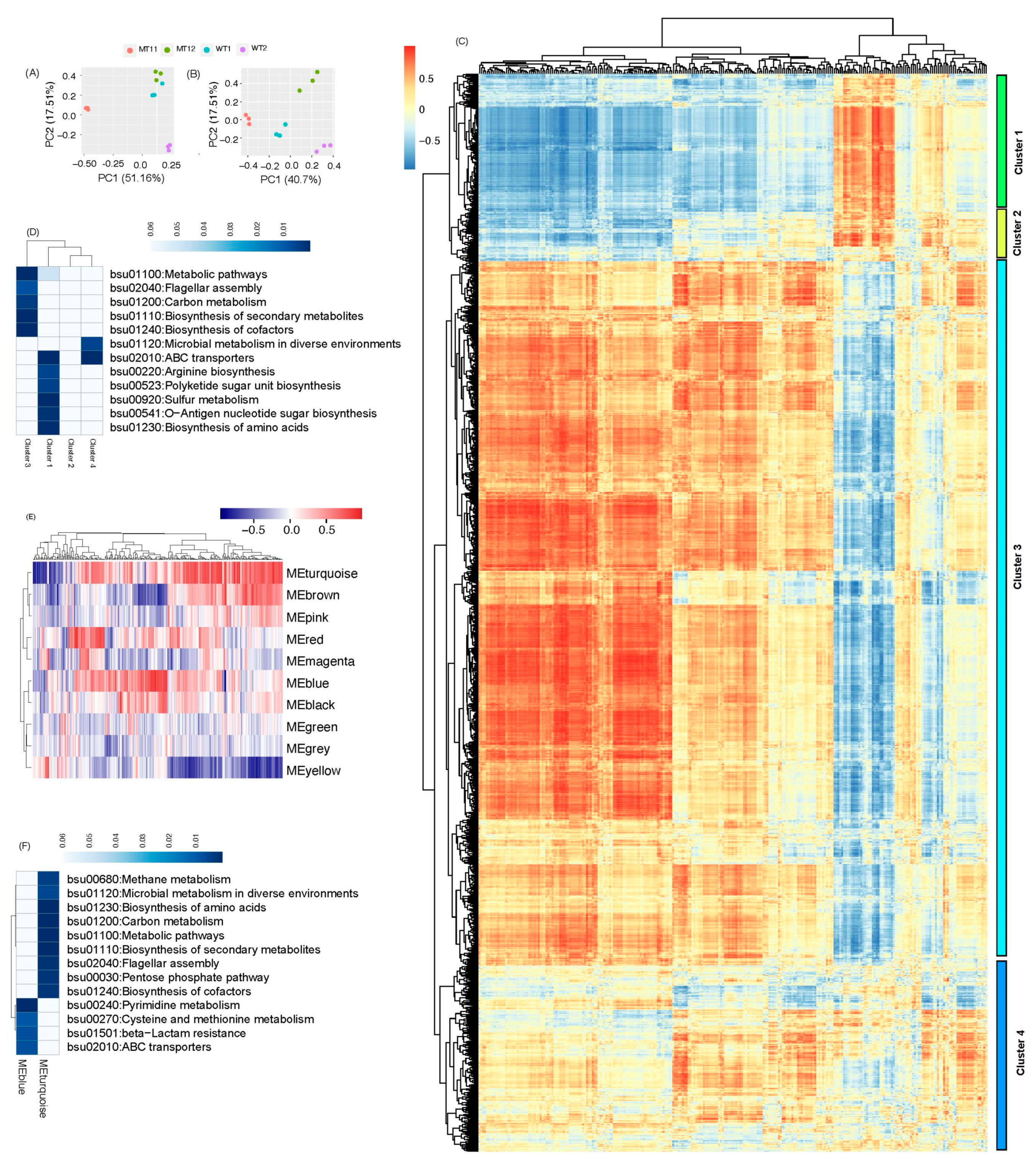
3. Results
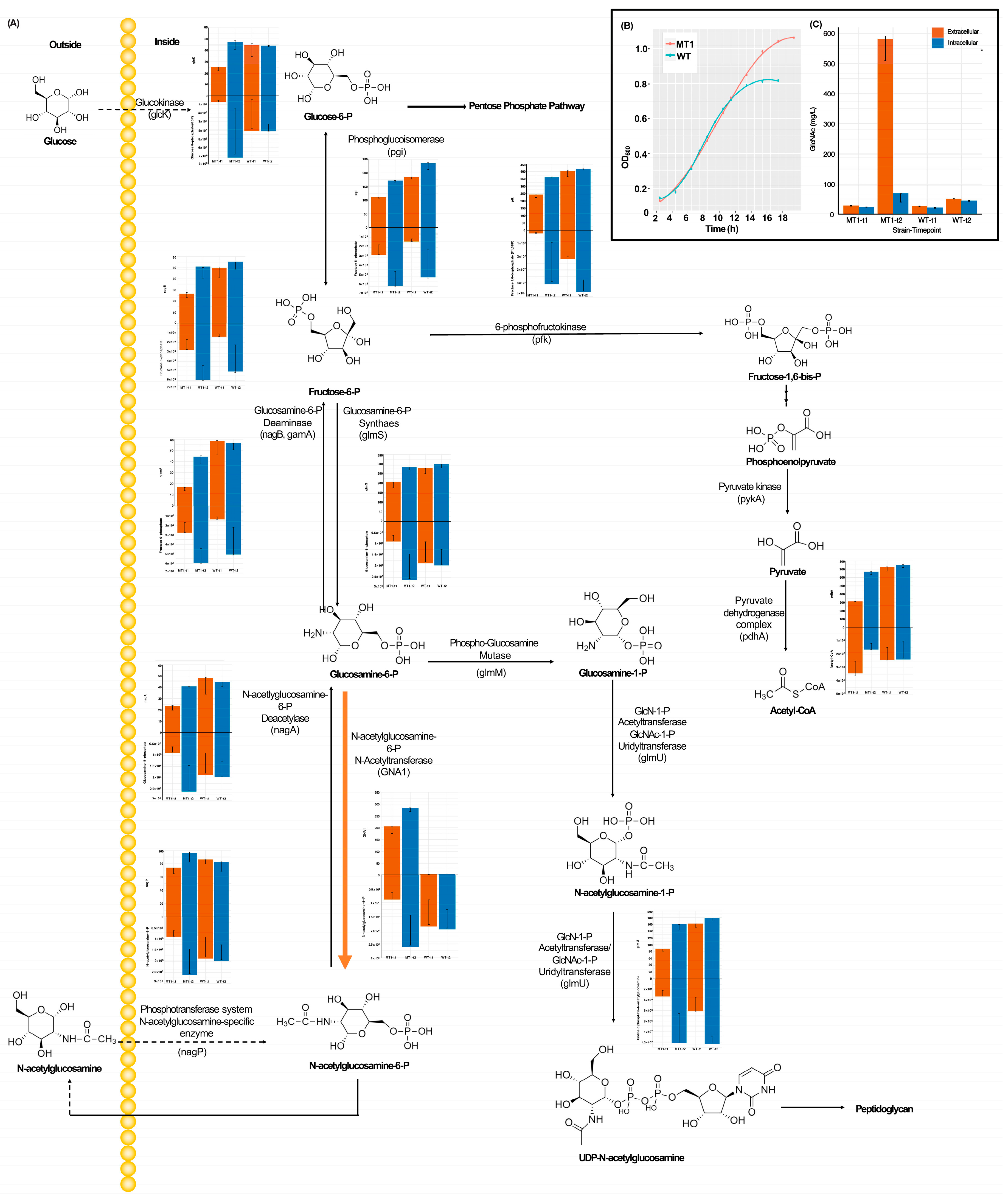
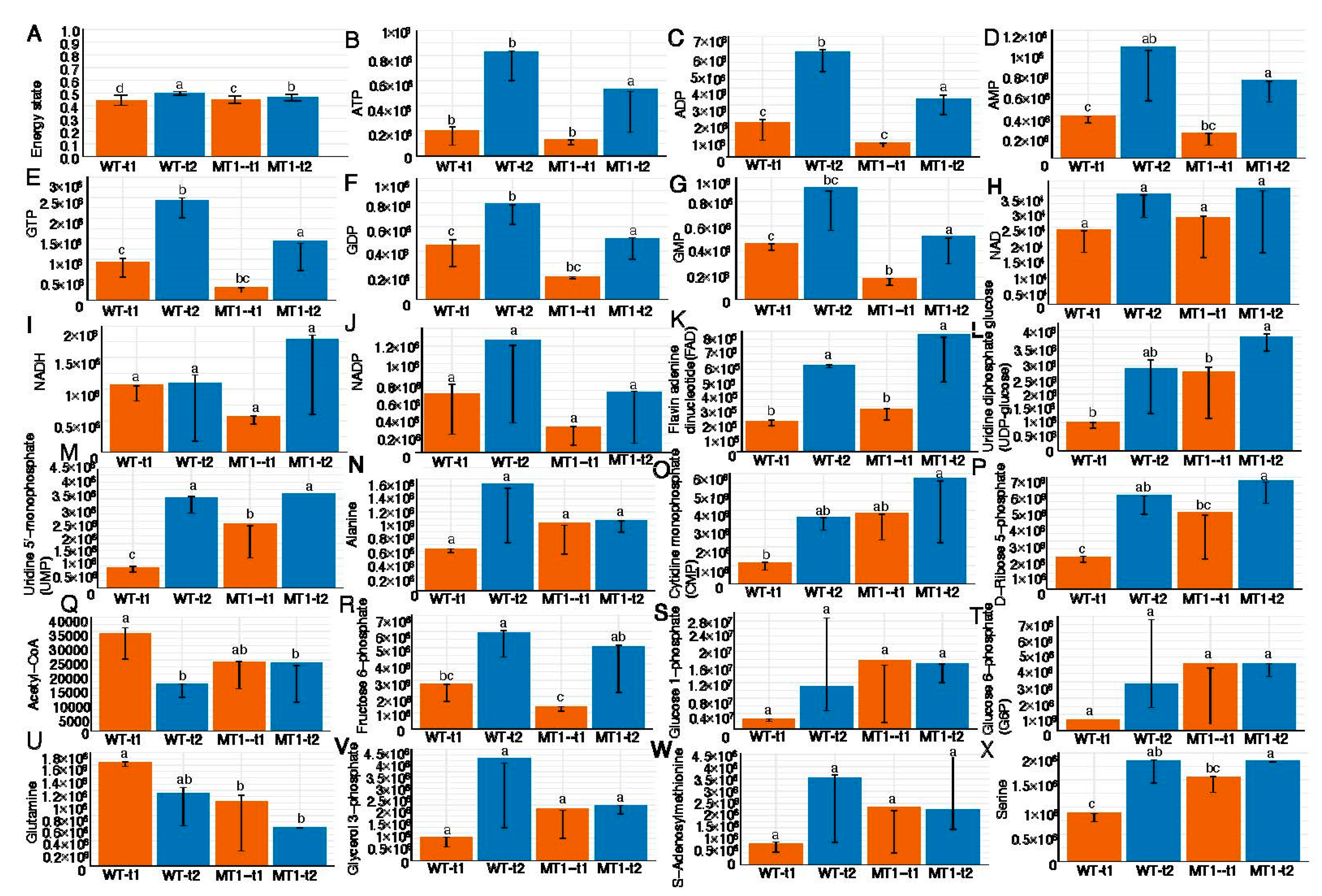
3.1. Systems Biology Responses in Central Metabolism with GlcNAc Production
3.2. Global Metabolomic Changes between Four Growth Conditions
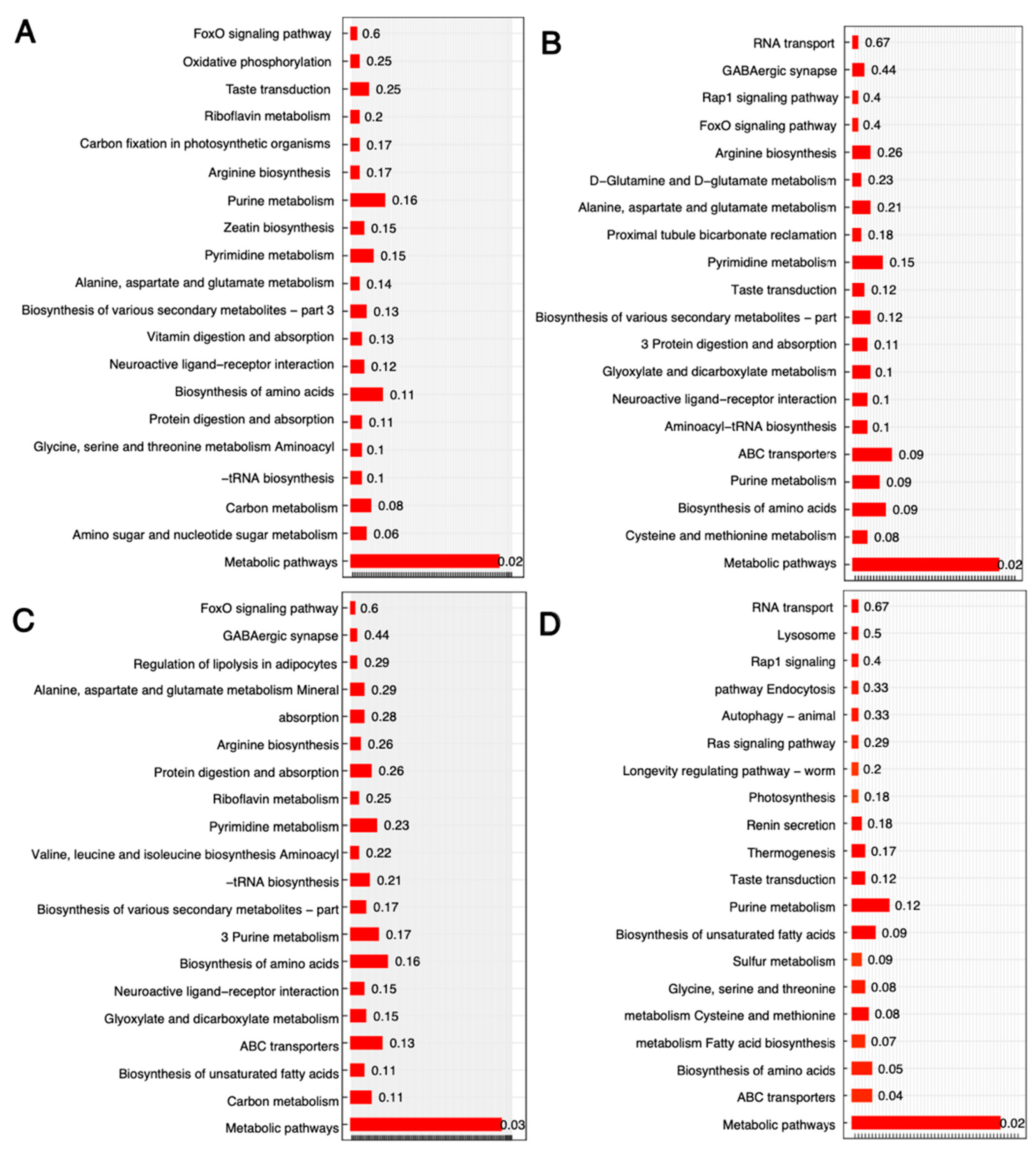
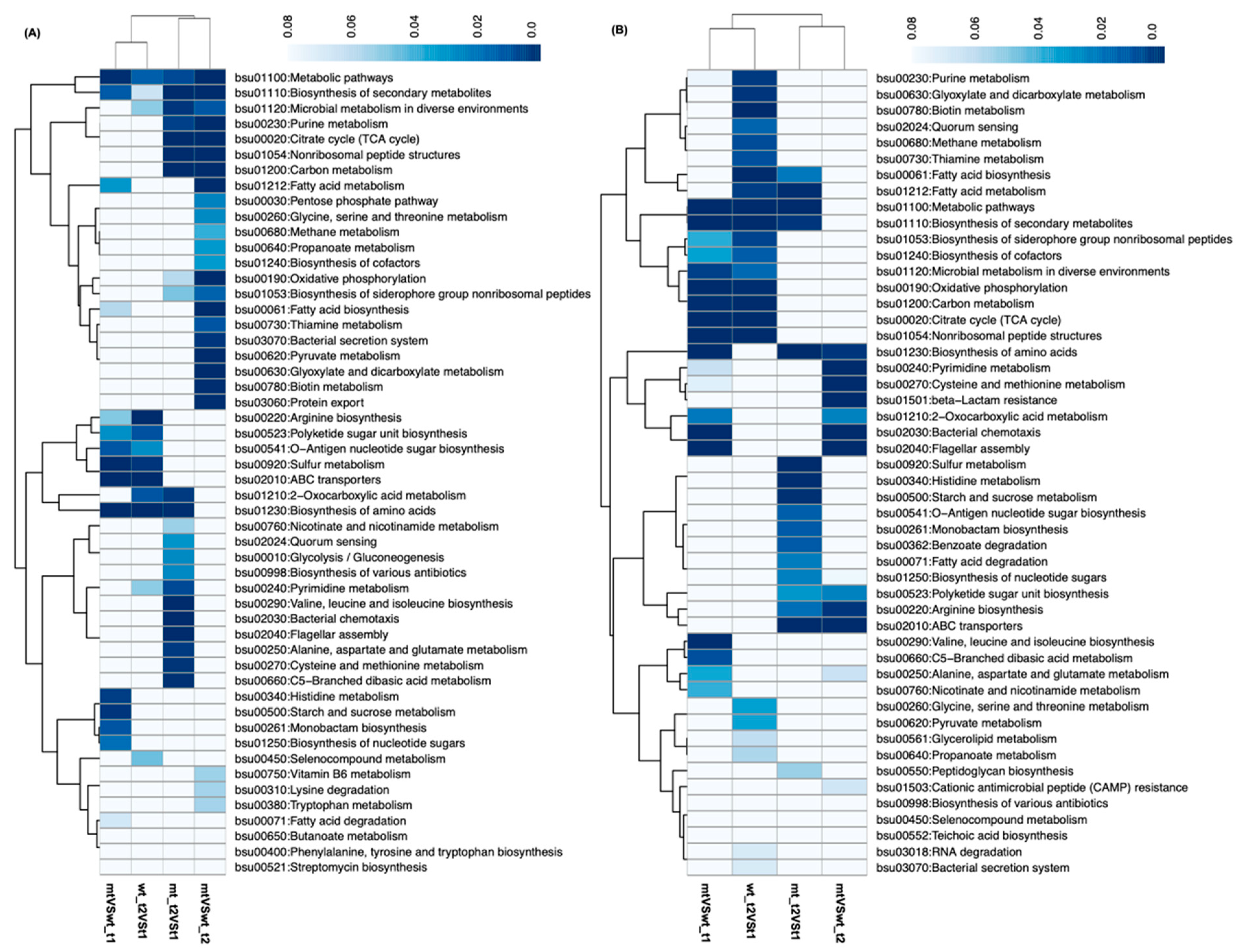
3.3. Transcriptional Changes in B. subtilis between Four CONDITIONS
3.4. Integrated Transcriptomic and Metabolomic Responses to Genetic and Environmental Perturbations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, H.; Wei, D.; Yang, Y.; Shang, Y.; Li, G.; Zhou, Y.; Ma, Q.; Xu, Y. Systems-level understanding of ethanol-induced stresses and adaptation in E. coli. Sci. Rep. 2017, 7, 44150. [Google Scholar] [CrossRef] [PubMed]
- Francine, P. Systems Biology: New Insight into Antibiotic Resistance. Microorganisms 2022, 10, 2362. [Google Scholar] [CrossRef]
- Gautam, P.; Pandey, A.K.; Dubey, S.K. Multi-omics approach reveals elevated potential of bacteria for biodegradation of imidacloprid. Environ. Res. 2023, 221, 115271. [Google Scholar] [CrossRef]
- Wu, Y.; Tao, Y.; Jin, J.; Tong, S.; Li, S.; Zhang, L. Multi-omics analyses of the mechanism for the formation of soy sauce-like and soybean flavor in Bacillus subtilis BJ3-2. BMC Microbiol. 2022, 22, 142. [Google Scholar] [CrossRef]
- Agostini, F.; Sinn, L.; Petras, D.; Schipp, C.J.; Kubyshkin, V.; Berger, A.A.; Dorrestein, P.C.; Rappsilber, J.; Budisa, N.; Koksch, B. Multiomics Analysis Provides Insight into the Laboratory Evolution of Escherichia coli toward the Metabolic Usage of Fluorinated Indoles. ACS Cent. Sci. 2021, 7, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Ropers, D.; Couté, Y.; Faure, L.; Ferré, S.; Labourdette, D.; Shabani, A.; Trouilh, L.; Vasseur, P.; Corre, G.; Ferro, M.; et al. Multiomics Study of Bacterial Growth Arrest in a Synthetic Biology Application. ACS Synth. Biol. 2021, 10, 2910–2926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Shin, H.-d.; Chen, R.R.; Li, J.; Du, G.; Chen, J. Pathway engineering of Bacillus subtilis for microbial production of N-acetylglucosamine. Metab. Eng. 2013, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Noor, E.; Eden, E.; Milo, R.; Alon, U. Central Carbon Metabolism as a Minimal Biochemical Walk between Precursors for Biomass and Energy. Mol. Cell 2010, 39, 809–820. [Google Scholar] [CrossRef]
- Kim, P.-J.; Lee, D.-Y.; Kim, T.Y.; Lee, K.H.; Jeong, H.; Lee, S.Y.; Park, S. Metabolite essentiality elucidates robustness of Escherichia coli metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 13638–13642. [Google Scholar] [CrossRef]
- Li, G.; Cao, H.; Xu, Y. Structural and functional analyses of microbial metabolic networks reveal novel insights into genome-scale metabolic fluxes. Brief. Bioinform. 2019, 20, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R. Bacillus subtilis as a Model for Bacterial Systems Biology. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Akashi, H.; Gojobori, T. Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2002, 99, 3695–3700. [Google Scholar] [CrossRef]
- Kohlstedt, M.; Sappa, P.K.; Meyer, H.; Maaß, S.; Zaprasis, A.; Hoffmann, T.; Becker, J.; Steil, L.; Hecker, M.; van Dijl, J.M.; et al. Adaptation of Bacillus subtilis carbon core metabolism to simultaneous nutrient limitation and osmotic challenge: A multi-omics perspective. Environ. Microbiol. 2014, 16, 1898–1917. [Google Scholar] [CrossRef]
- Ravasz, E.; Somera, A.L.; Mongru, D.A.; Oltvai, Z.N.; Barabási, A.L. Hierarchical Organization of Modularity in Metabolic Networks. Science 2002, 297, 1551–1555. [Google Scholar] [CrossRef]

| Strains | Characteristics | Reference |
|---|---|---|
| WT: B. subtilis 168 | Bacillus subtilis subsp. subtilis str. 168 | Lab stock |
| MT1: BNZR0.00/pP43-GNA1 | Bacillus subtilis 168 derivate, epr::PxylA-comK, trpC0, aprE::gamR-Psg2-lacO-RBS4-T7rnap-PT7gam-egfp, pP43-GNA1 | This work |
| MT2: BNZR1.06/pP43-GNA1 | BNZR0.00 derivate, ∆gamA∆nagABP∆ldh∆ackA, PlysC-glmS, pP43-GNA1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, G.; Cao, H. The Multiomics Response of Bacillus subtilis to Simultaneous Genetic and Environmental Perturbations. Microorganisms 2023, 11, 1949. https://doi.org/10.3390/microorganisms11081949
Liu L, Li G, Cao H. The Multiomics Response of Bacillus subtilis to Simultaneous Genetic and Environmental Perturbations. Microorganisms. 2023; 11(8):1949. https://doi.org/10.3390/microorganisms11081949
Chicago/Turabian StyleLiu, Li, Gaoyang Li, and Huansheng Cao. 2023. "The Multiomics Response of Bacillus subtilis to Simultaneous Genetic and Environmental Perturbations" Microorganisms 11, no. 8: 1949. https://doi.org/10.3390/microorganisms11081949
APA StyleLiu, L., Li, G., & Cao, H. (2023). The Multiomics Response of Bacillus subtilis to Simultaneous Genetic and Environmental Perturbations. Microorganisms, 11(8), 1949. https://doi.org/10.3390/microorganisms11081949






