Abstract
Antimicrobial resistance (AMR) is a public health problem threatening human, animal, and environmental safety. This study assessed the AMR profiles and risk factors associated with Escherichia coli in hospital and environmental settings in Lusaka, Zambia. This cross-sectional study was conducted from April 2022 to August 2022 using 980 samples collected from clinical and environmental settings. Antimicrobial susceptibility testing was conducted using BD PhoenixTM 100. The data were analysed using SPSS version 26.0. Of the 980 samples, 51% were from environmental sources. Overall, 64.5% of the samples tested positive for E. coli, of which 52.5% were from clinical sources. Additionally, 31.8% were ESBL, of which 70.1% were clinical isolates. Of the 632 isolates, 48.3% were MDR. Most clinical isolates were resistant to ampicillin (83.4%), sulfamethoxazole/trimethoprim (73.8%), and ciprofloxacin (65.7%) while all environmental isolates were resistant to sulfamethoxazole/trimethoprim (100%) and some were resistant to levofloxacin (30.6%). The drivers of MDR in the tested isolates included pus (AOR = 4.6, CI: 1.9–11.3), male sex (AOR = 2.1, CI: 1.2–3.9), and water (AOR = 2.6, CI: 1.2–5.8). This study found that E. coli isolates were resistant to common antibiotics used in humans. The presence of MDR isolates is a public health concern and calls for vigorous infection prevention measures and surveillance to reduce AMR and its burdens.
1. Introduction
Antimicrobial resistance (AMR) is a growing public health crisis that affects both human and animal health and has a strong relationship with the environment [1,2,3,4,5]. The concerns regarding AMR have been steadily increasingworldwide, endangering a wide variety of effective medical interventions (e.g., surgery, chemotherapy, intensive care), and the ability to effectively prevent and cure infectious diseases [6,7]. It has been established by numerous studies that the overuse and misuse of antibiotics in the agricultural, veterinary, and human medical sectors promote the development and spread of multidrug-resistant (MDR) pathogens and allow for the emergence of novel resistance mechanisms [8,9,10,11]. Additionally, antimicrobial-resistant bacteria and their resistomes spread between humans, animals, and their environment [12,13,14]. In real-world settings, infections caused by MDR bacteria are associated with increased morbidity and mortality [4,6,7,15]. The significance of MDR infections has been estimated by the Global Burden of Disease (GBD) study, where it was shown that ~1.27 million (95% UI: 0.91–1.71 million) deaths were directly attributable to bacterial MDR globally in the year 2019 alone [6,16]. Among these MDR bacteria, Escherichia coli (E. coli), Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa were the most significant and associated with ~0.93 million (95% UI: 0.66–1.27 million) directly attributable deaths [6,7,16]. Alongside these consequences, other AMR implications include increased medical costs with a negative impact on the economy (both due to direct and indirect costs), and limited options to treat infections, endangering sustainable development globally [15,17,18,19,20,21].
E. coli, a gram-negative rod belonging to the Enterobacterales order, is a lactose and non-lactose fermenting microbe [22,23,24]. It is among the microorganisms that have developed considerable levels of resistance to most antimicrobials used in humans, animals, and agriculture, and has the potential to spread effectively in the environment [1,25,26,27,28,29,30,31]. The injudicious handling of antimicrobials in the One Health ecology further exacerbates the resistance situation [32,33,34,35,36,37]. E. coli is considered a major cause of pediatric infections that result in adverse outcomes [37,38]. It has been reported as one of the most common pathogens responsible for infections, particularly in countries with unstable healthcare and surveillance systems [6,39,40,41,42]. Despite being a common member of the intestinal microbiota in humans and animals, E. coli is also found in water, soil, and around plants, and is the leading cause of several common bacterial infections, including gastroenteritis, urinary tract infections (UTIs), septicemia, and neonatal meningitis [2,43,44,45,46,47,48,49]. In recent years, the rise of MDR E. coli has been documented in almost all countries worldwide [43,46,50,51,52,53]. The spread of extended-spectrum β-lactamase (ESBL)-producing bacteria (including ESBL-E. coli; ESBL-EC) through the environment—a major cause of healthcare problems (i.e., in healthcare-associated infections) and community-acquired infections—is caused by the increasing global dependence and use of β-lactam antibiotics (i.e., penicillins, cephalosporins, carbapenems, and monobactams), which necessitates urgent action [54,55,56,57].
ESBL-EC possesses various β-lactamase enzymes that rapidly evolve through the ability to hydrolyze antimicrobials and cause increased resistance to β-lactam antibiotics [57,58]. Extended-spectrum cephalosporins—such as cefotaxime, ceftriaxone, and ceftazidime—and monobactams (such as aztreonam) are susceptible to hydrolysis by ESBLs [59,60]. Resistance to other antimicrobial classes, such as aminoglycosides, macrolides, tetracyclines, quinolones, and sulfonamides, may be acquired by plasmid-encoded resistance determinants—coexisting in bacteria-harbouring ESBLs—rapidly reaching the phenotype of MDR, which further limits therapeutic options and poses a therapeutic conundrum [2,16,60,61]. β-lactam antibiotics are among the most commonly administered drugs globally, as they have an advantageous side effect profile and in many patient populations (i.e., children, the elderly, and pregnant women), they are the only suitable antimicrobials [62,63,64,65]. However, the development of resistance to these agents in recent years has become a serious public health concern [66,67,68]; this is especially true in low-income countries, such as Zambia, where β-lactam antibiotics are overused and misused and are often readily available without a medical prescription [63,64,69]. In addition to β-lactamases, the modification of penicillin-binding proteins (PBPs), the decreased permeability of the bacterial outer membrane, and the co-existence of several resistance mechanisms contributed to this phenomenon [57]. One of the direct causes of the development of ESBL strains in resource-constrained healthcare settings includes the empirical and symptomatic (without a diagnosis) use of antibiotics [70]. Despite multiple cases of nosocomial outbreaks attributed to these pathogens, there is limited information regarding the frequency of ESBL-producing bacteria in most Zambian hospitals.
Due to the isolation of MDR bacteria—such as E. coli—from humans, animals, and the environment, it is being increasingly understood that a One Health approach is required to address this problem [45,71,72]. This is because there is a clear interaction between humans and animals in the environment that can facilitate the transmission of E. coli from humans to animals or the environment and vice-versa [73]. Moreover, the presence of antimicrobial-resistant E. coli and other pathogens in humans, animals, and the environment calls for a holistic, multi-disciplinary collaborative action guided by the World Health Organization Global Action Plan (GAP) on AMR [74]. The One Health approach aims to address AMR across all the abovementioned domains (i.e., humans, animals, and the environment), as there is a higher transmission potential at the human and animal interface in the environment [1,5,75,76]. Therefore, a One Health approach (i.e., the systems thinking within ecological systems) promotes, and is an integral part of, antimicrobial stewardship (AMS) programmes for the prudent of antimicrobials in humans, animals, and the environment [77,78,79,80,81]. Most AMR data comes from high-income countries (HICs), while the AMR burden of sub-Saharan Africa (SSA)—including Zambia—is inadequately documented. In Zambia, the National Action Plan (NAP) on AMR was developed in 2017 in line with the GAP on AMR to tackle this problem using a One Health approach [82,83]. Alongside this, there have been some studies published to promote AMS in human and animal health in this geographical region [27,28,63,64,84,85,86,87,88,89,90,91]. However, there is still very little information on the isolation, resistance patterns, and risk factors associated with ESBL-producing and/or MDR E. coli originating in humans, food-producing animals, other food products, and the environment. With this in mind, this study aimed to comprehensively assess the AMR patterns and risk factors associated with ESBL-producing and MDR E. coli in hospital and environmental settings in Lusaka, Zambia.
2. Materials and Methods
2.1. Study Design and Site Location
The present cross-sectional study was conducted between April and August 2022 at the main referral University Teaching Hospital (UTH) and townships (administrative sub-districts) in the capital city of Zambia, Lusaka. The samples collected and included in the data analysis were (i) clinical samples (i.e., urine, stool, blood, cerebrospinal fluid) from inpatients and outpatients and (ii) environmental samples (i.e., meat, fruits and vegetables, water, and those isolated from hospital equipment). The UTH has a bed capacity of 1665 beds, acting as a national and the largest tertiary referral hospital in Lusaka that provides specialised patient care for patients from all over Zambia. Lusaka (360 km2) is the capital city of Zambia, with an estimated 687,923 households [92], and a human population of approximately 3,079,964 [93].
2.2. Data Collection
Data collection for clinical samples included the date and time of sample collection, sample type, anonymised identification code, and the age and sex of the patients. Information corresponding to the environmental samples included the source, type and area sampled. Only the samples kept at an ambient temperature for no longer than two hours were included in the study.
2.3. Specimen Collection and Processing
The environmental samples were first swabbed and enriched in buffered peptone water (BPW) (Oxoid Ltd., Basingstoke, Hampshire, UK) and incubated for 3 h at 37 °C. A sterile loop was dipped into the enriched BPW, where the sample was incubated. Afterwards, a 0.5 mL sample of incubated BPW was inoculated on CHROMagar™ ECC (E. coli and coliforms; HiMedia Laboratories Pvt. Ltd., Mumbai MS, India) agar plates at 37 °C for 18 to 24 h for the isolation of E. coli. Presumptive E. coli colonies were streaked on Eosin Methylene Blue (EMB) agar (Oxoid™, Basingstoke, Hampshire, UK) for the identification of E. coli.
For clinical specimens, the presumptive identification of E. coli colonies was defined as the growth of lactose-fermenting, donut-shaped colonies on Xylose Lysine Deoxycholate (XLD) agar (Oxoid Ltd., Basingstoke, Hampshire, UK), MacConkey agar (Oxoid Ltd., Basingstoke, Hampshire, UK), and Hichrome chromogenic UTI agar (HiMedia Laboratories Pvt. Ltd., Mumbai MS, India). Therefore, urine samples were inoculated directly onto Hichrome chromogenic UTI agar and incubated at 37 °C for 18 to 24 h. Presumptive E. coli colonies were characterised by the appearance of dark blue to violet colonies. The stool was inoculated and incubated on XLD for 24 h at 37 °C, and E. coli colonies were defined after the appearance of yellow colonies. Clinical specimens were inoculated directly on MacConkey agar (Oxoid Ltd., Basingstoke, Hampshire, UK) and incubated for 24 h at 37 °C. On MacConkey agar, lactose-fermenting colonies appeared pink in colour while non-lactose-fermenting colonies appeared off-white opaque. On EMB, greenish metallic colonies were presumed to be E. coli and were sub-cultured on nutrient agar (Oxoid Ltd., Basingstoke, Hampshire, UK), where they appeared large, thin, circular, and greyish-white after 24 h of aerobic incubation at 37 °C. To differentiate E. coli from other lactose-fermenting bacteria, phenotypic confirmation was performed on all pure colonies using a battery of biochemical tests, including triple sugar iron (TSI) agar, lysine iron agar (LIA), simmons citrate agar (SCA), and sulfide indole motility (SIM) agar, respectively. Only colonies that passed the biochemical tests were identified as E. coli. The identified presumptive colonies of E. coli were selected and cultured on nutrient agar for purification purposes and further analysis. For further confirmation, E. coli isolates were subjected to identification using the Becton Dickinson BD PhoenixTM 100 system (BD Diagnostic Systems, Sparks, MD, USA).
2.4. Antibiotic Susceptibility Testing
Antimicrobial susceptibility testing (AST) for the respective E. coli isolates was performed using disk diffusion and the BD PhoenixTM 100 Automated Microbiology System (BD Diagnostic Systems, Sparks, MD; based on minimum inhibitory concentrations). The following antibiotics were used for AST: ampicillin 10 µg (AMP), amoxicillin/clavulanic acid 10 µg (AMC), cefepime 30 µg (FEP), ceftazidime 30 µg (CAZ), cephazolin 30 µg (KZ), ceftriaxone 30 µg (CRO), cefuroxime 30 µg (CXM), ciprofloxacin 5 µg (CIP), ertapenem 30 µg (ETP), gentamicin 10 µg (CN), imipenem 10 µg (IPM), levofloxacin 5 µg (LEV), nitrofurantoin 30 µg (NIT), and sulfamethoxazole/trimethoprim 23 µg (SXT). Interpretation of the AST results (i.e., defined as susceptible, intermediate or resistant) was based on the standards and breakpoints as defined by the Clinical and Laboratory Standard Institute (CLSI) [94]. Furthermore, ESBL-producing isolates were confirmed by the combined double-disk test (with cefotaxime and ceftazidime alone, and in combination with cefotaxime/clavulanic acid) and the Becton Dickinson BD PhoenixTM 100 system (Becton, Dickinson Company, Sparks, MD, USA) as defined by CLSI guidelines [94]. Each batch incorporated a control strain of E. coli ATCC 25922 to ensure the validity and reliability of AST. Isolates were classfied as MDR (resistance to at least one agent in ≥3 different antibiotic classes), extensive drug resistance (XDR; susceptibility to 1 or 2 remaining antibiotics), and pan-drug resistance (non-susceptiblity to all classes of antibiotics) (PDR) [95].
2.5. Data Analysis
The raw data of the isolates was summarised, cleaned, and coded in Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA). Descriptive analysis was conducted to characterise the data using means, medians, ranges, and percentages. Various statistical tests were employed to determine the factors associated with ESBL and MDR E. coli isolates, including Chi-square tests, univariate and multiple logistic regression (ESBL), and multinomial (MDR) analyses. The backward elimination method (MDR: based on the likelihood ratio test) was utilised to select the most relevant variables, accounting for confounding factors. Adherence to the assumptions of the Chi-square tests was ensured, and if not met, Fisher’s exact test with Monte Carlo simulation (n = 1000) was used. The analyses were performed using the Statistical Package for Social Sciences (SPSS), version 26.0 (IBM Corp, Armonk, NY, USA). The normality of the data was assessed through the Kolmogorov-Smirnov test. All statistical tests were performed at a 95% confidence level with a p < 0.05 indicating statistical significance.
3. Results
3.1. Descriptive Characteristics of Clinical and Environmental E. coli Strains
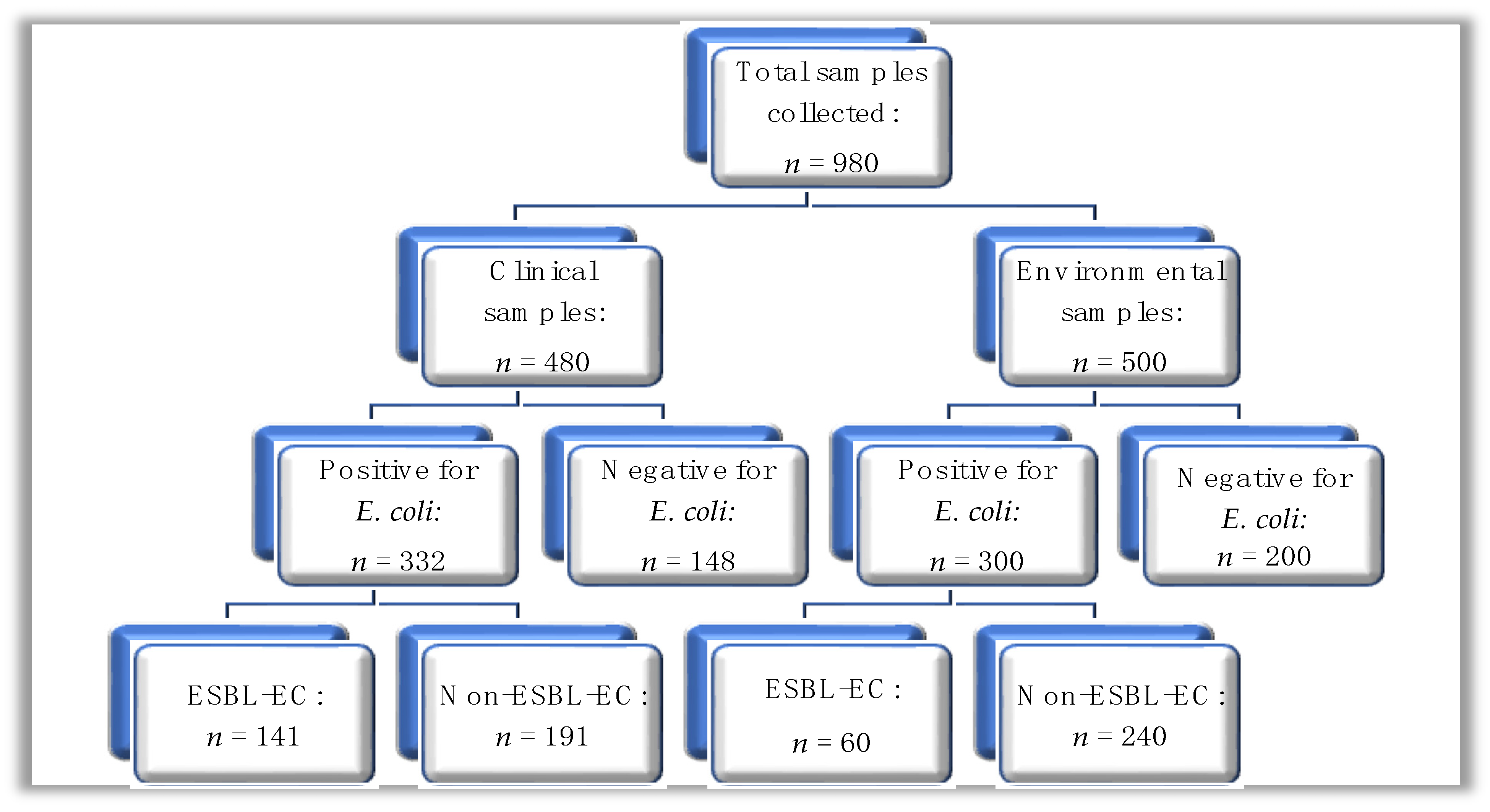
A total of n = 980 samples were collected and subjected to microbial culture for E. coli using phenotypic methods. Of the n = 980 samples, n = 480 were from clinical sources, while n = 500 were from environmental sources (Figure 1); out of the total sample number, E. coli was isolated from n = 632 (64.5%) of samples, where the distribution was n = 332 (69.2%) and n = 300 (60.0%) from clinical and environmental sources, respectively.
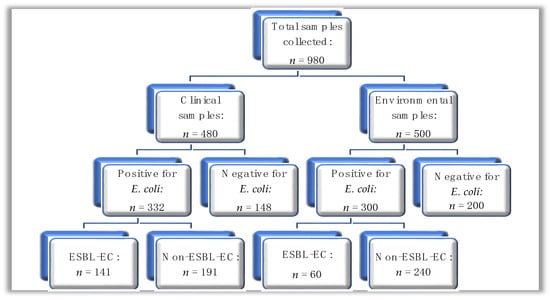
Figure 1.
A hierarchical diagram showing the summary of clinical and environmental samples processed for E. coli; ESBL-EC: ESBL-producing E. coli.
The characteristics of patients and specimens corresponding to positive clinical samples (n = 332) are summarised in Table 1. Most of the samples were from female participants (58.7%), patients aged 0 to 14 years, urine (74.4%), and outpatient department (35.5%) (Table 1).

Table 1.
Descriptive characteristics of clinical samples positive for E. coli.
The origins of the n = 300 environmental E. coli specimens are summarised in Table 2. The majority of the samples were from medical equipment, meat and fruits/vegetables (Table 2).

Table 2.
Descriptive characteristics of samples positive for E. coli from environmental sources.
3.2. Antibiotic Susceptibility Patterns of E. coli Isolated from Clinical Samples
The majority of the clinical E. coli isolates were highly resistant to AMP, SXT, CIP, KZ, and LEV (Table 3). However, the isolates were highly susceptible to ETP, IPM, NIT, and CN. Higher rates of resistance in clinical E. coli strains were shown against penicillin-derivatives, fluoroquinolones, cephalosporins, and SXT in specimens such as blood cultures, CSF, and urine; additionally, only two that were resistant to carbapenems were from pus samples (Supplementary Table S1). Most of the E. coli isolates with extensive resistance originated from general adults’ wards, the ICU (both adult and neonatal), surgical wards, and the pediatric unit; the isolates that were resistant to CL were from the adult medical ward (n = 1), the outpatient department (n = 1), and from paediatrics (n = 2); while the isolates that were resistant to carbapenems were from the outpatient department (n = 1) and surgical unit (n = 1), respectively (Supplementary Table S2).

Table 3.
Antibiotic susceptibility patterns of E. coli isolated from clinical samples.
3.3. Antibiotic Susceptibility Patterns of E. coli Isolated from Environmental Samples
Isolates of E. coli from environmental samples were highly resistant to SXT, followed by LEV and KZ. However, the isolates were highly susceptible to ETP, IPM, CN, AMP, and CRO (Table 4). Environmental E. coli showing higher rates of non-susceptibility was isolated from water, fruits/vegetables and medical equipment (Supplementary Table S3).

Table 4.
Antibiotic susceptibility patterns of E. coli isolated from environmental samples.
3.4. Prevalence of ESBL-Producing, and MDR/XDR E. coli from Clinical and Environmental Sources
Overall, 48.3% (n = 304/632) of E. coli were MDR (clinical: 67.4% [n = 205/304], environmental 32.5% [n = 99/304]), while 13.2% (n = 40/304) were XDR (clinical: 32.5% [n = 13/40], environmental 67.5% [n = 27/40]); MDR isolates were more common among E. coli from clinical sources (p = 0.021), while this association was not found for XDR isolates (p = 0.729). The detailed distribution of MDR and XDR E. coli among environmental and clinical samples is presented in Supplementary Figures S1–S3. The overall prevalence of ESBL-EC was 31.8% (n = 201), out of which, 70.1% (n = 141) were of clinical, while 29.9% n = 60 were of environmental origin; ESBL-EC were significantly more common in clinical than environmental samples (p = 0.0328).
The largest number of ESBL-EC were from samples of patients aged between 0 and 14 years, females (54.6%), urine (56.7%), pus (34%), outpatient department (27.7%), and medical equipment (43.4%) (Table 5). Statistical significance was found among isolates from CSF, urine, surgical ward, and meat (Table 5).

Table 5.
Epidemiological characteristics of ESBL-positive and negative E. coli among clinical and environmental isolates.
This study found that isolates from samples of individuals aged between 45 and 54 years (AOR = 0.175, CI: 0.047–0.651) were less likely to be ESBL-EC compared to those aged between 0 and 14 years. Additionally, isolates from CSF were less likely to be ESBL-EC (AOR = 0.050, CI: 0.005–0.363) compared to those from blood. Finally, isolates from urine were less likely to be ESBL-EC (AOR = 0.093, CI: 0.014–0.388) compared to those from blood (Table 6).

Table 6.
Risk factors associated with ESBL-producing E. coli.
Most MDR E. coli were isolated from samples of patients aged between 0 and 14 years (24.9%), males (52.7%), urine (66.3%), outpatient department (29.3%), and fruits/vegetables (44.4%). This study revealed that MDR E. coli isolates were significantly associated with age, sex, specimen type, hospital department, and environmental samples (Table 7).

Table 7.
Distribution of MDR E. coli isolates among clinical and environmental samples.
Table 8 summarises the results of the logistic regression analysis for the factors significantly associated with MDR in E. coli isolates: notably, clinical isolates originating from pus and male patients were significantly associated with the MDR phenotype; in the case of environmental sources, isolates from water were significantly associated with the MDR phenotype (Table 8).

Table 8.
Risk factors for MDR E. coli isolates from both clinical and environmental sources.
4. Discussion
AMR has become a rising global burden, endangering global public health and sustainable healthcare for both developing and developed countries [6,7]. According to the O’Neill report, AMR may become the second leading cause of death by 2050, responsible for over 10 million deaths worldwide [96]. To establish effective regional, national, and global strategies to curb AMR, it is essential to investigate the prevalence of this problem and to develop empirical treatment strategies (local antibiograms). The objective of the present study was to examine ESBL-producing E. coli and the antimicrobial susceptibility patterns of isolates from various clinical and environmental sources to a wide range of antibiotic groups. Production of β-lactamase enzymes—especially ESBLs, owing to their rapid and successful spread across the globe, is one of the most significant mediators conferring resistance to a wide range of β-lactams in E. coli [43,97]. These enzymes form a large class of resistance determinants that are frequently encoded on plasmids and are a major driver in the emergence of MDR that confers resistance to penicillins and cephalosporins. Due to their considerable prevalence, clinicians are now often forced to use carbapenems (the last of the β-lactams), or other antibiotics with more disadvantageous adverse effects [98].
This present study investigated the AMR profiles and risk factors associated with ESBL-producing E. coli in hospital and environmental settings in Zambia. This study found that the prevalence of E. coli was 64.5%, of which 52.5% were from clinical sources. Additionally, 31.8% were ESBL, of which 70.1% were clinical isolates. Of the 632 isolates, 48.3% were MDR. Most clinical isolates were resistant to AMP (83.4%), SXT (73.8%), and CIP (65.7%) while most environmental isolates were resistant to SXT (100%). The risk factors associated with MDR of the tested E. coli isolates included pus, male sex, and water. Finally, E. coli isolates from samples of patients aged from 45 to 54 years and urine were less likely to be ESBL-producers.
The present study found the prevalence of E. coli to be 64.5%, of which 52.5% were isolated from clinical samples and 47.5% from environmental samples. Our low prevalence of E. coli isolated from the environment compared to the hospital setting could be due to the challenges in isolation methods of E. coli from environmental samples [99]. The prevalence of E. coli found in our study is higher than that reported in Pakistan where 23.75% of E. coli were isolated from urine samples [100]. A study in Poland reported a higher E. coli isolation rate of 78% (identified using 16S rRNA sequencing) and 82% (identified using MALDI Biotype) from river water and wastewater [100]. However, our isolation rate of E. coli from water samples was lower compared to that reported in Pakistan where the researchers found an isolation rate of 26.7% [100]. These differences in isolation rates could be due to technical differences and slight variability in methods. Interestingly, the isolation of E. coli from environmental and clinical samples demonstrates the need for a One Health approach in the surveillance of infections and AMR [73,101]. Additionally, genomic surveillance of priority pathogens should be promoted [102].
The current study found that most clinical E. coli isolates were highly resistant to AMP. Our findings corroborate findings from other studies where E. coli isolates from clinical and environmental samples were highly resistant to penicillins such as AMP [85,103,104,105,106,107,108,109]. The high resistance of E. coli to ampicillin can be attributed to its potential to develop intrinsic resistance against penicillins. Additionally, exposure to antibiotics such as penicillins also contributes to the high resistance of E. coli reported in many studies [47,48,50,85,89,91,110]. Conversely, a lower resistance rate of E. coli to ampicillin has been reported in other studies’ findings. The lower resistance can be due to the low use of antibiotics in other settings. Other studies found a high resistance of E. coli isolated from clinical and environmental samples to SXT [103,109,111,112,113]. The high resistance of E. coli to SXT could be due to its overuse and misuse in humans and animals [85,114,115,116]. However, a low resistance rate of E. coli to SXT was reported in a study that was conducted in Turkey among outpatients [117]. Similarly, low resistance of E. coli to SXT was reported in Australia due to the restriction of antibiotic use [118]. Hence, restricting the use of antibiotics may help curb AMR [119,120].
Our study also found high resistance of E. coli to quinolones such as CIP and LEV. The high resistance of E. coli to quinolones has been reported in other studies [114,121,122,123,124,125,126]. This high resistance could be due to the overuse and misuse of quinolones in human and animal health systems [114]. This is a huge problem because quinolones are largely used to treat urinary tract infections, respiratory tract infections, and other infections. However, lower resistance rates of E. coli to quinolones have been reported in similar settings [103,127]. This low use could be due to the effective implementation of AMS programmes in healthcare facilities. High resistance of E. coli to cephalosporins such as cefuroxime was found in our study. This is similar to a study that was conducted in Uganda and Nigeria, where E. coli isolates were 100% resistant to cefuroxime [109,125], and in South Africa where high resistance of E. coli to cephalothin was reported [128]. High resistance to Ceftriaxone [129,130], Ceftazidime [125], and Nalidixic acid [125] has also been reported. In Zambia, there is an overuse and misuse of antibiotics such as cephalosporins, which could be a driver of the high resistance [63,64,90,131].
The present study found that E. coli isolates were highly susceptible to CN, ETP, IPM, and NIT. This was observed for both clinical and environmental isolates. These findings corroborate reports from a meta-analysis where E. coli was highly susceptible to antibiotics such as amikacin, IPM, and NIT [103]. High susceptibility of E. coli to gentamicin was also reported in South Africa [128] and other similar studies [106,132]. The high susceptibility of E. coli isolates to IPM was also reported in other studies [129,133,134]. The high susceptibility of E. coli to these antibiotics suggests that they are the most effective drugs for the treatment of infections caused by E. coli, such as UTIs [132].
The current study found that 48.3% of E. coli isolates were MDR. A comparable E. coli MDR prevalence of 49.48% was reported in Ghana [135]. However, the finding in our study is lower than the 52% MDR reported in South Africa [128], 63.3% in Mexico [136], 68.3% in Ethiopia [43], 80% in Brazil [137], 91.4% in the United Kingdom [133], 97% in another Mexican study [138], and 98% in Bangladesh [139]. It is well known that susceptibility patterns can change over time and can differ between geographical locations [140]. Further, the high MDR among the E. coli isolates in hospital and environmental settings is partially due to the misuse and overuse of antibiotics both in humans and the environment [141]. It is also critical to note that MDR pathogens limit antibiotic treatment options, contributing to increased morbidity and mortality globally [141]. Therefore, the study of bacterial resistance to multiple antibiotics is essential for determining the most effective therapy for the subsequent infection, as the rise of MDR bacterial strains poses a significant threat to the health of people of all ages.
ESBL-producing E. coli may arise from interactions between ESBL type, strain genetic background, and selective pressures in various ecologic niches [54,142,143,144,145]. ESBL-producing E. coli is an important cause of both nosocomial and community-onset infections globally [146]. Additionally, ESBL-producing E. coli often shows resistance to multiple drugs, which limits treatment options [56,147,148,149]. Commonly used treatments for severely ill patients, such as fluoroquinolones, aminoglycosides, and trimethoprim, are often associated with co-resistance, resulting in higher rates of morbidity and mortality [150].
In this present study, the prevalence of ESBL-producing E. coli was found to be 31.8%. A low prevalence of ESBL-producing E. coli was reported in other studies [58,151,152]. Consequently, a higher prevalence of ESBL-producing E. coli was reported in other studies, including 38% in Sudan [124], 38.07% in China [153], 42.5% in Thailand [154], 50% in Brazil [137], 55.5% in India [134], 57.7% in Ethiopia [43], 62% in Jordan [155], and 88.8% in the United Kingdom [133]. The overuse and misuse of antibiotics, especially cephalosporins and fluoroquinolones in humans, animals, and the environment, have contributed to the emergence of ESBL-producing E. coli [153]. The increased ESBL-producing E. coli indicates a greater extent of resistance to antibiotics. Consequently, increased rates of ESBL producers limit treatment options [156].
The present study found that most ESBL producers were isolated from urine (56.7%). This finding is different from a study that was conducted in India that found that most of the ESBL-producing strains were isolated from blood (66.67%) [134]. Further, our study revealed that most ESBL producers were isolated from the outpatient department, in contrast with findings from a similar study where most ESBL producers were isolated from in-patients [134]. A study in the United Arabs Emirates (UAE) reported that ESBL-producing E. coli were responsible for 75% of UTIs in communities, indicating their high prevalence in outpatients [157]. Our study revealed that E. coli isolates from samples of patients aged between 45 and 54 years, CSF, and urine were less likely to be ESBL-producers. Older age was found to be a risk factor for ESBL-producing E. coli [158]. Similar studies have reported other risk factors of ESBL-producing E. coli, including previous hospitalisations, and use of urinary catheters [155].
In this study, most MDR E. coli isolates were isolated from samples of patients aged between 0 and 14 years, males, urine, outpatient department, and fruits/vegetables. The isolation of MDR E. coli from similar samples has been reported in other studies [159]. Additionally, E. coli isolates from males were more likely to be MDR than those from female patients. This is in line with other studies that reported similar results of males having higher odds of harbouring MDR E. coli isolates than females [160,161]. The impact of sex on the pattern of resistance was solely dependent on the clinical factors and location of the samples within the clinical isolates. Further, the risk of isolating MDR E. coli in our study was noted from pus samples. Our findings are similar to reports from previous studies which reported a larger fraction of MDR E. coli from pus [162,163]. However, some studies revealed that urine had a high prevalence of MDR E. coli [164,165,166,167,168]. Additionally, the present study found that water (drinking water from the community taps, boreholes, and wells) was significantly associated with MDR. This may be due to contaminated water sources within the communities or poor water quality. This is similar to a study in Zambia that reported that shallow water in peri-urban areas was significantly more contaminated with E. coli [169]. Our findings conform to other studies that have demonstrated the presence of high rates of MDR E. coli in water samples [170,171,172,173,174,175]. These findings indicate the presence of MDR E. coli in various samples.
We are aware of the limitations of our study: This study was conducted in the Lusaka province of Zambia; therefore, generalisation of the findings should be performed with caution. Additionally, we did not collect equal numbers of clinical and environmental samples, which may affect the comparison of results. However, we believe that the obtained results on the AMR patterns of E. coli isolated from clinical and environmental settings require heightened surveillance programs. Additionally, the identified risk factors, including isolates from pus, male sex, and water samples, emphasise the need for a One Health approach, which is critical to the surveillance of AMR across the human, animal, and environmental sectors.
5. Conclusions
This study reported a high prevalence rate of ESBL-producing E. coli among clinical and environmental samples. Most of these E. coli strains showed multiple AMR patterns to commonly used antibiotics, most of which were MDR and potential XDR strains. Significantly, risk factors in ESBL strains were associated with pus and blood specimens, with most isolates showing high resistance to cephalosporins, fluoroquinolones, ampicillin, and colistin, and only a few isolates being sensitive to aminoglycosides and carbapenems. The importance of these findings was the identification of ESBL-producing E. coli in humans, animals, and the environment. This suggests that surveillance and routine screening for MDR and ESBL-producing E. coli is important to control the spread of resistant strains as part of a One Health approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11081951/s1, Table S1. Antimicrobial Susceptibility of Clinical E. coli categorised according to sample types; Table S2. Antimicrobial Susceptibility of Clinical E. coli categorised according to hospital departments; Table S3. Antimicrobial Susceptibility of Environmental E. coli categorised according to sample types; Figure S1. Distribution of MDR and XDR environmental E. coli isolates categorised according to sample types; Figure S2. Distribution of MDR and XDR clinical E. coli isolates categorised according to sample types; Figure S3. Distribution of MDR and XDR clinical E. coli isolates categorised according to hospital departments.
Author Contributions
Conceptualization: M.K. (Maisa Kasanga), J.W., D.C. and G.K.; Methodology: M.K (Maisa Kasanga), S.M., M.K. (Maika Kasanga), Z.M. and G.K.; Formal analysis: S.M., R.C., J.W., D.C., M.K. (Maisa Kasanga) and G.K.; Investigation: B.Y., M.S., E.M., D.M.S., Z.M., B.B.S., C.P., M.J.M. and M.K. (Maika Kasanga); Resources: D.C., M.K. (Maika Kasanga) and Z.M.; Supervision: G.K, D.C. and S.M.; Project administration: M.K. (Maisa Kasanga), J.W., D.C. and G.K.; Funding acquisition: D.C.; Writing—original draft: M.K. (Maisa Kasanga), J.W., D.C., S.M. and G.K.; Writing—review and editing: G.K., M.K. (Maisa Kasanga), J.W., D.C., S.M. and M.K. (Maika Kasanga). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union Horizon 2020 research and innovation programme/Trials of Excellence in Southern Africa (EDCTP2/TESA III), Key Project of TESA III (CSA2020NOE3104).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and national and institutional ethical standards. Ethical approval for the study protocol was obtained from the Excellence in Research Ethics and Science (ERES) Converge (reference number: 2022-May-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during the study are presented in this paper and its Supplementary Materials.
Acknowledgments
The authors are thankful to EDCTP2 and TESA III and ECDP for the financial support. We are aso grateful to the University of Zambia e-library for providing us with access to most of the articles cited in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iramiot, J.S.; Kajumbula, H.; Bazira, J.; Kansiime, C.; Asiimwe, B.B. Antimicrobial resistance at the human-animal interface in the Pastoralist Communities of Kasese District, South Western Uganda. Sci. Rep. 2020, 10, 14737. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ding, Y.; Yao, K.; Gao, W.; Wang, Y. Antimicrobial Resistance Analysis of Clinical Escherichia coli Isolates in Neonatal Ward. Front. Pediatr. 2021, 9, 670470. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Chang, D.; Sharma, L.; Dela Cruz, C.S.; Zhang, D. Clinical Epidemiology, Risk Factors, and Control Strategies of Klebsiella pneumoniae Infection. Front. Microbiol. 2021, 12, 750662. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Abass, A.; Ahmed, M.; Ibrahim, I.; Yahia, S. Bacterial Resistance to Antibiotics: Current Situation in Sudan. J. Adv. Microbiol. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Nariya, H.; Matsumoto, T.; Shimamoto, T. High Prevalence of Antimicrobial Resistance in Gram-Negative Bacteria Isolated from Clinical Settings in Egypt: Recalling for Judicious Use of Conventional Antimicrobials in Developing Nations. Microb. Drug Resist. 2019, 25, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Nossair, M.A.; Abd El Baqy, F.A.; Rizk, M.S.Y.; Elaadli, H.; Mansour, A.M.; El-Aziz, A.H.A.; Alkhedaide, A.; Soliman, M.M.; Ramadan, H.; Shukry, M.; et al. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamases and AmpC β-lactamase-Producing Enterobacteriaceae among Human, Cattle, and Poultry. Pathogens 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.M.; Ahmed, S.F.; Klena, J.D.; Mohamed, Z.K.; Moussa, T.A.A.; Ghenghesh, K.S. Enteroaggregative Escherichia coli in diarrheic children in Egypt: Molecular characterization and antimicrobial susceptibility. J. Infect. Dev. Ctries. 2014, 8, 589–596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Azab, K.S.M.; Abdel-Rahman, M.A.; El-Sheikh, H.H.; Azab, E.; Gobouri, A.A.; Farag, M.M.S. Distribution of extended-spectrum β-lactamase (Esbl)-encoding genes among multidrug-resistant gram-negative pathogens collected from three different countries. Antibiotics 2021, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, T.M.; Dyda, A.; Merlo, G.; Hall, L. Disease burden, associated mortality and economic impact of antimicrobial resistant infections in Australia. Lancet Reg. Health-West. Pacific 2022, 27, 100521. [Google Scholar] [CrossRef]
- Innes, G.K.; Randad, P.R.; Korinek, A.; Davis, M.F.; Price, L.B.; So, A.D.; Heaney, C.D. External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock. Annu. Rev. Public Health 2019, 41, 141–157. [Google Scholar] [CrossRef]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P. Drug-resistant infections: A Threat to Our Economic Future. World Bank Rep. 2017, 2, 1–132. [Google Scholar]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Mazumder, R.; Hussain, A.; Phelan, J.E.; Campino, S.; Haider, S.M.A.; Mahmud, A.; Ahmed, D.; Asadulghani, M.; Clark, T.G.; Mondal, D. Non-lactose fermenting Escherichia coli: Following in the footsteps of lactose fermenting E. coli high-risk clones. Front. Microbiol. 2022, 13, 1027494. [Google Scholar] [CrossRef]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef] [PubMed]
- Yaratha, G.; Perloff, S.; Changala, K. Lactose vs Non-Lactose Fermenting E. coli: Epidemiology, Clinical Outcomes, and Resistance. Open Forum Infect. Dis. 2017, 4, S589–S590. [Google Scholar] [CrossRef]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef]
- Muloi, D.; Kiiru, J.; Ward, M.J.; Hassell, J.M.; Bettridge, J.M.; Robinson, T.P.; van Bunnik, B.A.D.; Chase-Topping, M.; Robertson, G.; Pedersen, A.B.; et al. Epidemiology of antimicrobial-resistant Escherichia coli carriage in sympatric humans and livestock in a rapidly urbanizing city. Int. J. Antimicrob. Agents 2019, 54, 531–537. [Google Scholar] [CrossRef]
- Phiri, N.; Mainda, G.; Mukuma, M.; Sinyangwe, N.N.; Banda, L.J.; Kwenda, G.; Muonga, E.M.; Flavien, B.N.; Mwansa, M.; Yamba, K.; et al. Antibiotic-resistant Salmonella species and Escherichia coli in broiler chickens from farms, abattoirs, and open markets in selected districts of Zambia. J. Epidemiol. Res. 2020, 6, 13–21. [Google Scholar] [CrossRef]
- Muligisa-Muonga, E.; Mainda, G.; Mukuma, M.; Kwenda, G.; Hang’ombe, B.; Flavien, B.N.; Phiri, N.; Mwansa, M.; Munyeme, M.; Muma, J.B. Antimicrobial resistance of Escherichia coli and Salmonella isolated from retail broiler chicken carcasses in Zambia. J. Epidemiol. Res. 2021, 6, 35–43. [Google Scholar] [CrossRef]
- Kabali, E.; Pandey, G.S.; Munyeme, M.; Kapila, P.; Mukubesa, A.N.; Ndebe, J.; Muma, J.B.; Mubita, C.; Muleya, W.; Muonga, E.M.; et al. Identification of Escherichia coli and related enterobacteriaceae and examination of their phenotypic antimicrobial resistance patterns: A pilot study at a wildlife-livestock interface in Lusaka, Zambia. Antibiotics 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Nomamiukor, B.O.; Horner, C.; Kirby, A.; Hughes, G.J. Living conditions are associated with increased antibiotic resistance in community isolates of Escherichia coli. J. Antimicrob. Chemother. 2015, 70, 3154–3158. [Google Scholar] [CrossRef]
- Leski, T.A.; Taitt, C.R.; Bangura, U.; Stockelman, M.G.; Ansumana, R.; Cooper, W.H.; Stenger, D.A.; Vora, G.J. High prevalence of multidrug-resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect. Dis. 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Chem, E.D.; Anong, D.N.; Akoachere, J.F.K.T. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS ONE 2018, 13, e0193353. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chusri, S.; Sangthong, R.; McNeil, E.; Hu, J.; Du, W.; Li, D.; Fan, X.; Zhou, H.; Chongsuvivatwong, V.; et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS ONE 2018, 14, e0214779. [Google Scholar] [CrossRef]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Tola, M.A.; Abera, N.A.; Gebeyehu, Y.M.; Dinku, S.F.; Tullu, K.D. High prevalence of extended-spectrum beta-lactamase- producing Escherichia coli and Klebsiella pneumoniae fecal carriage among children under five years in Addis Ababa, Ethiopia. PLoS ONE 2021, 16, e0258117. [Google Scholar] [CrossRef]
- MacKinnon, M.C.; McEwen, S.A.; Pearl, D.L.; Lyytikäinen, O.; Jacobsson, G.; Collignon, P.; Gregson, D.B.; Valiquette, L.; Laupland, K.B. Increasing incidence and antimicrobial resistance in Escherichia coli bloodstream infections: A multinational population-based cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 131. [Google Scholar] [CrossRef]
- Tadesse, S.; Mulu, W.; Genet, C.; Kibret, M.; Belete, M.A. Emergence of High Prevalence of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Enterobacteriaceae Species among Patients in Northwestern Ethiopia Region. Biomed Res. Int. 2022, 2022, 5727638. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Irek, E.O.; Amupitan, A.A.; Obadare, T.O.; Aboderin, A.O. A systematic review of healthcare-associated infections in Africa: An antimicrobial resistance perspective. Afr. J. Lab. Med. 2018, 7, a796. [Google Scholar] [CrossRef] [PubMed]
- Teklu, D.S.; Negeri, A.A.; Legese, M.H.; Bedada, T.L.; Woldemariam, H.K.; Tullu, K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 2019, 8, 39. [Google Scholar] [CrossRef]
- Park, S.; So, H.J.; Kim, M.N.; Lee, J. Initial empirical antibiotics of non-carbapenems for ESBL-producing E. coli and K. pneumoniae bacteremia in children: A retrospective medical record review. BMC Infect. Dis. 2022, 22, 866. [Google Scholar] [CrossRef] [PubMed]
- Robert, E.; Grippa, M.; Nikiema, D.E.; Kergoat, L.; Koudougou, H.; Auda, Y.; Rochelle-Newall, E. Environmental determinants of E. coli, link with the diarrheal diseases, and indication of vulnerability criteria in tropical west africa (kapore, burkina faso). PLoS Neglected Trop. Dis. 2021, 15, e0009634. [Google Scholar] [CrossRef] [PubMed]
- Mfoutou Mapanguy, C.C.; Adedoja, A.; Kecka, L.G.V.; Vouvoungui, J.C.; Nguimbi, E.; Velavan, T.P.; Ntoumi, F. High prevalence of antibiotic-resistant Escherichia coli in Congolese students. Int. J. Infect. Dis. 2021, 103, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Valentão, P.; Falco, V.; Poeta, P. Antimicrobial Resistance and Clonal Lineages of Escherichia coli from Food-Producing Animals. Antibiotics 2023, 12, 1061. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended-spectrum β-lactamase (ESBL) production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Ribeiro, J.; Silva, V.; Monteiro, A.; Vieira-Pinto, M.; Igrejas, G.; Reis, F.S.; Barros, L.; Poeta, P. Antibiotic Resistance among Gastrointestinal Bacteria in Broilers: A Review Focused on Enterococcus spp. and Escherichia coli. Animals 2023, 13, 1362. [Google Scholar] [CrossRef]
- Bumbangi, F.N.; Llarena, A.-K.; Skjerve, E.; Hang’ombe, B.M.; Mpundu, P.; Mudenda, S.; Mutombo, P.B.; Muma, J.B. Evidence of Community-Wide Spread of Multi-Drug Resistant Escherichia coli in Young Children in Lusaka and Ndola Districts, Zambia. Microorganisms 2022, 10, 1684. [Google Scholar] [CrossRef]
- Choudhuri, A.H.; Ahuja, B.; Biswas, P.S.; Uppal, R. Epidemiology of multidrug-resistant infections after inter ICU transfer in India. Indian J. Crit. Care Med. 2019, 23, 1–6. [Google Scholar] [CrossRef]
- Ema, F.A.; Shanta, R.N.; Rahman, M.Z.; Islam, M.A.; Khatun, M.M. Isolation, identification, and antibiogram studies of Escherichia coli from ready-to-eat foods in Mymensingh, Bangladesh. Vet. World 2022, 15, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, Z.H.; Kabir, M.H.; Ali, S.; Moniruzzaman, M.; Imran, K.M.; Nafiz, T.N.; Islam, M.S.; Hussain, A.; Hakim, S.A.I.; Worth, M.; et al. Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Drinking Water Samples From a Forcibly Displaced, Densely Populated Community Setting in Bangladesh. Front. Public Health 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Chen, L.; Nobrega, D.; Finn, T.J.; Kreiswirth, B.N.; DeVinney, R.; Pitout, J.D.D. Genomic Epidemiology of Global Carbapenemase-Producing Escherichia coli, 2015–2017. Emerg. Infect. Dis. 2022, 28, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Martischang, R.; François, P.; Cherkaoui, A.; Gaïa, N.; Renzi, G.; Agostinho, A.; Perez, M.; Graf, C.E.; Harbarth, S. Epidemiology of ESBL-producing Escherichia coli from repeated prevalence studies over 11 years in a long-term-care facility. Antimicrob. Resist. Infect. Control 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Mora-Ochomogo, M.; Lohans, C.T. β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med. Chem. 2021, 12, 1623–1639. [Google Scholar] [CrossRef]
- Kayastha, K.; Dhungel, B.; Karki, S.; Adhikari, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella Species in Pediatric Patients Visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect. Dis. Res. Treat. 2020, 13, 117863372090979. [Google Scholar] [CrossRef]
- Franiczek, R.; Sobieszczańska, B.; Turniak, M.; Kasprzykowska, U.; Krzyzanowska, B.; Jermakow, K.; Mokracka-Latajka, G. ESBL-producing Escherichia coli isolated from children with acute diarrhea—Antimicrobial susceptibility, adherence patterns and phylogenetic background. Adv. Clin. Exp. Med. 2012, 21, 187–192. [Google Scholar]
- Kumar, N.; Chatterjee, K.; Deka, S.; Shankar, R.; Kalita, D. Increased Isolation of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli From Community-Onset Urinary Tract Infection Cases in Uttarakhand, India. Cureus 2021, 13, e13837. [Google Scholar] [CrossRef]
- Mboya, E.A.; Sanga, L.A.; Ngocho, J.S. Irrational use of antibiotics in the moshi municipality Northern Tanzania: A cross-sectional study. Pan Afr. Med. J. 2018, 31, 165. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Mudenda, S.; Nsofu, E.; Chisha, P.; Daka, V.; Chabalenge, B.; Mufwambi, W.; Kainga, H.; Kanaan, M.H.G.; Mfune, R.L.; Mwaba, F.; et al. Prescribing Patterns of Antibiotics According to the WHO AWaRe Classification during the COVID-19 Pandemic at a Teaching Hospital in Lusaka, Zambia: Implications for Strengthening of Antimicrobial Stewardship Programmes. Pharmacoepidemiology 2023, 2, 42–53. [Google Scholar] [CrossRef]
- Mudenda, S.; Chomba, M.; Chabalenge, B.; Hikaambo, C.N.; Banda, M.; Daka, V.; Zulu, A.; Mukesela, A.; Kasonde, M.; Lukonde, P.; et al. Antibiotic Prescribing Patterns in Adult Patients According to the WHO AWaRe Classification: A Multi-Facility Cross-Sectional Study in Primary Healthcare Hospitals in Lusaka, Zambia. Pharmacol. Pharm. 2022, 13, 379–392. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, M.; Mudenda, S.; Siyanga, M.; Chileshe, M.; Mwiikisa, M.J.; Kasanga, M.; Solochi, B.B.; Gondwe, T.; Kantenga, T.; L Shibemba, A.; et al. Antimicrobial susceptibility patterns of bacteria that commonly cause bacteremia at a tertiary hospital in Zambia. Future Microbiol. 2020, 15, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, M.; Mukosha, R.; Kasanga, M.; Siyanga, M.; Mudenda, S.; Solochi, B.B.; Chileshe, M.; Mwiikisa, M.J.; Gondwe, T.; Kantenga, T.; et al. Antimicrobial resistance patterns of bacterial pathogens their distribution in university teaching hospitals in Zambia. Future Microbiol. 2020, 16, 811–824. [Google Scholar] [CrossRef]
- Badulla, W.F.S.; Alshakka, M.; Mohamed Ibrahim, M.I. Antimicrobial Resistance Profiles for Different Isolates in Aden, Yemen: A Cross-Sectional Study in a Resource-Poor Setting. Biomed Res. Int. 2020, 2020, 1810290. [Google Scholar] [CrossRef]
- Kasanga, M.; Chileshe, M.; Mudenda, S.; Mukosha, R.; Kasanga, M.; Daka, V.; Mudenda, T.; Chisembele, M.; Musuku, J.; Solochi, B.B.; et al. Antibiotic Prescribing Patterns and Prevalence of Surgical Site Infections in Caesarean Section Deliveries at Two Tertiary Hospitals in Lusaka, Zambia. Pharmacol. Pharm. 2022, 13, 313–330. [Google Scholar] [CrossRef]
- Seman, A.; Mihret, A.; Sebre, S.; Awoke, T.; Yitayew, B.; Aseffa, A.; Asrat, D.; Abebe, T.; Yeshitela, B. Prevalence and Molecular Characterization of Extended Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae Isolates from Bloodstream Infection Suspected Patients in Addis Ababa, Ethiopia. Infect. Drug Resist. 2022, 15, 1367–1382. [Google Scholar] [CrossRef]
- Hassell, J.M.; Ward, M.J.; Muloi, D.; Bettridge, J.M.; Robinson, T.P.; Kariuki, S.; Ogendo, A.; Kiiru, J.; Imboma, T.; Kang’ethe, E.K.; et al. Clinically relevant antimicrobial resistance at the wildlife–livestock–human interface in Nairobi: An epidemiological study. Lancet Planet. Health 2019, 3, e259–e269. [Google Scholar] [CrossRef]
- Enany, M.E.; Algammal, A.M.; Nasef, S.A.; Abo-Eillil, S.A.M.; Bin-Jumah, M.; Taha, A.E.; Allam, A.A. The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express 2019, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Butaye, P.; Dockrell, D.H.; Fitzgerald, J.R.; Kuijper, E.J. One Health: A multifaceted concept combining diverse approaches to prevent and control antimicrobial resistance. Clin. Microbiol. Infect. 2020, 26, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; pp. 1–28. [Google Scholar]
- Rhouma, M.; Soufi, L.; Cenatus, S.; Archambault, M.; Butaye, P. Current Insights Regarding the Role of Farm Animals in the Spread of Antimicrobial Resistance from a One Health Perspective. Vet. Sci. 2022, 9, 480. [Google Scholar] [CrossRef]
- Meier, H.; Spinner, K.; Crump, L.; Kuenzli, E.; Schuepbach, G.; Zinsstag, J. State of Knowledge on the Acquisition, Diversity, Interspecies Attribution and Spread of Antimicrobial Resistance between Humans, Animals and the Environment: A Systematic Review. Antibiotics 2023, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Godman, B.; Cook, A.; Khan, M.A.; Campbell, S.M.; Seaton, R.A.; Siachalinga, L.; Haseeb, A.; Amir, A.; Kurdi, A.; et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics 2022, 11, 1824. [Google Scholar] [CrossRef]
- Siachalinga, L.; Mufwambi, W.; Lee, I.H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: A systematic review and meta-analysis. J. Hosp. Infect. 2022, 129, 124–143. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- Lammie, S.L.; Hughes, J.M. Antimicrobial Resistance, Food Safety, and One Health: The Need for Convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Republic of Zambia NAP on AMR Multi-Sectoral National Action Plan on Antimicrobial Resistance. Available online: https://www.afro.who.int/publications/multi-sectoral-national-action-plan-antimicrobial-resistance-2017-2027 (accessed on 24 July 2022).
- Kapona, O. Zambia successfully launches the first multi-sectoral national action plan on antimicrobial resistance (AMR). Health Press Zamb. Bull 2017, 1, 5–7. [Google Scholar]
- Mwikuma, G.; Kainga, H.; Kallu, S.A.; Nakajima, C.; Suzuki, Y.; Hang’ombe, B.M. Determination of the Prevalence and Antimicrobial Resistance of Enterococcus Faecalis and Enterococcus Faecium Associated with Poultry in Four Districts in Zambia. Antibiotics 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S.; Malama, S.; Munyeme, M.; Matafwali, S.K.; Kapila, P.; Katemangwe, P.; Mainda, G.; Mukubesa, A.N.; Hadunka, M.A.; Muma, J.B. Antimicrobial Resistance Profiles of Escherichia Coli Isolated from Laying Hens in Zambia: Implications and Significance on One Health. JAC-Antimicrob. Resist. 2023, 5, dlad060. [Google Scholar] [CrossRef]
- Mudenda, S.; Hankombo, M.; Saleem, Z.; Sadiq, M.J.; Banda, M.; Munkombwe, D.; Mwila, C.; Kasanga, M.; Zulu, A.C.; Hangoma, J.M.; et al. Knowledge, Attitude, and Practices of Community Pharmacists on Antibiotic Resistance and Antimicrobial Stewardship in Lusaka, Zambia. J. Biomed. Res. Environ. Sci. 2021, 2, 1005–1014. [Google Scholar] [CrossRef]
- Tembo, N.; Mudenda, S.; Banda, M.; Chileshe, M.; Matafwali, S. Knowledge, Attitudes and Practices on Antimicrobial Resistance among Pharmacy Personnel and Nurses at a Tertiary Hospital in Ndola, Zambia: Implications for Antimicrobial Stewardship Programmes. JAC-Antimicrob. Resist. 2022, 4, dlac107. [Google Scholar] [CrossRef]
- Mudenda, S.; Malama, S.; Munyeme, M.; Hang’ombe, B.M.; Mainda, G.; Kapona, O.; Mukosha, M.; Yamba, K.; Bumbangi, F.N.; Mfune, R.L.; et al. Awareness of Antimicrobial Resistance and Associated Factors among Layer Poultry Farmers in Zambia: Implications for Surveillance and Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 383. [Google Scholar] [CrossRef]
- Mudenda, S.; Matafwali, S.K.; Malama, S.; Munyeme, M.; Yamba, K.; Katemangwe, P.; Siluchali, G.; Mainda, G.; Mukuma, M.; Bumbangi, F.N.; et al. Prevalence and Antimicrobial Resistance Patterns of Enterococcus Species Isolated from Laying Hens in Lusaka and Copperbelt Provinces of Zambia: A Call for AMR Surveillance in the Poultry Sector. JAC-Antimicrob. Resist. 2022, 4, dlac126. [Google Scholar] [CrossRef]
- Mudenda, S.; Mukosha, M.; Godman, B.; Fadare, J.; Malama, S.; Munyeme, M.; Hikaambo, C.N.; Kalungia, A.C.; Hamachila, A.; Kainga, H.; et al. Knowledge, Attitudes and Practices of Community Pharmacy Professionals on Poultry Antimicrobial Dispensing, Use and Resistance in Zambia: Implications on Antibiotic Stewardship and WHO AWaRe Classification of Antibiotics. Antibiotics 2022, 11, 1210. [Google Scholar] [CrossRef]
- Yamba, K.; Lukwesa-Musyani, C.; Samutela, M.T.; Kapesa, C.; Hang’ombe, M.B.; Mpabalwani, E.; Hachaambwa, L.; Fwoloshi, S.; Chanda, R.; Mpundu, M.; et al. Phenotypic and Genotypic Antibiotic Susceptibility Profiles of Gram-Negative Bacteria Isolated from Bloodstream Infections at a Referral Hospital, Lusaka, Zambia. PLOS Glob. Public Health 2023, 3, e0001414. [Google Scholar] [CrossRef]
- ZamStats Total Number of Households by Province, Zambia 2022. Available online: https://www.zamstats.gov.zm/total-number-of-household-by-province-zambia-2022/ (accessed on 24 January 2023).
- ZamStats Population Size by Province, Zambia 2010 and 2022. Available online: https://www.zamstats.gov.zm/population-size-by-province-zambia-2010-and-2022/# (accessed on 24 January 2023).
- Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, Thirtieth Edition: M100. Available online: https://unitedvrg.com/2021/05/20/m100-performance-standards-for-antimicrobial-susceptibility-testing-30th-edition-2020-pdf/ (accessed on 26 August 2021).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016, pp. 1–84. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 27 May 2023).
- Bush, K.; Bradford, P.A. Epidemiology of β-lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Ali, S. Antimicrobial Resistance in Escherichia coli. In Escherichia coli—Old and New Insights; IntechOpen: London, UK, 2023; ISBN 978-1-83969-870-5. [Google Scholar]
- Osińska, A.; Korzeniewska, E.; Korzeniowska-Kowal, A.; Wzorek, A.; Harnisz, M.; Jachimowicz, P.; Buta-Hubeny, M.; Zieliński, W. The challenges in the identification of Escherichia coli from environmental samples and their genetic characterization. Environ. Sci. Pollut. Res. 2023, 30, 11572–11583. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Naeem, W.; Liaqat, F.; Sadiq, M.B.; Shafee, M.; Gul, Z.; Khan, S.A.; Mengal, H.; Chein, S.H.; Qasim, S.; et al. Hospital Acquired Pathogenic Escherichia coli from Clinical and Hospital Water Samples of Quetta Balochistan. J. Trop. Med. 2022, 2022, 6495044. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- Roberts, L.W.; Hoi, L.T.; Khokhar, F.A.; Hoa, N.T.; Van Giang, T.; Bui, C.; Ninh, T.H.; Co, D.X.; Binh, N.G.; Long, H.B.; et al. Genomic characterisation of multidrug-resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii in two intensive care units in Hanoi, Viet Nam: A prospective observational cohort study. Lancet Microbe 2022, 3, e857–e866. [Google Scholar] [CrossRef] [PubMed]
- Reza Mortazavi-Tabatabaei, S.; Ghaderkhani, J.; Nazari, A.; Sayehmiri, K.; Sayehmiri, F.; Pakzad, I. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 2019, 10, 169. [Google Scholar] [CrossRef]
- Dela Peña, L.B.R.O.; Nacario, M.A.G.; Bolo, N.R.; Rivera, W.L. Multiple Antibiotic Resistance in Escherichia coli Isolates from Fecal and Water Sources in Laguna Lake, Philippines. Water 2022, 14, 1517. [Google Scholar] [CrossRef]
- Salvador-Membreve, D.M.; Rivera, W.L. Predominance of blatem and teta genes in antibiotic-resistant escherichia coli isolates from laguna lake, Philippines. J. Water Sanit. Hyg. Dev. 2021, 11, 814–823. [Google Scholar] [CrossRef]
- Naqid, I.A.; Balatay, A.A.; Hussein, N.R.; Saeed, K.A.; Ahmed, H.A.; Yousif, S.H. Antibiotic Susceptibility Pattern of Escherichia coli Isolated from Various Clinical Samples in Duhok City, Kurdistan Region of Iraq. Int. J. Infect. 2020, 7, e103740. [Google Scholar] [CrossRef]
- Ramatla, T.; Ramaili, T.; Lekota, K.E.; Ndou, R.; Mphuti, N.; Bezuidenhout, C.; Thekisoe, O. A systematic review and meta-analysis on prevalence and antimicrobial resistance profile of Escherichia coli isolated from water in africa (2000–2021). Heliyon 2023, 9, e16123. [Google Scholar] [CrossRef]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef]
- Agbagwa, O.E.; Okorafor, O.N.; Horsfall, S.J. Multidrug Resistant Pattern and Plasmid Detection of Escherichia coli from Various Sources within the University of Port Harcourt. Open J. Med. Microbiol. 2022, 12, 11–23. [Google Scholar] [CrossRef]
- Chiyangi, H.; Muma, B.; Malama, S.; Manyahi, J.; Abade, A.; Kwenda, G.; Matee, M. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0–59 months at the University Teaching Hospital, Lusaka, Zambia: A prospective cross-sectional study. BMC Infect. Dis. 2017, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Yang, L.; Wu, X.; Wu, Y.; Shao, B. Prevalence of Escherichia coli and Antibiotic Resistance in Animal-Derived Food Samples—Six Districts, Beijing, China, 2020. China CDC Wkly. 2021, 3, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, Y.J.; Binn-Kim; Seo, K.H. Spread of multidrug-resistant Escherichia coli harboring integron via swine farm waste water treatment plant. Ecotoxicol. Environ. Saf. 2018, 149, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sula, I.; Alreshidi, M.A.; Alnasr, N.; Hassaneen, A.M.; Saquib, N. Urinary Tract Infections in the Kingdom of Saudi Arabia, a Review. Microorganisms 2023, 11, 952. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Harrell, E.; Thakur, S. Quinolone-resistant Escherichia coli at the interface between humans, poultry and their shared environment- a potential public health risk. One Heal. Outlook 2023, 5, 2. [Google Scholar] [CrossRef]
- Almagor, J.; Temkin, E.; Benenson, I.; Fallach, N.; Carmeli, Y. The impact of antibiotic use on transmission of resistant bacteria in hospitals: Insights from an agent-based model. PLoS ONE 2018, 13, e0197111. [Google Scholar] [CrossRef]
- Mwansa, M.; Mukuma, M.; Mulilo, E.; Kwenda, G.; Mainda, G.; Yamba, K.; Bumbangi, F.N.; Muligisa-Muonga, E.; Phiri, N.; Silwamba, I.; et al. Determination of antimicrobial resistance patterns of Escherichia coli isolates from farm workers in broiler poultry production and assessment of antibiotic resistance awareness levels among poultry farmers in Lusaka, Zambia. Front. Public Health 2023, 10, 998860. [Google Scholar] [CrossRef]
- Öztürk, R.; Tazegul, G. Bacteria Causing Community-Acquired Urinary Tract Infections and Their Antibiotic Susceptibility Patterns in Outpatients Attending at a State Hospital in Turkey. Cureus 2021, 13, e17753. [Google Scholar] [CrossRef]
- Abraham, R.; Allison, H.S.; Lee, T.; Pavic, A.; Chia, R.; Hewson, K.; Lee, Z.Z.; Hampson, D.J.; Jordan, D.; Abraham, S. A national study confirms that Escherichia coli from Australian commercial layer hens remain susceptible to critically important antimicrobials. PLoS ONE 2023, 18, e0281848. [Google Scholar] [CrossRef]
- Schuts, E.C.; Boyd, A.; Muller, A.E.; Mouton, J.W.; Prins, J.M. The effect of antibiotic restriction programs on prevalence of antimicrobial resistance: A systematic review and meta-analysis. Open Forum Infect. Dis. 2021, 8, ofab070. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Bhatnagar, K.; Wong, A. The mutational landscape of quinolone resistance in Escherichia coli. PLoS ONE 2019, 14, e0224650. [Google Scholar] [CrossRef]
- Röderova, M.; Halova, D.; Papousek, I.; Dolejska, M.; Masarikova, M.; Hanulik, V.; Pudova, V.; Broz, P.; Htoutou-Sedlakova, M.; Sauer, P.; et al. Characteristics of quinolone resistance in Escherichia coli isolates from humans, animals, and the environment in the Czech Republic. Front. Microbiol. 2017, 7, 2147. [Google Scholar] [CrossRef] [PubMed]
- Slettemeås, J.S.; Sunde, M.; Ulstad, C.R.; Norström, M.; Wester, A.L.; Urdahl, A.M. Occurrence and characterization of quinolone resistant Escherichia coli from Norwegian Turkey meat and complete sequence of an IncX1 plasmid encoding qnrS1. PLoS ONE 2019, 14, e0212936. [Google Scholar] [CrossRef] [PubMed]
- Dirar, M.H.; Bilal, N.E.; Ibrahim, M.E.; Hamid, M.E. Prevalence of extended-spectrum β-lactamase (Esbl) and molecular detection of bla TEM, bla SHV and bla CTX-M genotypes among enterobacteriaceae isolates from patients in khartoum, sudan. Pan Afr. Med. J. 2020, 37, 213. [Google Scholar] [CrossRef]
- Odongo, I.; Ssemambo, R.; Kungu, J.M. Prevalence of Escherichia Coli and Its Antimicrobial Susceptibility Profiles among Patients with UTI at Mulago Hospital, Kampala, Uganda. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 8042540. [Google Scholar] [CrossRef]
- Bhowmik, A.; Shah, S.T.; Goswami, S.; Sirajee, A.S.; Ahsan, S. Predominance of Multidrug-Resistant Escherichia coli of Environmental Phylotype in Different Environments of Dhaka, Bangladesh. Trop. Med. Infect. Dis. 2023, 8, 226. [Google Scholar] [CrossRef]
- Gottesman, B.S.; Carmeli, Y.; Shitrit, P.; Chowers, M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin. Infect. Dis. 2009, 49, 869–875. [Google Scholar] [CrossRef]
- Malema, M.S.; Abia, A.L.K.; Tandlich, R.; Zuma, B.; Kahinda, J.M.M.; Ubomba-Jaswa, E. Antibiotic-resistant pathogenic escherichia coli isolated from rooftop rainwater-harvesting tanks in the Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 892. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.B.; Al Atbee, M.Y.N. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J. Clin. Lab. Anal. 2022, 36, e24619. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, L.Y.; Bitew, A.A.; Gashey, E.M.; Ademe, M.N. Ceftriaxone resistance among patients at GAMBY teaching general hospital. Sci. Rep. 2022, 12, 12000. [Google Scholar] [CrossRef]
- Chilawa, S.; Mudenda, S.; Daka, V.; Chileshe, M.; Matafwali, S.; Chabalenge, B.; Mpundu, P.; Mufwambi, W.; Mohamed, S.; Mfune, R.L.; et al. Knowledge, Attitudes, and Practices of Poultry Farmers on Antimicrobial Use and Resistance in Kitwe, Zambia: Implications on Antimicrobial Stewardship. Open J. Anim. Sci. 2023, 13, 60–81. [Google Scholar] [CrossRef]
- Joya, M.; Aalemi, A.K.; Baryali, A.T. Prevalence and Antibiotic Susceptibility of the Common Bacterial Uropathogen Among UTI Patients in French Medical Institute for Children. Infect. Drug Resist. 2022, 15, 4291–4297. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.R.; Dodd, C.E.R.; Stekel, D.J.; Meshioye, R.T.; Diggle, M.; Lister, M.; Hobman, J.L. Multidrug-Resistant ESBL-Producing E. coli in Clinical Samples from the UK. Antibiotics 2023, 12, 169. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.K.; Ali, M.R.; Chander, Y. Antimicrobial Susceptibility Profile of Extended Spectrum β-Lactamase (ESBL) Producing Escherichia coli from Various Clinical Samples. Infect. Dis. Res. Treat. 2014, 7, IDRT-S13820. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Addo, K.K. Prevalence of Multidrug-Resistant Escherichia coli Isolated from Drinking Water Sources. Int. J. Microbiol. 2018, 2018, 7204013. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.D.; Meirelles-Pereira, F.; Cataldo, M.; Fonseca, B.D.O.; Nogueira, B.A.; Olivella, J.G.B.; Esteves, F.D.A.; Mattos-Guaraldi, A.L.; de Andrade, A.F.B.; Bello, A.R.; et al. Detection of multidrug-resistant Enterobacteria isolated from river waters flowing to Guanabara Bay (Rio de Janeiro, Brazil) and from clinical samples of hospital origin. Biomedica 2019, 39, 135–149. [Google Scholar] [CrossRef]
- Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Rodríguez-Moctezuma, J.R.; Domínguez-Trejo, P.; Vaca-Paniagua, F.; Vaca, S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community-acquired urinary tract infection patients in Mexico. J. Microbiol. Immunol. Infect. 2017, 50, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Bepari, A.K.; Sen, P.K.; Rafe, T.; Imtiaz, R.; Hossain, M.; Reza, H.M. High prevalence of multiple antibiotic resistance in clinical E. coli isolates from Bangladesh and prediction of molecular resistance determinants using WGS of an XDR isolate. Sci. Rep. 2021, 11, 22859. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Rafaque, Z.; Ahmed, S.; Malik, S.; Dasti, J.I. Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac. J. Trop. Biomed. 2016, 6, 60–66. [Google Scholar] [CrossRef]
- Fani, R.; Dioli, C.; Pappa, O.; Siatravani, E.; Bratakou, S.; Tatsiopoulos, A.; Giakkoupi, P.; Miriagou, V.; Beloukas, A. Molecular Characterization and Prevalence of Antimicrobial-Resistant Escherichia coli Isolates Derived from Clinical Specimens and Environmental Habitats. Microorganisms 2023, 11, 1399. [Google Scholar] [CrossRef]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef]
- Brolund, A. Overview of ESBL-producing Enterobacteriaceae from a Nordic perspective. Infect. Ecol. Epidemiol. 2014, 4, 24555. [Google Scholar] [CrossRef]
- Erb, S.; D’Mello-Guyett, L.; Malebo, H.M.; Njee, R.M.; Matwewe, F.; Ensink, J.; Hinic, V.; Widmer, A.; Frei, R. High prevalence of ESBL-Producing E. coli in private and shared latrines in an informal urban settlement in Dar es Salaam, Tanzania. Antimicrob. Resist. Infect. Control 2018, 7, 3. [Google Scholar] [CrossRef]
- Peirano, G.; Pitout, J.D.D. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: The worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 2010, 35, 316–321. [Google Scholar] [CrossRef]
- Quan, J.; Dai, H.; Liao, W.; Zhao, D.; Shi, Q.; Zhang, L.; Shi, K.; Akova, M.; Yu, Y. Etiology and prevalence of ESBLs in adult community-onset urinary tract infections in East China: A prospective multicenter study. J. Infect. 2021, 83, 175–181. [Google Scholar] [CrossRef]
- Yadav, K.K.; Adhikari, N.; Khadka, R.; Pant, A.D.; Shah, B. Multidrug-resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: A cross-sectional study in National Kidney Center, Nepal. Antimicrob. Resist. Infect. Control 2015, 4, 42. [Google Scholar] [CrossRef]
- Mukherjee, M.; Basu, S.; MuKherjee, S.K.M.; Majumder, M. Multidrug-resistance and extended spectrum beta-lactamase production in uropathogenic E. coli which were isolated from hospitalized patients in Kolkata, India. J. Clin. Diagn. Res. 2013, 7, 449–453. [Google Scholar] [CrossRef]
- Tian, G.B.; Wang, H.N.; Zhang, A.Y.; Zhang, Y.; Fan, W.Q.; Xu, C.W.; Zeng, B.; Guan, Z.B.; Zou, L.K. Detection of clinically important β-lactamases in commensal Escherichia coli of human and swine origin in western China. J. Med. Microbiol. 2012, 61, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Tigabie, M.; Biset, S.; Belachew, T.; Amare, A.; Moges, F. Multidrug-resistant and extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated from chicken droppings in poultry farms at Gondar City, Northwest Ethiopia. PLoS ONE 2023, 18, e0287043. [Google Scholar] [CrossRef]
- Ludden, C.; Coll, F.; Gouliouris, T.; Restif, O.; Blane, B.; Blackwell, G.A.; Kumar, N.; Naydenova, P.; Crawley, C.; Brown, N.M.; et al. Defining nosocomial transmission of Escherichia coli and antimicrobial resistance genes: A genomic surveillance study. Lancet Microbe 2021, 2, e472–e480. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhu, Y.; Li, X.; Kudinha, T.; Yang, Y.; Zhang, G.; Zhang, J.; Xu, Y.; Yang, Q. High Prevalence of Extended-Spectrum Beta-Lactamases in Escherichia coli Strains Collected From Strictly Defined Community-Acquired Urinary Tract Infections in Adults in China: A Multicenter Prospective Clinical Microbiological and Molecular Study. Front. Microbiol. 2021, 12, 663033. [Google Scholar] [CrossRef]
- Siriphap, A.; Kitti, T.; Khuekankaew, A.; Boonlao, C.; Thephinlap, C.; Thepmalee, C.; Suwannasom, N.; Khoothiam, K. High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates: A 5-year retrospective study at a Tertiary Hospital in Northern Thailand. Front. Cell. Infect. Microbiol. 2022, 12, 955774. [Google Scholar] [CrossRef]
- Al-Jamei, S.A.; Albsoul, A.Y.; Bakri, F.G.; Al-Bakri, A.G. Extended-spectrum β-lactamase producing E. coli in urinary tract infections: A two-center, cross-sectional study of prevalence, genotypes and risk factors in Amman, Jordan. J. Infect. Public Health 2019, 12, 21–25. [Google Scholar] [CrossRef]
- Hays, J.P.; Safain, K.S.; Almogbel, M.S.; Habib, I.; Khan, M.A. Extended Spectrum- and Carbapenemase-Based β-Lactam Resistance in the Arabian Peninsula—A Descriptive Review of Recent Years. Antibiotics 2022, 11, 1354. [Google Scholar] [CrossRef]
- Ranjan Dash, N.; Albataineh, M.T.; Alhourani, N.; Khoudeir, A.M.; Ghanim, M.; Wasim, M.; Mahmoud, I. Community-acquired urinary tract infections due to extended-spectrum β-lactamase-producing organisms in United Arab Emirates. Travel Med. Infect. Dis. 2018, 22, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Psutka, R.; Carter, A.; Priest, P. An outbreak of multi-drug resistant Escherichia coli urinary tract infection in an elderly population: A case-control study of risk factors. BMC Infect. Dis. 2015, 15, 224. [Google Scholar] [CrossRef]
- Aabed, K.; Moubayed, N.; Alzahrani, S. Antimicrobial resistance patterns among different Escherichia coli isolates in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 3776–3782. [Google Scholar] [CrossRef]
- Shrestha, A.; Shrestha, R.; Koju, P.; Tamrakar, S.; Rai, A.; Shrestha, P.; Madhup, S.K.; Katuwal, N.; Shrestha, A.; Shrestha, A.; et al. The Resistance Patterns in E. coli Isolates among Apparently Healthy Adults and Local Drivers of Antimicrobial Resistance: A Mixed-Methods Study in a Suburban Area of Nepal. Trop. Med. Infect. Dis. 2022, 7, 133. [Google Scholar] [CrossRef]
- Sahuquillo-Arce, J.M.; Selva, M.; Perpiñán, H.; Gobernado, M.; Armero, C.; López-Quílez, A.; González, F.; Vanaclocha, H. Antimicrobial resistance in more than 100,000 Escherichia coli isolates according to culture site and patient age, gender, and location. Antimicrob. Agents Chemother. 2011, 55, 1222–1228. [Google Scholar] [CrossRef]
- Pariyar, M.; Adhikari, S.; Regmi, R.S.; Dhungel, B.; Banjara, M.R.; Rijal, B.P.; Rijal, K.R.; Ghimire, P. Beta-Lactamase-Producing Gram-Negative Bacterial Isolates Among the Patients Attending a Tertiary Care Hospital, Kathmandu, Nepal. Microbiol. Insights 2023, 16, 117863612211507. [Google Scholar] [CrossRef]
- Bora, A.; Sanjana, R.; Jha, B.K.; Narayan Mahaseth, S.; Pokharel, K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res. Notes 2014, 7, 557. [Google Scholar] [CrossRef]
- Bao, D.; Xu, X.; Wang, Y.; Zhu, F. Emergence of a Multidrug-Resistant Escherichia coli Co-Carrying a New mcr-1.33 Variant and blaNDM-5 Genes Recovered from a Urinary Tract Infection. Infect. Drug Resist. 2022, 15, 1499–1503. [Google Scholar] [CrossRef]
- Niranjan, V.; Malini, A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J. Med. Res. 2014, 139, 945–948. [Google Scholar]
- Kourtis, A.P.; Sheriff, E.A.; Weiner-Lastinger, L.M.; Elmore, K.; Preston, L.E.; Dudeck, M.; McDonald, L.C. Antibiotic Multidrug Resistance of Escherichia coli Causing Device- and Procedure-related Infections in the United States Reported to the National Healthcare Safety Network, 2013–2017. Clin. Infect. Dis. 2021, 73, E4552–E4559. [Google Scholar] [CrossRef]
- Mutters, N.T.; Mampel, A.; Kropidlowski, R.; Biehler, K.; Günther, F.; Bălu, I.; Malek, V.; Frank, U. Treating urinary tract infections due to MDR E. coli with Isothiocyanates—A phytotherapeutic alternative to antibiotics? Fitoterapia 2018, 129, 237–240. [Google Scholar] [CrossRef]
- Kaye, K.S.; Gupta, V.; Mulgirigama, A.; Joshi, A.V.; Scangarella-Oman, N.E.; Yu, K.; Ye, G.; Mitrani-Gold, F.S. Antimicrobial Resistance Trends in Urine Escherichia coli Isolates From Adult and Adolescent Females in the United States From 2011 to 2019: Rising ESBL Strains and Impact on Patient Management. Clin. Infect. Dis. 2021, 73, 1992–1999. [Google Scholar] [CrossRef]
- Reaver, K.M.; Levy, J.; Nyambe, I.; Hay, M.C.; Mutiti, S.; Chandipo, R.; Meiman, J. Drinking Water Quality and Provision in Six Low-Income, Peri-Urban Communities of Lusaka, Zambia. GeoHealth 2021, 5, e2020GH000283. [Google Scholar] [CrossRef] [PubMed]
- Seguni, N.Z.; Kimera, Z.I.; Msafiri, F.; Mgaya, F.X.; Joachim, A.; Mwingwa, A.; Matee, M.I. Multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolated from hospital sewage flowing through community sewage system and discharging into the Indian Ocean. Bull. Natl. Res. Cent. 2023, 47, 66. [Google Scholar] [CrossRef]
- Farrell, M.L.; Chueiri, A.; O’Connor, L.; Duane, S.; Maguire, M.; Miliotis, G.; Cormican, M.; Hooban, B.; Leonard, A.; Gaze, W.H.; et al. Assessing the impact of recreational water use on carriage of antimicrobial resistant organisms. Sci. Total Environ. 2023, 888, 164201. [Google Scholar] [CrossRef]
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef]
- Lyimo, B.; Buza, J.; Subbiah, M.; Smith, W.; Call, D.R. Comparison of antibiotic-resistant Escherichia coli obtained from drinking water sources in northern Tanzania: A cross-sectional study. BMC Microbiol. 2016, 16, 254. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, D.; He, S.; Ye, H.; Zhang, L.; Wen, Y.; Zhang, W.; Shu, L.; Chen, S. Prevalence of antibiotic-resistant Escherichia coli in drinking water sources in Hangzhou City. Front. Microbiol. 2017, 8, 267763. [Google Scholar] [CrossRef]
- Kalakonda, S.P.; Parameswarreddy, G.; Skariah, E.N.; George, B.; Suchithra, T.V.; Sindhu, T.K. Treatment of Escherichia coli contaminated water with different pulse-powered NTP configurations and analysis for post treatment efficacy. Sci. Rep. 2022, 12, 20380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).