Markers of Epstein–Barr Virus Infection in Association with the Onset and Poor Control of Rheumatoid Arthritis: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Samples
2.3. Determination of Anti-EBV Antibodies Presence and Titer
2.4. Viral DNA Extraction, Amplification and Quantification of EBV DNA
2.5. Sequencing of EBV EBNA1 and LMP1 Genes
2.6. Data Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Status and Markers of EBV Infection in RA Patients and Controls
3.3. Association of Anti-EBV Antibodies Titers and RA Activity/Severity, Inflammation and Immunological Parameters in RA Patients
3.4. Association of Anti-EBV Antibodies Titers and Therapy in RA Patients
3.5. EBV Viremic vs. Non-Viremic RA Patients and Disease Characteristics
3.6. EBNA1 Variants in RA Patients
3.7. The Impact of EBV Infection on Disease Onset, Having Disease and Poor Disease Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Westergaard, M.W.; Draborg, A.H.; Troelsen, L.; Jacobsen, S.; Houen, G.; Pan, H.F. Isotypes of Epstein-Barr Virus Antibodies in Rheumatoid Arthritis: Association with Rheumatoid Factors and Citrulline-Dependent Antibodies. BioMed Res. Int. 2015, 2015, 472174. [Google Scholar]
- Picerno, V.; Ferro, F.; Adinolfi, A.; Valentini, E.; Tani, C.; Alunno, A. One Year in Review: The Pathogenesis of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2015, 33, 551–558. [Google Scholar]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar]
- Costenbader, K.H.; Karlson, E.W. Epstein-Barr Virus and Rheumatoid Arthritis: Is There a Link? Arthritis Res. Ther. 2006, 8, 204. [Google Scholar] [CrossRef]
- Erre, G.L.; Mameli, G.; Cossu, D.; Muzzeddu, B.; Piras, C.; Paccagnini, D.; Passiu, G.; Sechi, L.A. Increased Epstein-Barr Virus DNA Load and Antibodies against EBNA1 and EA in Sardinian Patients with Rheumatoid Arthritis. Viral Immunol. 2015, 28, 385–390. [Google Scholar] [CrossRef]
- De Paschale, M. Serological Diagnosis of Epstein-Barr Virus Infection: Problems and Solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar]
- Ball, R.J.; Avenell, A.; Aucott, L.; Hanlon, P.; Vickers, M.A. Systematic Review and Meta-Analysis of the Sero-Epidemiological Association between Epstein-Barr Virus and Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 274. [Google Scholar] [CrossRef]
- Alspaugh, M.A.; Henle, G.; Lennette, E.T.; Henle, W.; Stokes, J. Elevated Levels of Antibodies to Epstein-Barr Virus Antigens in Sera and Synovial Fluids of Patients with Rheumatoid Arthritis. J. Clin. Investig. 1981, 67, 1134–1140. [Google Scholar]
- Ferrell, P.B.; Aitcheson, C.T.; Pearson, G.R.; Tan, E.M. Seroepidemiological Study of Relationships between Epstein-Barr Virus and Rheumatoid Arthritis. J. Clin. Investig. 1981, 67, 681–687. [Google Scholar]
- Roudier, J.; Petersen, J.; Rhodes, G.H.; Lukat, J.; Carson, D.A. Susceptibility to Rheumatoid Arthritis Maps to a T-Cell Epitope Shared by the HLA-Dw4 DR F8-1 Chain and the Epstein-Barr Virus Glycoprotein GpllO (Histocompatibility Antigen). Proc. Nail. Acad. Sci. USA 1989, 86, 5104–5108. [Google Scholar] [CrossRef]
- Birkenfeld, P.; Haratz, N.; Klein, G.; Sulitzeanu, D. Cross-Reactivity between EBNA 1p107 Peptide, Collagen and Keratin: Implications for the Pathogenesis of Rheumatoid Arthritis. Clin. Immunol. Immunopathol. 1990, 54, 14–25. [Google Scholar]
- Toussirot, É.; Roudier, J. Epstein-Barr Virus in Autoimmune Diseases. Best Pract. Res. Clin. Rheumatol. 2008, 22, 883–896. [Google Scholar] [CrossRef]
- McDermott, M.; Molloy, M.; Buckley, J.; Greally, J. Antibodies to Epstein-Barr Viral Antigens in Familial Rheumatoid Arthritis. Ir. J. Med. Sci. 1989, 158, 203–205. [Google Scholar] [CrossRef]
- Balandraud, N.; Meynard, J.B.; Auger, I.; Sovran, H.; Mugnier, B.; Reviron, D.; Roudier, J.; Roudier, C. Epstein-Barr Virus Load in the Peripheral Blood of Patients with Rheumatoid Arthritis: Accurate Quantification Using Real-Time Polymerase Chain Reaction. Arthritis Rheum. 2003, 48, 1223–1228. [Google Scholar] [CrossRef]
- Tosato, G.; Steinberg, A.D.; Yarchoan, R.; Heilman, C.A.; Pike, S.E.; De Seau, V.; Blaese, R.M. Rapid Publication Abnormally Elevated Frequency of Epstein-Barr Virus-Infected B Cells in the Blood of Patients with Rheumatoid Arthritis. J. Clin. Investig. 1984, 73, 1789–1795. [Google Scholar] [CrossRef]
- Takeda, T.; Mizugaki, Y.; Matsubara, L.; Imai, S.; Koike, T.; Takada, K. Lytic Epstein-Barr Virus Infection in the Synovial Tissue of Patients with Rheumatoid Arthritis. Arthritis Rheum. 2000, 43, 1218–1225. [Google Scholar]
- Masuoka, S.; Kusunoki, N.; Takamatsu, R.; Takahashi, H.; Tsuchiya, K.; Kawai, S.; Nanki, T. Epstein-Barr Virus Infection and Variants of Epstein-Barr Nuclear Antigen-1 in Synovial Tissues of Rheumatoid Arthritis. PLoS ONE 2018, 13, e0208957. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar]
- Li, D.J.; Bei, J.X.; Mai, S.J.; Xu, J.F.; Chen, L.Z.; Zhang, R.H.; Yu, X.J.; Hong, M.H.; Zeng, Y.X.; Kang, T. The dominance of China 1 in the spectrum of Epstein-Barr virus strains from Cantonese patients with nasopharyngeal carcinoma. J. Med. Virol. 2009, 81, 1253–1260. [Google Scholar] [CrossRef]
- Lorenzetti, M.A.; Altcheh, J.; Moroni, S.; Moscatelli, G.; Chabay, P.A.; Preciado, M.V. EBNA1 sequences in Argentinean pediatric acute and latent Epstein-Barr virus infection reflect circulation of novel South American variants. J. Med. Virol. 2010, 82, 1730–1738. [Google Scholar]
- Jakovljevic, A.; Knezevic, A.; Nikolic, N.; Soldatovic, I.; Jovanovic, T.; Milasin, J.; Andric, M. Herpesviruses Viral Loads and Levels of Proinflammatory Cytokines in Apical Periodontitis. Oral Dis. 2018, 24, 840–846. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95, 98, NT. Nucl. Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Balandraud, N.; Guis, S.; Meynard, J.B.; Auger, I.; Roudier, J.; Roudier, C. Long-Term Treatment with Methotrexate or Tumor Necrosis Factor α Inhibitors Does Not Increase Epstein-Barr Virus Load in Patients with Rheumatoid Arthritis. Arthritis Care Res. 2007, 57, 762–767. [Google Scholar] [CrossRef]
- Kuusela, E.; Kouri, V.P.; Olkkonen, J.; Koivuniemi, R.; Äyräväinen, L.; Rajamäki, K.; Valleala, H.; Nordström, D.; Leirisalo-Repo, M.; Ainola, M.; et al. Serum Epstein-Barr Virus DNA, Detected by Droplet Digital PCR, Correlates with Disease Activity in Patients with Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2018, 36, 778–784. [Google Scholar]
- Miceli-Richard, C.; Gestermann, N.; Amiel, C.; Sellam, J.; Ittah, M.; Pavy, S.; Urrutia, A.; Girauld, I.; Carcelain, G.; Venet, A.; et al. Effect of Methotrexate and Anti-TNF on Epstein-Barr Virus T-Cell Response and Viral Load in Patients with Rheumatoid Arthritis or Spondylarthropathies. Arthritis Res. Ther. 2009, 11, R77. [Google Scholar] [CrossRef]
- Draborg, A.H.; Lydolph, M.C.; Westergaard, M.; Larsen, S.O.; Nielsen, C.T.; Duus, K.; Jacobsen, S.; Houen, G. Elevated Concentrations of Serum Immunoglobulin Free Light Chains in Systemic Lupus Erythematosus Patients in Relation to Disease Activity, Inflammatory Status, B Cell Activity and Epstein-Barr Virus Antibodies. PLoS ONE 2015, 10, e0138753. [Google Scholar]
- Esen, B.A.; YIlmaz, G.; Uzun, S.; Özdamar, M.; Aksözek, A.; KamalI, S.; Türkoǧlu, S.; Gül, A.; Öcal, L.; Aral, O.; et al. Serologic Response to Epstein-Barr Virus Antigens in Patients with Systemic Lupus Erythematosus: A Controlled Study. Rheumatol. Int. 2012, 32, 79–83. [Google Scholar] [CrossRef]
- Fechtner, S.; Berens, H.; Bemis, E.; Johnson, R.L.; Guthridge, C.J.; Carlson, N.E.; Demoruelle, M.K.; Harley, J.B.; Edison, J.D.; Norris, J.A.; et al. Antibody Responses to Epstein-Barr Virus in the Preclinical Period of Rheumatoid Arthritis Suggest the Presence of Increased Viral Reactivation Cycles. Arthritis Rheumatol. 2022, 74, 597–603. [Google Scholar] [CrossRef]
- Simon, K.; IAF van der Mei, S.; Munger, K.; Ponsonby, S.A.; Dickinson, J.; Dwyer, T.; Sundström, P.; Ascherio, A. Combined Effects of Smoking, Anti-EBNA Antibodies, and HLA-DRB1*1501 on Multiple Sclerosis Risk. Neurology 2010, 74, 1365–1371. [Google Scholar]
- Shirodaria, P.V.; Haire, M.; Fleming, E.; Merrett, J.D.; Hawkins, S.A.; Roberts, S.D. Viral Antibody Titers Comparison in Patients With Multiple Sclerosis and Rheumatoid Arthritis. Arch. Neurol. 1987, 44, 1237–1241. [Google Scholar]
- Svendsen, A.J.; Westergaard, M.C.W.; Draborg, A.H.; Holst, R.; Kyvik, K.O.; Jakobsen, M.A.; Junker, P.; Houen, G. Altered Antibody Response to Epstein-Barr Virus in Patients With Rheumatoid Arthritis and Healthy Subjects Predisposed to the Disease. A Twin Study. Front. Immunol. 2021, 12, 650713. [Google Scholar] [CrossRef]
- Sherina, N.; Hreggvidsdottir, H.S.; Bengtsson, C.; Hansson, M.; Israelsson, L.; Alfredsson, L.; Lundberg, K. Low Levels of Antibodies against Common Viruses Associate with Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis; Implications for Disease Aetiology. Arthritis Res. Ther. 2017, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hakoda, M.; Iwabuchi, K.; Takeda, T.; Koike, T.; Kamatani, N.; Takada, K. Rheumatoid Factors Induce Signaling from B Cells, Leading to Epstein-Barr Virus and B-Cell Activation. J. Virol. 2004, 78, 9918–9923. [Google Scholar] [CrossRef]
- Rowe, M.; Lear, A.L.; Croom-Carter, D.; Davies, A.H.; Rickinson, A.A.B. Three Pathways of Epstein-Barr Virus Gene Activation from EBNAl-Positive Latency in B Lymphocytes. J. Virol. 1992, 66, 122–131. [Google Scholar] [CrossRef]
- Chang, C.M.; Yu, K.J.; Mbulaiteye, S.M.; Hildesheim, A.; Bhatia, K. The Extent of Genetic Diversity of Epstein-Barr Virus and Its Geographic and Disease Patterns: A Need for Reappraisal. Virus Res. 2009, 143, 209–221. [Google Scholar]
- Chen, J.-N.; Zhang, N.-N.; Jiang, Y.; Hui, D.-Y.; Wen, Z.-J.; Li, H.-G.; Ding, Y.-G.; Du, H.; Shao, C.-K. Variations of Epstein-Barr Virus Nuclear Antigen 1 in Epstein-Barr Virus-Associated Gastric Carcinomas from Guangzhou, Southern China. PLoS ONE 2012, 7, e50084. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Raj, A.; Spangler, G.; Sharma, A.; Hussain, A.; Judde, J.; Tsao, S.; Yuen, P.; Joab, I.; Magrath, I.; et al. Sequence Variations in EBNA-1 May Dictate Restriction of Tissue Distribution of Epstein-Barr Virus in Normal and Tumour Cells. J. Gen. Virol. 1997, 78, 1663–1670. [Google Scholar] [CrossRef]
- Banko, A.V.; Lazarevic, I.B.; Folic, M.M.; Djukic, V.B.; Cirkovic, A.M.; Karalic, D.Z.; Cupic, M.D.; Jovanovic, T.P. Characterization of the Variability of Epstein-Barr Virus Genes in Nasopharyngeal Biopsies: Potential Predictors for Carcinoma Progression. PLoS ONE 2016, 11, 0153498. [Google Scholar]
- Sandvej, K.; Zhou, X.-G.; Hamilton-Dutoit, S.; Sandvej, D.K. EBNA-1 Sequence Variation in Danish and Chinese EBV-Associated Tumours: Evidence for Geographical Polymorphism but Not for Tumour-Specific Subtype Restriction. J. Pathol. 2000, 191, 127–131. [Google Scholar] [CrossRef]



| Primers | Sequence (5′-3′) | Nucleotide Position a | Reference |
|---|---|---|---|
| LMP1 gene | |||
| Fw1 (outer) | GCTAAGGCATTCCCAGTAAA | 168081–168100 | Li et al., 2009 [20] |
| Rev1 (outer) | GATGAACACCACCACGATG | 168726–168774 | Li et al., 2009 [20] |
| Fw2 (inner) | CGGAACCAGAAGAACCCA | 169679–169658 | Li et al., 2009 [20] |
| Rev2 (inner) | TCCCGCACCCTCAACAAG | 168702–168719 | Li et al., 2009 [20] |
| EBNA1 gene | |||
| Fw1 (outer) | AGATGGTGAGCCTGACGTG | 109218–109236 | Lorenzetti et al., 2010 [21] |
| Rev1 (outer) | GCATCCTTCAAAACCTCAGC | 109663–109682 | Lorenzetti et al., 2010 [21] |
| Fw2 (inner) | CCCGCAGATGACCCAGGAGA | 109261–109280 | Lorenzetti et al., 2010 [21] |
| Rev2 (inner) | GGGTCCAGGGGCCATTCCAAA | 109570–109590 | Lorenzetti et al., 2010 [21] |
| Characteristic | Group | p * | Subgroups | p * | ||
|---|---|---|---|---|---|---|
| RA n = 133 | Control n = 50 | RA A n = 80 | RA B n = 53 | |||
| Sociodemographics | ||||||
| Age (years), mean ± sd | 58.86 ± 11.78 | 45.40 ± 8.78 | <0.001 £ | 59.45 ± 12.73 | 57.96 ± 10.21 | 0.478 £ |
| Gender, n (%) | ||||||
| Male | 37 (27.8) | 6 (12.0) | 0.024 § | 23 (28.7) | 14 (26.4) | 0.769 § |
| Female | 96 (72.2) | 44 (88.0) | 57 (71.3) | 39 (73.6) | ||
| Educational level, n (%) | ||||||
| Primary | 25 (18.8) | 1 (2.0) | <0.001 § | 14 (17.5) | 11 (20.8) | 0.058 § |
| Secondary | 78 (58.6) | 11 (22.4) | 53 (66.3) | 25 (47.2) | ||
| Tertiary or higher | 30 (22.6) | 37 (75.5) | 13 (16.3) | 17 (32.1) | ||
| BMI, mean ± sd | 25.21 ± 4.32 | 26.12 ± 4.49 | 0.213 £ | 25.34 ± 4.34 | 25.03 ± 4.34 | 0.684 £ |
| Smoking status, n (%) | ||||||
| Smoker | 63 (47.4) | 26 (52.0) | 0.576 § | 51 (63.7) | 19 (35.8) | 0.002 § |
| Non-smoker | 70 (52.6) | 24 (48.0) | 29 (36.3) | 34 (64.2) | ||
| Smoking duration (years), med (min–max) | 7.0 (0.0–60.0) | 10.0 (0.0–35.0) | 0.494 ¥ | 20.0 (0–60.0) | 0.0 (0.0–53.0) | 0.012 ¥ |
| Disease characteristics | ||||||
| Duration of RA (years), med (min–max) | 8.0 (1.0–40.0) | / | NA | / | 8.0 (1.0–40.0) | NA |
| Family history of RA, n (%) | 54 (40.6) | 1 (2.0) | <0.001 § | 32 (40.0) | 22 (41.5) | 0.862 § |
| VAS, med (min–max) | ||||||
| Patient | 60 (10–100) | / | NA | 60 (10–100) | 70 (30–100) | 0.168 ¥ |
| Physician | 50 (10–90) | / | NA | 50 (10–90) | 50 (10–90) | 0.917 ¥ |
| Pain | 60 (10–90) | / | NA | 60 (10–100) | 60 (30–90) | 0.694 ¥ |
| Ultrasound examination, med (min–max) | ||||||
| Total number of swollen joints | 7 (0–32) | / | NA | 6 (0–32) | 9 (0–30) | <0.001 ¥ |
| Total number of tender joints | 11 (0–33) | / | NA | 10 (0–33) | 14 (0–31) | <0.001 ¥ |
| RA activity/severity | ||||||
| DAS28 (SE), mean ± sd | 5.24 ± 1.12 | / | NA | 4.96 ± 1.33 | 5.67 ± 0.42 | <0.001 £ |
| DAS28 (CRP), mean ± sd | 4.72 ± 1.10 | / | NA | 4.47 ± 1.23 | 5.10 ± 0.75 | <0.001 £ |
| CDAI, med (min–max) | 18 (1–40) | / | NA | 12 (1–26) | 22 (7–40) | <0.001 § |
| SDAI, med (min–max) | 16 (0–63) | / | NA | 12.3 (0–63) | 22.8 (6–62) | <0.001 § |
| RAID, med (min–max) | 5 (1–18) | / | NA | 5 (1–16) | 5 (1–18) | 0.351 § |
| RAQoL, med (min–max) | 12 (1–28) | / | NA | 10.25 (1–28) | 13.0 (1–27) | 0.017 § |
| HAQ, med (min–max) | 1.125 (0.125–2.625) | / | NA | 0.935 (0.125–2.125) | 1.250 (0.280–2.625) | <0.001 § |
| ESR, med (min–max) | 35 (6–120) | / | NA | 37 (6–100) | 34 (10–120) | 0.711 § |
| CRP, med (min–max) | 15 (0–152.4) | / | NA | 12.8 (0–84.1) | 19.3 (1.7–152.4) | 0.042 § |
| Immunological parameters | ||||||
| ANA, med (min–max) | 640.0 (40.0–640.0) | / | NA | 20.0 (0.0–640.0) | 40.0 (0.0–640.0) | 0.984 § |
| aCL-IgM, med (min–max) | 0.0 (0.0–7.4) | / | NA | 0.0 (0.0–1.0) | 0.0 (0.0–31.2) | 0.590 § |
| aCL-IgG, med (min–max) | 0.0 (0.0–15.1) | / | NA | 0.0 (0.0–7.8) | 0.0 (0.0–15.1) | 0.138 § |
| RF, med (min–max) | 151 (0.0–641.6) | / | NA | 127.5 (2.0–641.6) | 162.0 (0.0–500.0) | 0.198 § |
| ACPA, med (min–max) | 320.0 (0.0–500.0) | / | NA | 284.5 (3.5–500.0) | 350.0 (0.0–500.0) | 0.866 § |
| Current RA therapy | ||||||
| Corticosteroids, n (%) | 75 (56.4) | / | NA | 35 (43.8) | 40 (75.5) | <0.001 § |
| Antimalarials, n (%) | 25 (18.8) | / | NA | 0 (0.0) | 25 (47.2) | <0.001 § |
| Sulfasalazine, n (%) | 9 (6.8) | / | NA | 0 (0.0) | 9 (17.0) | <0.001 § |
| Methotrexate (MTX) | 52 (39.1) | / | NA | 0 (0.0) | 52 (98.1) | <0.001 § |
| Paracetamol, n (%) | 53 (39.8) | / | NA | 30 (37.5) | 23 (43.4) | 0.497 § |
| NSAID | 106 (57.9) | / | NA | 59 (73.8) | 47 (88.7) | 0.036 § |
| Comorbidities, n (%) | ||||||
| AH | 61 (45.9) | 10 (20.0) | 0.001 § | 36 (45.0) | 25 (47.2) | 0.806 § |
| DM | 18 (13.5) | 2 (4.0) | 0.065 § | 11 (13.8) | 7 (13.2) | 0.929 § |
| Cardiovascular events (CVI, TIA, MI, AP) | 37 (27.8) | 1 (2.0) | <0.001 § | 24 (30.0) | 13 (24.5) | 0.491 § |
| EBV Infection Parameter | Group | p * | Subgroups | p * | ||
|---|---|---|---|---|---|---|
| RA n = 133 | Control n = 50 | RA A n = 80 | RA B n = 53 | |||
| EBV DNA (EBNA1 and/or LMP1), n (%) | 9 (6.8) | 0 (0.0) | 0.059 § | 7 (8.8) | 2 (3.8) | 0.263 § |
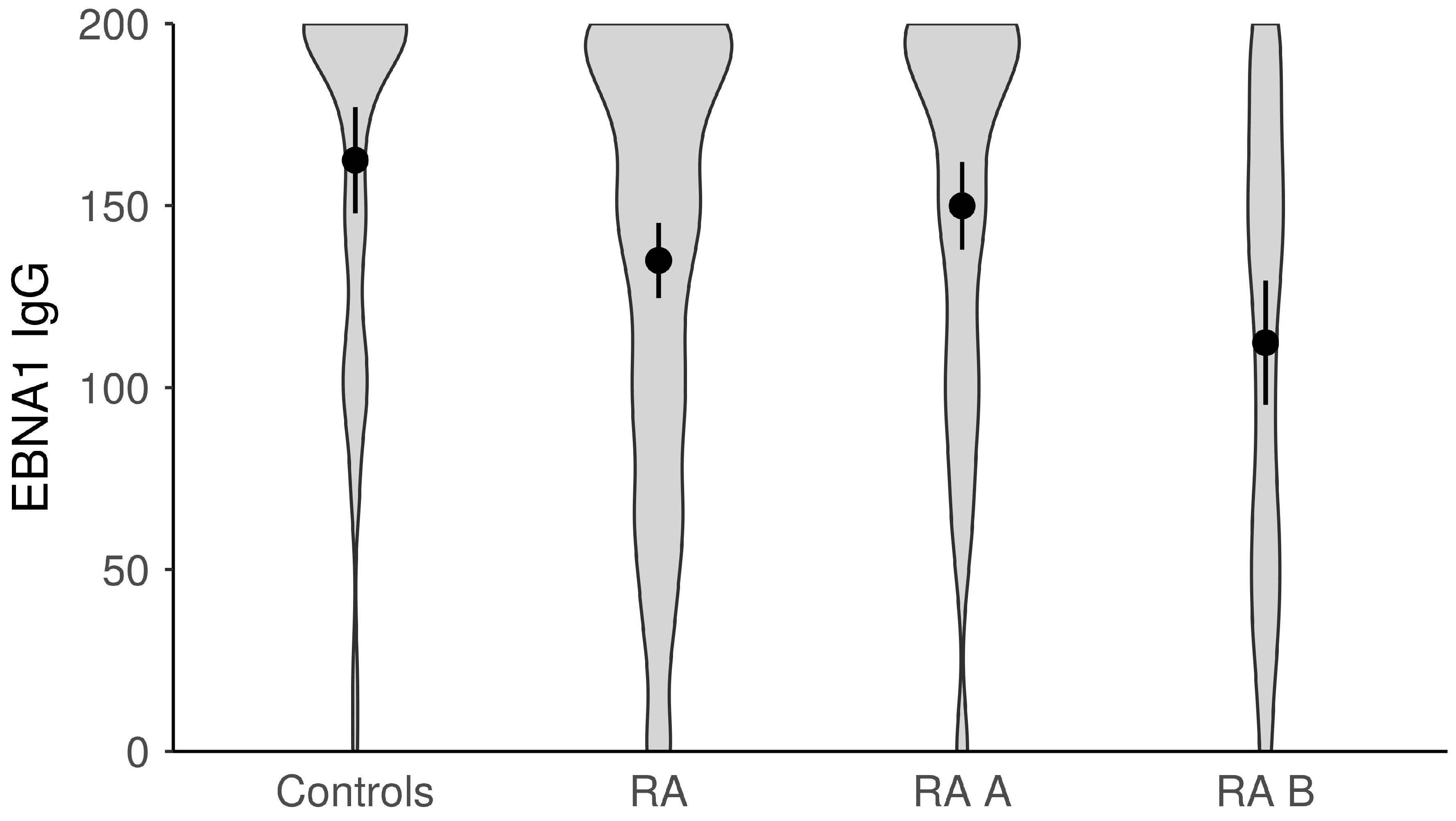
| Anti-EBV-EBNA1 IgG, med (min–max) | 149.0 (0.0–200.0) | 195.0 (0.0–200.0) | 0.001 | 172.5 (0–200) | 124.0 (0–200) | <0.001 |
| Anti-EBV-EBNA1 IgG | 127 (95.5) | 49 (98.0) | 0.430 § | 77 (96.3) | 50 (94.3) | 0.603 § |
| Anti-EBV-CA IgG, med (min–max) | 184.0 (0.0–200.0) | 199.0 (0.0–200.0) | 0.104 € | 194.5 (0–200) | 160.0 (18–200) | <0.001 |
| Anti-EBV-CA IgG | 132 (99.2) | 49 (98.0) | 0.473 | 79 (98.8) | 53 (100.0) | 1.000 € |
| Anti-EBV-CA IgM, med (min–max) | 0.0 (0.0–5.7) | 0.0 (0.0–1.4) | 0.003 | 0.0 (0.0–4.4) | 0.0 (0.0–5.6) | 0.132 |
| Anti-EBV-CA IgM | 26 (19.5) | 1 (2.0) | 0.003 § | 19 (23.8) | 7 (13.2) | 0.133 § |
| Anti-EBV-EA(D) IgG, med (min–max) | 0.0 (0.0–200.0) | 0.0 (0.0–90.0) | 0.023 | 0.0 (0–200.0) | 0.0 (0–175.0) | 0.612 |
| Anti-EBV-EA(D) IgG | 26 (19.5) | 3 (6.0) | 0.025 § | 17 (21.3) | 9 (17.0) | 0.543 § |
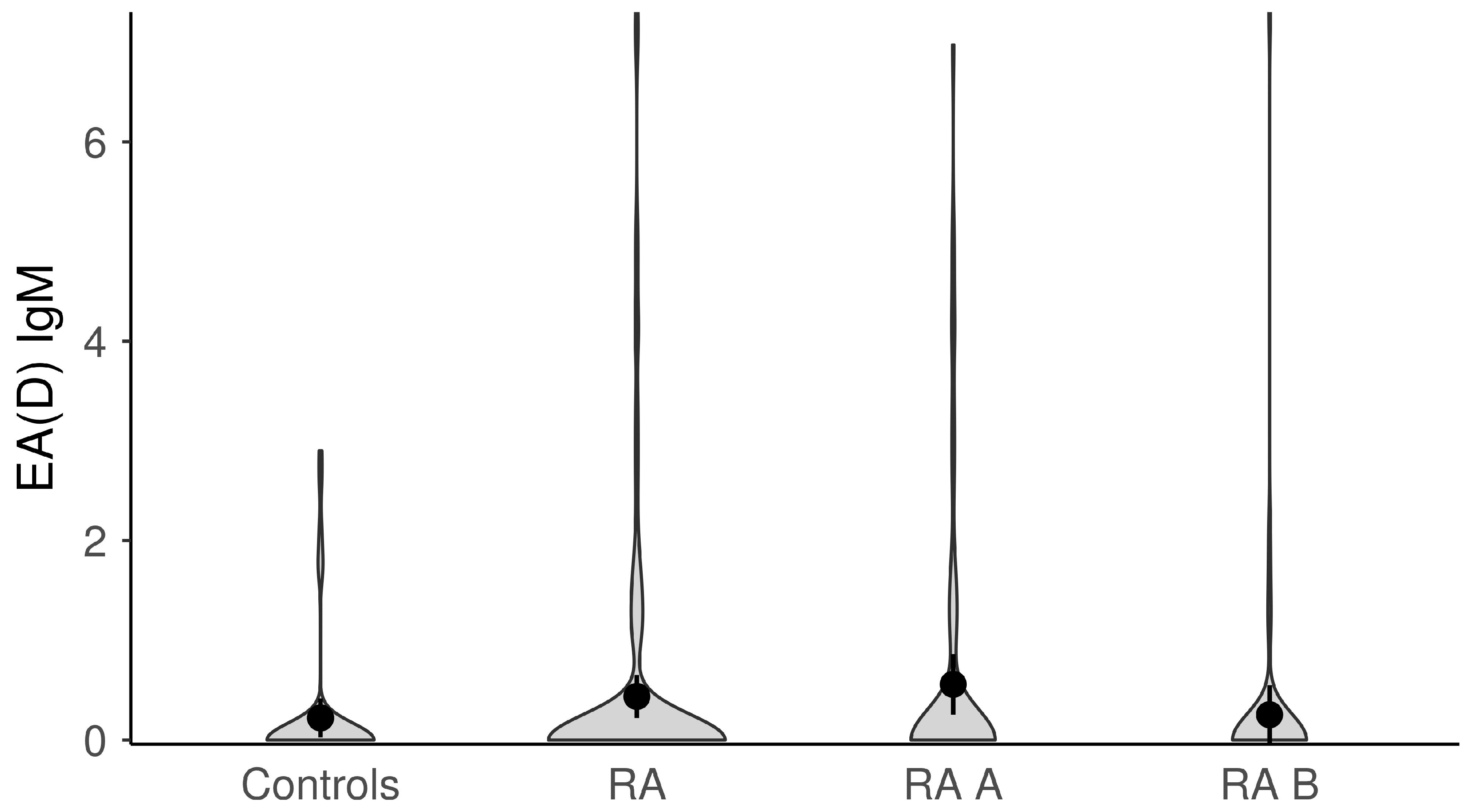
| Anti-EBV-EA(D) IgM, med (min–max) | 0.0 (0.0–7.3) | 0.0 (0.0–2.9) | 0.335 | 0.0 (0.0–7.0) | 0.0 (0.0–7.3) | 0.099 |
| Anti-EBV-EA(D) IgM | 21 (15.8) | 5 (10.0) | 0.317 § | 16 (20.0) | 5 (9.4) | 0.102 § |
| EBV infection status, n (%) | ||||||
| Active/recent | 56 (42.1) | 8 (16.0) | <0.001 § | 41 (51.25) | 15 (28.3) | 0.009 § |
| past | 77 (57.9) | 42 (84.0) | 39 (48.75) | 38 (71.7) | ||
| Disease Characteristics | RA Patients with Positive EBV DNA in Blood (Viremic RA Patients) | RA Patients with Negative EBV DNA in Blood (Non-viremic RA Patients) | p * |
|---|---|---|---|
| DAS28CRP mean ± sd | 4.98 ± 1.47 | 5.26 ± 1.09 | 0.313 |
| CDAI med (min–max) | 14.0 (6.0–29.0) | 18.0 (1.0–40.0) | 0.536 |
| SDAI med (min–max) | 15.0 (6.0–23.0) | 16.0 (0.0–63.0) | 0.387 |
| RAID med (min–max) | 6.0 (2.0–10.0) | 5.0 (1.0–18.0) | 0.615 |
| HAQ med (min–max) | 1.1 (0.3–1.1) | 1.12 (0.1–2.6) | 0.250 |
| CRP med (min–max) | 10.1 (1.1–66.6) | 15.1 (0.0–152.4) | 0.402 |
| ANA med (min–max) | 0.00 (0.0–80.0) | 40.0 (0.0–640.0) | 0.565 |
| ACPA med (min–max) | 500.0 (3.8–500.0) | 309.0 (0.0–500.0) | 0.182 |
| RF med (min–max) | 219.0 (113.0–500.0) | 149.0 (0.0–641.6) | 0.033 |
| Anticardiolipin IgG | 0.0 (0.0–0.1) | 0.0 (0.0–15.1) | 0.148 |
| Anticardiolipin IgM | 0.0 (0.0–0.2) | 0.0 (0.0–31.2) | 0.226 |
| Position of Amino Acid/Codon Based on Ref. Sequence of EBV B95.8 (P-ala) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EBV B95.8 (P-ala) | 471 Gln/CAA | 476 Pro/CCG | 483 Glu/GAA | 487 Ala/GCT | 492 Ser/AGT | 499 Asp/GAC | 502 Thr/ACT | 520 Leu/CTA | 524 Thr/ACT | 529 Pro/CCA | 533 Leu/CTT |
| Patient RA33A (P-thr) | * | Gln/CAG | * | Thr/ACT | Cys/TGT | Asp/GAT | * | Leu/CTC | Ile/ATT | * | * |
| Patient RA35A (P-thr) | * | Gln/CAG | * | Thr/ACT | Cys/TGT | Asp/GAT | * | Leu/CTC | Ile/ATT | * | * |
| Patient RA37A (P-ala-sv-2) | * | * | * | * | * | Glu/GAA | * | * | Val/GTT | * | * |
| Patient RA41A (P-thr) | * | Gln/CAG | * | Thr/ACT | Cys/TGT | Asp/GAT | * | Leu/CTC | Ile/ATT | * | * |
| Patient RA62A (P-thr) | * | Gln/CAG | * | Thr/ACT | Cys/TGT | Asp/GAT | * | Leu/CTC | Ile/ATT | * | * |
| Factor Associated with | Multivariate Logistic Regression | ||
|---|---|---|---|
| OR/RR | 95% CI OR | p * | |
| Having RA | |||
| Active/recent EBV infection | 4.645 | 1.33–16.19 | 0.016 |
| The onset of RA | |||
| Active/recent EBV infection | 5.471 | 1.28–23.35 | 0.022 |
| Poor control of RA | |||
| Active/recent EBV infection | 0.451 | 0.21–0.98 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljanovic, D.; Cirkovic, A.; Jermic, I.; Basaric, M.; Lazarevic, I.; Grk, M.; Miskovic, R.; Despotovic, A.; Banko, A. Markers of Epstein–Barr Virus Infection in Association with the Onset and Poor Control of Rheumatoid Arthritis: A Prospective Cohort Study. Microorganisms 2023, 11, 1958. https://doi.org/10.3390/microorganisms11081958
Miljanovic D, Cirkovic A, Jermic I, Basaric M, Lazarevic I, Grk M, Miskovic R, Despotovic A, Banko A. Markers of Epstein–Barr Virus Infection in Association with the Onset and Poor Control of Rheumatoid Arthritis: A Prospective Cohort Study. Microorganisms. 2023; 11(8):1958. https://doi.org/10.3390/microorganisms11081958
Chicago/Turabian StyleMiljanovic, Danijela, Andja Cirkovic, Ivica Jermic, Milica Basaric, Ivana Lazarevic, Milka Grk, Rada Miskovic, Aleksa Despotovic, and Ana Banko. 2023. "Markers of Epstein–Barr Virus Infection in Association with the Onset and Poor Control of Rheumatoid Arthritis: A Prospective Cohort Study" Microorganisms 11, no. 8: 1958. https://doi.org/10.3390/microorganisms11081958
APA StyleMiljanovic, D., Cirkovic, A., Jermic, I., Basaric, M., Lazarevic, I., Grk, M., Miskovic, R., Despotovic, A., & Banko, A. (2023). Markers of Epstein–Barr Virus Infection in Association with the Onset and Poor Control of Rheumatoid Arthritis: A Prospective Cohort Study. Microorganisms, 11(8), 1958. https://doi.org/10.3390/microorganisms11081958







