Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolle, L.E. Urinary Tract Infections in the Older Adult. Clin. Geriatr. Med. 2016, 32, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.J.; Nicolle, L.E. Urinary Tract Infections in Older Men. N. Engl. J. Med. 2016, 374, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.A.; McKoy, J.M. Sepsis in Older Adults. Infect. Dis. Clin. North Am. 2017, 31, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Oh, S.K.; Cho, S.U.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Cho, Y.C.; Ahn, H.J.; Kang, C. A novel predictive tool for prognosis in elderly patients with urinary tract infection: Modified PRACTICE. Am. J. Emerg. Med. 2020, 38, 2002–2006. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital Deaths in Patients With Sepsis From 2 Independent Cohorts. JAMA 2014, 312, 90. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Moss, M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006, 34, 15–21. [Google Scholar] [CrossRef]
- Martín, S.; Pérez, A.; Aldecoa, C. Sepsis and immunosenescence in the elderly patient: A review. Front. Med. 2017, 4, 20. [Google Scholar] [CrossRef]
- Van Vught, L.A.V.; Klouwenberg, P.M.C.K.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nürnberg, P.; Bonten, M.J.M.; Cremer, O.L.; et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA J. Am. Med. Assoc. 2016, 315, 1469–1479. [Google Scholar] [CrossRef]
- Prescott, H.C.; Osterholzer, J.J.; Langa, K.M.; Angus, D.C.; Iwashyna, T.J. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016, 353, i2375. [Google Scholar] [CrossRef]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-term mortality and quality of life in sepsis: A systematic review. Crit. Care Med. 2010, 38, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA J. Am. Med. Assoc. 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review HHS Public Access. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Li, Y.; Ji, M.; Yang, J. Current Understanding of Long-Term Cognitive Impairment after Sepsis. Front. Immunol. 2022, 13, 855006. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181. [Google Scholar] [CrossRef]
- Abernethy, J.K.; Johnson, A.P.; Guy, R.; Hinton, N.; Sheridan, E.A.; Hope, R.J. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin. Microbiol. Infect. 2015, 21, 251.e1–251.e8. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, M.H.; Park, Y.S.; Lee, H.S.; Kim, D.; Lee, H.; Shin, K.S.; Shin, J.H.; Uh, Y.; Kim, Y.A.; et al. Impact of host-pathogen-treatment tripartite components on early mortality of patients with Escherichia coli bloodstream infection: Prospective observational study. eBioMedicine 2018, 35, 76–86. [Google Scholar] [CrossRef]
- Chin, B.S.; Kim, M.S.; Han, S.H.; Shin, S.Y.; Choi, H.K.; Chae, Y.T.; Jin, S.J.; Baek, J.H.; Choi, J.Y.; Song, Y.G.; et al. Risk factors of all-cause in-hospital mortality among Korean elderly bacteremic urinary tract infection (UTI) patients. Arch. Gerontol. Geriatr. 2011, 52, e50–e55. [Google Scholar] [CrossRef]
- Esparcia, A.; Artero, A.; Eiros, J.M.; Balaguer, M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. Eur. J. Intern. Med. 2014, 25, 523–527. [Google Scholar] [CrossRef]
- Holmbom, M.; Andersson, M.; Grabe, M.; Peeker, R.; Saudi, A.; Styrke, J.; Aljabery, F. Community-onset urosepsis: Incidence and risk factors for 30-day mortality–a retrospective cohort study. Scand. J. Urol. 2022, 56, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, M. Aplicación de los Criterios de Sepsis-3 en la Infección Urinaria; Universitat de València: València, Spain, 2020. [Google Scholar]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a newdefinition and assessing newclinical criteria for Septic shock: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Leone, M.; Bourgoin, A.; Cambon, S.; Dubuc, M.; Albanèse, J.; Martin, C. Empirical antimicrobial therapy of septic shock patients: Adequacy and impact on the outcome*. Crit. Care Med. 2003, 31, 462–467. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Davis, J.S.; He, V.; Anstey, N.M.; Condon, J.R. Long term outcomes following hospital admission for sepsis using relative survival analysis: A prospective cohort study of 1,092 patients with 5 year follow up. PLoS ONE 2014, 9, e112224. [Google Scholar] [CrossRef]
- Sacanella, E.; Pérez-Castejón, J.M.; Nicolás, J.M.; Masanés, F.; Navarro, M.; Castro, P.; López-Soto, A. Functional status and quality of life 12 months after discharge from a medical ICU in healthy elderly patients: A prospective observational study. Crit. Care 2011, 15, R105. [Google Scholar] [CrossRef]
- Villar, J.; Short, J.H.; Lighthall, G. Lactate Predicts Both Short- and Long-Term Mortality in Patients With and Without Sepsis. Infect. Dis. Res. Treat. 2019, 12, 117863371986277. [Google Scholar] [CrossRef]
- Park, D.W.; Chunx, B.C.; Kim, J.M.; Sohn, J.W.; Peck, K.R.; Kim, Y.S.; Choi, Y.H.; Choi, J.Y.; Kim, S.I.; Eom, J.S.; et al. Epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock: A prospective observational study in 12 university hospitals in Korea. J. Korean Med. Sci. 2012, 27, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.G.M.; Spronk, P.E.; Van Stel, H.F.; Schrijvers, A.J.P.; Rommes, J.H.; Bakker, J. The impact of severe sepsis on health-related quality of life: A long-term follow-up study. Anesth. Analg. 2008, 107, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shou, Z.; Zhang, P.; He, Q.; Xiao, H.; Xu, Y.; Li, C.; Chen, J. Mitochondrial DNA haplogroup R predicts survival advantage in severe sepsis in the Han population. Genet. Med. 2008, 10, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Angus, D.C. Long-term outcomes from sepsis. Curr. Infect. Dis. Rep. 2007, 9, 382–386. [Google Scholar] [CrossRef]
- Dalager-Pedersen, M.; Thomsen, R.W.; Schønheyder, H.C.; Nielsen, H. Functional status and quality of life after community-acquired bacteraemia: A matched cohort study. Clin. Microbiol. Infect. 2016, 22, 78.e1–78.e8. [Google Scholar] [CrossRef]
- Leibovici, L. Long-term consequences of severe infections. Clin. Microbiol. Infect. 2013, 19, 510–512. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Netzer, G.; Langa, K.M.; Cigolle, C. Spurious inferences about long-term outcomes: The case of severe sepsis and geriatric conditions. Am. J. Respir. Crit. Care Med. 2012, 185, 835–841. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Ambler, M.; Mahalingasivam, V.; Jones, A.; Rowan, K.; Rubenfeld, G.D. Evidence for a causal link between sepsis and long-term mortality: A systematic review of epidemiologic studies. Crit. Care 2016, 20, 101. [Google Scholar] [CrossRef]
- Rowe, T.; Araujo, K.L.B.; Van Ness, P.H.; Pisani, M.A.; Juthani-Mehta, M. Outcomes of older adults with sepsis at admission to an intensive care unit. Open Forum Infect. Dis. 2016, 3, ofw010. [Google Scholar] [CrossRef]
- Singer, M.; Inada-Kim, M.; Shankar-Hari, M. Sepsis hysteria: Excess hype and unrealistic expectations. Lancet 2019, 394, 1513–1514. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, M.; López-Cruz, I.; Piles, L.; Viñola, S.; Alberola, J.; Eiros, J.M.; Artero, A. Risk Factors and the Impact of Multidrug-Resistant Bacteria on Community-Acquired Urinary Sepsis. Microorganisms 2023, 11, 1278. [Google Scholar] [CrossRef]
- Karve, S.; Ryan, K.; Peeters, P.; Baelen, E.; Rojas-Farreras, S.; Potter, D.; Rodríguez-Baño, J. The impact of initial antibiotic treatment failure: Real-world insights in patients with complicated urinary tract infection. J. Infect. 2018, 76, 121–131. [Google Scholar] [CrossRef] [PubMed]
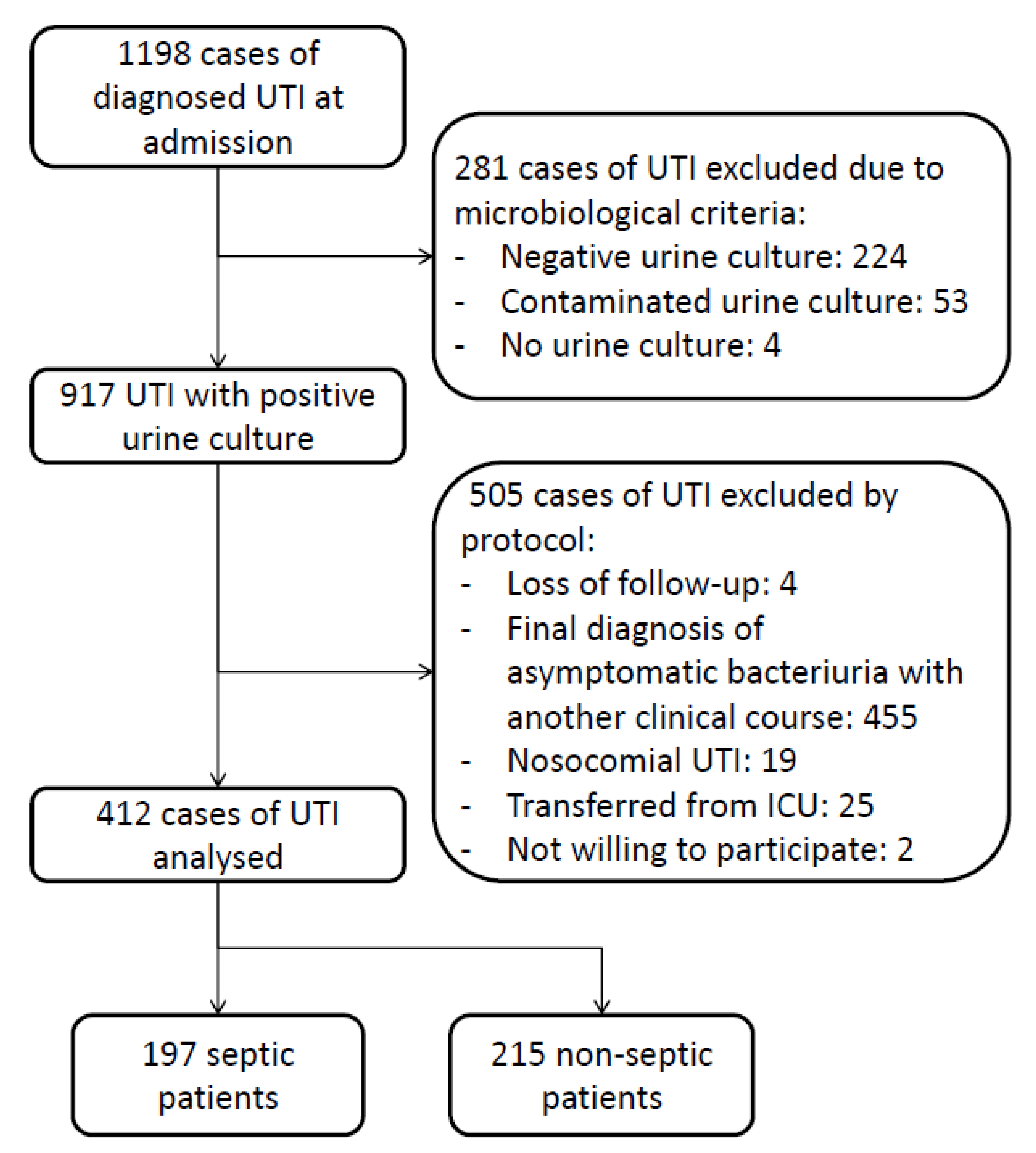
| Total N 412 | Septic Patients N 197 (47.8%) | Non-Septic Patients N 215 (52.2%) | p | |
|---|---|---|---|---|
| Female sex, n (%) | 209 (50.7) | 99 (49.7) | 110 (51.2) | 0.854 |
| Age (years), median [IQR] | 81 [75–88] | 83 [77–89] | 80 [74–85] | <0.001 |
| Charlson ≥ 3, n (%) | 402 (97.6) | 191 (97) | 211 (98.1) | 0.435 |
| Barthel < 40, n (%) | 169 (41) | 90 (45.7) | 79 (36.7) | 0.065 |
| Comorbidities | ||||
| Dementia, n (%) | 136 (33) | 69 (35) | 68 (31.4) | 0.396 |
| Diabetes mellitus, n (%) | 161 (39.1) | 75 (38.1) | 86 (40) | 0.689 |
| COPD, n (%) | 59 (14.3) | 25 (12.8) | 34 (15.8) | 0.377 |
| CKD, n (%) | 149 (36.2) | 71 (36.2) | 78 (36.4) | 0.962 |
| Cancer, n (%) | 93 (22.6) | 40 (20.3) | 53 (24.7) | 0.292 |
| Indwelling urinary catheter, n (%) | 87 (21.1) | 39 (19.8) | 48 (22.3) | 0.530 |
| HCA-UTI, n (%) | 248 (60.2) | 114 (57.9) | 134 (62.3) | 0.356 |
| Previous hospitalization, n (%) | 142 (34.5) | 69 (35) | 73 (34) | 0.819 |
| Previous antimicrobial therapy, n (%) | 212 (51.5) | 92 (46.7) | 120 (55.8) | 0.064 |
| Nursing home residence, n (%) | 29 (7) | 18 (9.1) | 11 (5.1) | 0.111 |
| Clinical characteristics | ||||
| APACHE II, median [IQR] | 12 [9–16] | 16 [12–20] | 10 [8–12] | <0.001 |
| APN, n (%) | 262 (63.6) | 110 (55.8) | 152 (70.7) | 0.002 |
| Altered mental status, n (%) | 189 (45.9) | 128 (65.3) | 61 (28.4) | <0.001 |
| RR ≥ 22 bpm, n (%) | 92 (22.3) | 78 (39.8) | 14 (6.5) | <0.001 |
| SBP < 100 mmHg, n (%) | 74 (18) | 68 (34.7) | 6 (2.8) | <0.001 |
| Fever, n (%) | 307 (74.5) | 139 (70.6) | 168 (78.1) | 0.078 |
| qSOFA ≥ 2, n (%) | 113 (27.4) | 109 (55.3) | 4 (1.9) | <0.001 |
| Septic shock-3, n (%) | 45 (10.9) | 43 (21.8) | 2 (0.9) | <0.001 |
| Lactate ≥ 2 mg/dL, n (%) | 171 (41.5) | 111 (56.3) | 60 (27.9) | <0.001 |
| Leukocytosis, median [IQR] | 12,900 [9200–17,975] | 13,800 [10,100–19,000] | 11,800 [8500–16,900] | 0.004 |
| Blood cultures positive/BC taken, n (%) | 95/228 (41.7) | 60/125 (48) | 35/103 (33.9) | 0.001 |
| MDR-B, n (%) | 148 (35.9) | 70 (35.5) | 78 (36.3) | 0.875 |
| Polymicrobial UTI, n (%) | 40 (9.7) | 22 (11.2) | 18 (8.4) | 0.338 |
| IEAT, n (%) | 107 (26) | 48 (24.4) | 59 (27.6) | 0.460 |
| Total N 412 | Septic Patients N 197 (47.8%) | Non-Septic Patients N 215 (52.2%) | p | |
|---|---|---|---|---|
| In-hospital case-fatality rate, n (%) | 39 (9.5) | 36 (18.3) | 3 (1.4) | <0.001 |
| 30-day case-fatality rate, n (%) | 57 (13.8) | 47 (23.9) | 10 (4.7) | <0.001 |
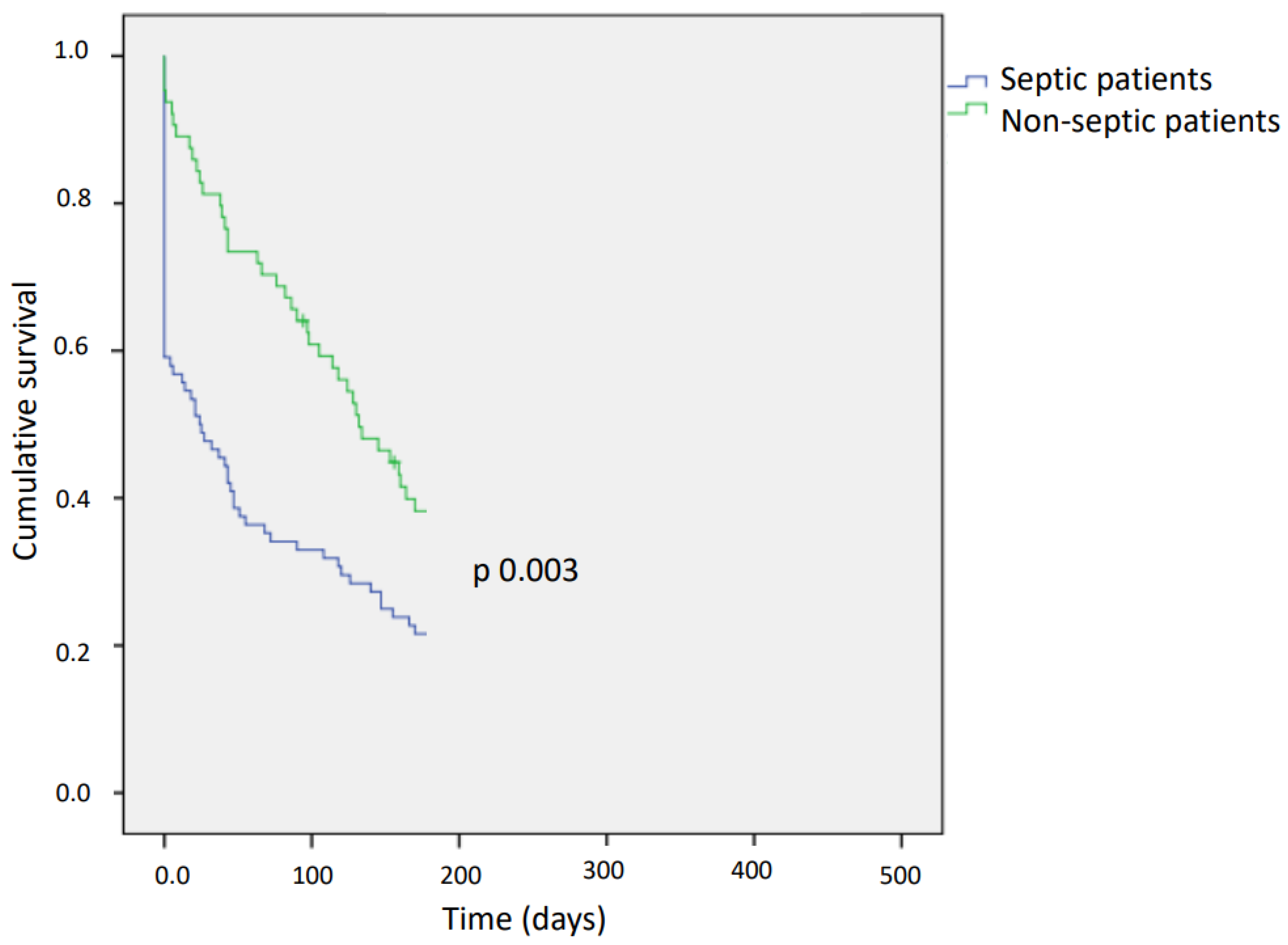
| 6-month case-fatality rate, n (%) | 107 (26) | 67 (34) | 40 (18.6) | 0.003 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | aOR (95% CI) | p | |
| Older than 75 years | 3.1 (1.6–5.9) | <0.001 | 2.5 (1.1–5.6) | 0.026 |
| Charlson ≥ 3 | 1.4 (1.1–1.8) | 0.048 | 4.8 (0.5–9.7) | 0.999 |
| Barthel ≤ 40 | 4.1 (2.8–5.9) | <0.001 | 5.1 (3–8.7) | <0.001 |
| Fever | 0.6 (0.4–0.8) | 0.006 | 0.5 (0.3–0.9) | 0.018 |
| HCA-UTI | 1.6 (1.1–2.3) | 0.008 | 1.7 (1.1–2.9) | 0.049 |
| Sepsis (SOFA ≥ 2) | 1.6 (1.2–2.4) | <0.001 | 1.9 (1.1–3.1) | 0.025 |
| Lactate ≥ 2 mg/dL | 1.7 (1.2–2.3) | 0.002 | 1.3 (0.8–2.3) | 0.246 |
| IEAT | 1.5 (1.1–2.1) | 0.019 | 1.3 (0.7–2.4) | 0.500 |
| Bacteremia | 1.1 (0.7–1.6) | 0.723 | 1.3 (0.7–2.4) | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artero, A.; López-Cruz, I.; Alberola, J.; Eiros, J.M.; Resa, E.; Piles, L.; Madrazo, M. Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People. Microorganisms 2023, 11, 1959. https://doi.org/10.3390/microorganisms11081959
Artero A, López-Cruz I, Alberola J, Eiros JM, Resa E, Piles L, Madrazo M. Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People. Microorganisms. 2023; 11(8):1959. https://doi.org/10.3390/microorganisms11081959
Chicago/Turabian StyleArtero, Arturo, Ian López-Cruz, Juan Alberola, José María Eiros, Elena Resa, Laura Piles, and Manuel Madrazo. 2023. "Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People" Microorganisms 11, no. 8: 1959. https://doi.org/10.3390/microorganisms11081959
APA StyleArtero, A., López-Cruz, I., Alberola, J., Eiros, J. M., Resa, E., Piles, L., & Madrazo, M. (2023). Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People. Microorganisms, 11(8), 1959. https://doi.org/10.3390/microorganisms11081959


_Di_Marco.png)



