Rice Husk—Cellulose-Based Agricultural Waste Enhances the Degradation of Synthetic Dyes Using Multiple Enzyme-Producing Extremophiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation of the Degrading Bacteria of Azo Dyes
2.2. Identification and Phylogenetic Analysis
2.3. Determination of Ligninolytic Enzyme Production of Strain FW2
2.4. Degradation and Decolorization Experiments of Dyes
2.5. Effects of Physicochemical Factors on Bacterial Growth and Dye Degradation
2.6. Effects of Rice Husks as a Special Carbon Source for FW2 Growth and Dye Degradation
2.7. Effects of Metal Ions on Enzyme Production and Dye Removal
2.8. Effect of Different Dye Concentrations
3. Results
3.1. 16S rRNA Gene Analysis
3.2. Optimal Conditions for Bacterial Growth, Enzyme Production, and Dye Degradation
3.2.1. Effect of pH, Temperature, and NaCl
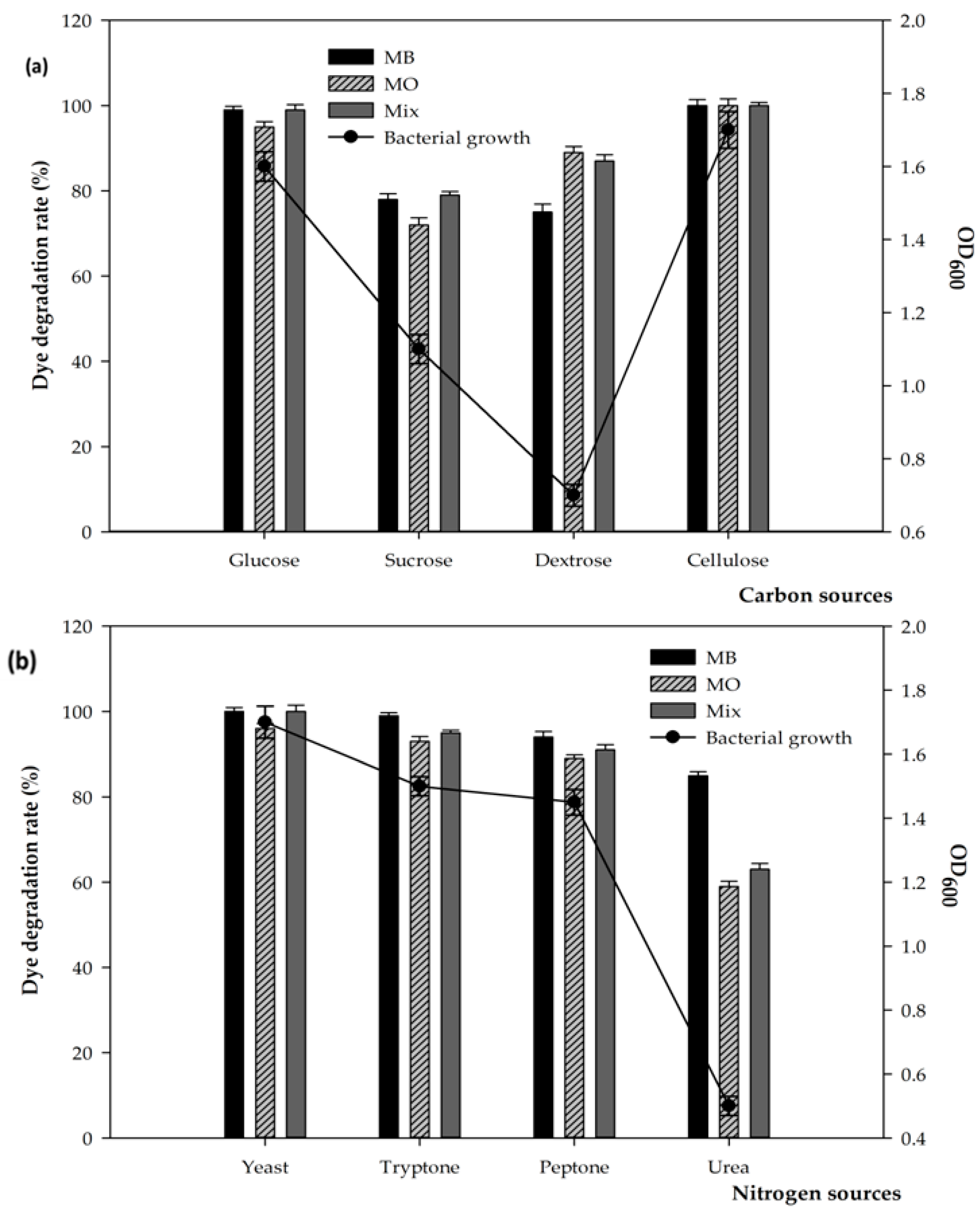
3.2.2. Effect of Carbon and Nitrogen Sources
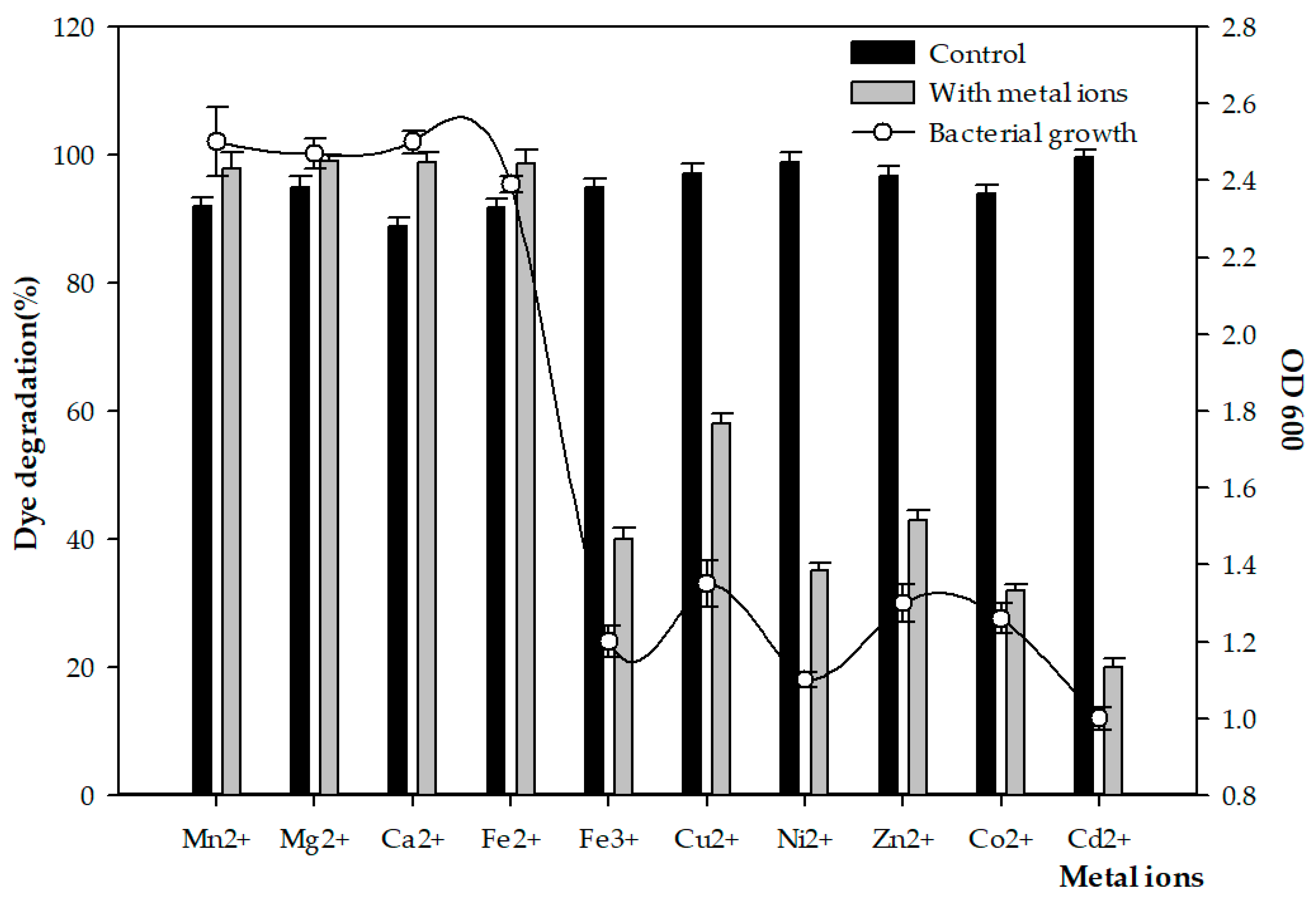
3.3. Effect of Metal Ions
3.4. Effect of Dye Concentrations on Bacterial Growth and Dye Removal Performance
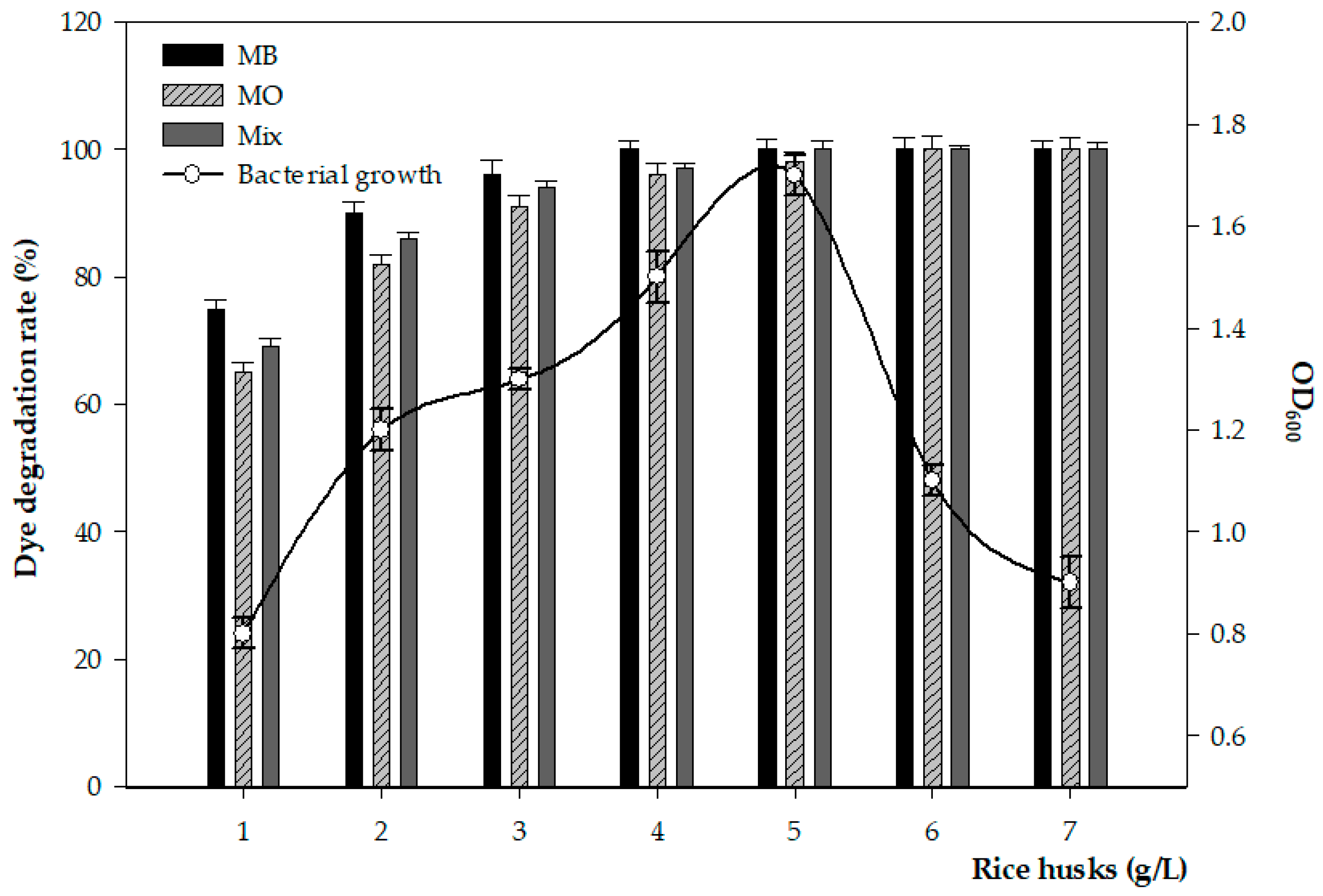
3.5. Effect of Rice Husk Concentrations on Bacterial Growth and Its Dye Degradation Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jameel, M.; Umar, K.; Parveen, T.; Ismail, I.M.I.; Qari, H.A.; Yaqoob, A.A.; Ibrahim, M.N.M. Chapter 12—Extraction of natural dyes from agro-industrial waste. In Extraction of Natural Products from Agro-Industrial Wastes; Bhawani, S., Khan, A., Ahmad, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–216. [Google Scholar]
- Parmar, S.; Daki, S.; Bhattacharya, S.; Shrivastav, A. Microorganism: An ecofriendly tool for waste management and environmental safety. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–193. [Google Scholar]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Akil Ahmad, A.; Oves, M.; Ismail, I.M.I.; Huda, A.; Qari, H.A.; Khalid Umar, K.; Ibrahim, M.N.M. Recent advances in metal decorated nanomaterials and their various biological applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Treatment of textile dye containing effluents. Curr. Environ. Eng. 2014, 1, 162–184. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Tohamy, R.A.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Asim Ali Yaqoob, A.A.; Claudia, G.B.; Ahmad, A.; Ibrahim, M.N.M.; Mohammed, B.; Alshammari, M.B. Advanced technologies for wastewater treatment. In Green Chemistry for Sustainable Water Purification; Willey: Hoboken, NJ, USA, 2023; pp. 179–202. [Google Scholar]
- Pham, V.H.T.; Kim, J.; Chang, S.; Bang, D. Investigating bio-inspired degradation of toxic dyes using potential multi-enzyme producing extremophiles. Microorganisms 2023, 11, 1273. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Biodegradation of Methylene Blue using a novel lignin peroxidase enzyme producing bacteria, named Bacillus sp. React3, as a promising candidate for dye-contaminated wastewater treatment. Fermentation 2022, 8, 190. [Google Scholar] [CrossRef]
- Sompark, C.; Singkhonrat, J.; Sakkayawong, N. Biotransformation of Reactive Red 141 by Paenibacillus terrigena KKW2-005 and examination of product toxicity. J. Microbiol. Biotechnol. 2021, 31, 967–977. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.L. Bio-removal of Azo Dyes: A Review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and prospects for a hazard-free conversion by microorganisms. Int. J. Environ. Res. Public Health. 2022, 19, 4740. [Google Scholar] [CrossRef] [PubMed]
- Fareed, A.; Zaffar, H.; Bilal, M.; Hussain, J.; Jackson, C.; Naqvi, T.A. Decolorization of azo dyes by a novel aerobic bacterial strain Bacillus cereus strain ROC. PLoS ONE 2022, 17, e0269559. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, Z.S.; Zahraei, Z.; Jookar Kashi, F. Decolorization of Reactive Black 5 and Reactive Red 152 azo dyes by new haloalkaliphilic bacteria isolated from the textile wastewater. Curr. Microbiol. 2020, 77, 2084–2092. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Goyal, N.; Gupta, A. Degradation of azo dye methyl red by alkaliphilic, halotolerant Nesterenkonia lacusekhoensis EMLA3: Application in alkaline and salt-rich dyeing effluent treatment. Extremophiles 2017, 21, 479–490. [Google Scholar] [CrossRef]
- Pandey, K.; Saha, P.; Rao, K.B. A study on the utility of immobilized cells of indigenous bacteria for biodegradation of reactive azo dyes. Prep. Biochem. Biotechnol. 2020, 50, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Sonu, K.; Sogani, M.; Syed, Z.; Rajvanshi, J.; Sengupta, N. Effectiveness of rice husk in the removal of methyl orange dye in constructed Wetland-Microbial Fuel Cell. Bioresour. Technol. Rep. 2022, 20, 101223. [Google Scholar] [CrossRef]
- Govindarao, V.M.H. Utilization of rice husk—A preliminary analysis. J. Sci. Ind. Res. India. 1980, 39, 495–515. [Google Scholar]
- Mladenovic, N.; Makreski, P.; Tarbuk, A.; Grgic, K.; Boev, B.; Mirakovski, D.; Toshikj, E.; Dimova, V.; Dimitrovski, D.; Jordanov, I. Improved dye removal ability of modified rice husk with effluent from alkaline scouring based on the circular economy concept. Processes 2020, 8, 653. [Google Scholar] [CrossRef]
- Forss, J.; Lindh, M.V.; Pinhassi, J.; Welander, U. Microbial biotreatment of actual textile wastewater in a continuous sequential rice husk biofilter and the microbial community involved. PLoS ONE 2017, 12, e0170562. [Google Scholar] [CrossRef] [Green Version]
- João, J.J.; Locks, L.; Vieira, J.L.; Lucia, E.A. Rice husks as a microbial source for wastewater treatment. Rev. Bras. Eng. Agríc. Ambient. 2020, 24, 343–347. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Purification and characterization of strong simultaneous enzyme production of Protease and α-Amylase from an extremophile-Bacillus sp. FW2 and Its Possibility in Food Waste Degradation. Fermentation 2022, 8, 12. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA Genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobis-(3- ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin peroxidase of Phanerochaete chrysosporium. Meth. Enzymol. 1988, 161, 238–249. [Google Scholar]
- Velayutham, K.; Madhava, A.K.; Pushparaj, M.; Thanarasu, A.; Devaraj, T.; Periyasamy, K.; Subramanian, S. Biodegradation of remazol brilliant blue R using isolated bacterial culture (Staphylococcus sp. K2204). Environ. Technol. 2018, 39, 2900–2907. [Google Scholar] [CrossRef]
- Hossen, M.Z.; Hussain, M.E.; Hakim, A.; Islam, K.; Uddin, M.N.; Azad, A.K. Biodegradation of reactive textile dye novacron super black G by free cells of newly isolated Alcaligenes faecalis AZ26 and bacillus sp. obtained from textile effluents. Heliyon 2019, 5, e02068. [Google Scholar] [CrossRef] [Green Version]
- Patil, N.P.; Bholay, A.D.; Kapadnis, B.P.; Gaikwad, V.B. Biodegradation of model azo dye methyl red and other textile dyes by isolate Bacillus circulans npp1. J. Pure Appl. Microbiol. 2018, 10, 2793–2800. [Google Scholar] [CrossRef]
- Chen, G.; An, X.; Li, H.; Lai, F.; Yuan, E.; Xia, X.; Zhang, Q. Detoxification of Azo Dye Direct Black G by Thermophilic Anoxybacillus Sp. PDR2 and its Application Potential in Bioremediation. Ecotoxicol. Environ. Saf. 2021, 214, 112084. [Google Scholar] [CrossRef]
- Khan, Z.; Datta, A.S.; Jain, M.K. Microaerophilic degradation of sulphonatedazo dye reactive red 195 by bacterial consortium AR1 through cometabolism. Int. Biodeter. Biodegr. 2014, 94, 167–175. [Google Scholar] [CrossRef]
- Sharma, V.; Vasanth, D. Lignocellulolytic enzymes from thermophiles. In Sustainable Biotechnology-Enzymatic Resources of Renewable Energy; Springer: Cham, Switzerland, 2018; pp. 205–217. [Google Scholar]
- Gianolini, J.E.; Britos, C.N.; Mulreedy, C.B.; Trelles, J.A. Hyperstabilization of a thermophile bacterial laccase and its application for industrial dyes degradation. 3 Biotech 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.A.; Tawabini, B.; Nazal, M.; AlThaqfi, J.; Khalil, A. Efficiency of thermophilic bacteria in wastewater treatment. Arab. J. Sci. Eng. 2021, 46, 123–128. [Google Scholar] [CrossRef]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2019, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, V.; Yogendra, K.; Mahadevan, K.M.; Mahesh, M.; Kalasaiah, M.; Aroonsrimorakot, S. Effect of carbon and nitrogen sources for the degradation of Red 2G by Bacillus. sp. Int. J. Pharm. Sci. Rev. Res. 2017, 47, 108–113. [Google Scholar]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Ullah, R.; Farraj, D.A.A.; Elshikh, M.S.; Zekker, I.; Gulfam, N. Biological degradation of the azo dye basic Orange 2 by Escherichia coli: A sustainable and ecofriendly approach for the treatment of textile wastewater. Water 2022, 14, 2063. [Google Scholar] [CrossRef]
- Jadhav, S.U.; Kalme, S.D.; Govindwar, S.P. Biodegradation of methyl red by galactomyces geotrichum MTCC 1360. Int. Biodeterior. Biodegrad. 2008, 62, 135–142. [Google Scholar] [CrossRef]
- Mishra, A.; Takkar, S.; Joshi, N.C.; Shukla, S.; Shukla, K.; Singh, A.; Manikonda, A.; Varma, A. An integrative approach to study Bacterial Enzymatic Degradation of Toxic Dyes. Front. Microbiol. 2022, 12, 802544. [Google Scholar] [CrossRef]
- Safa, Y.; Bhatti, H.N. Biosorption of direct Red-31 and direct Orange-26 dyes by rice husk: Application of factorial design analysis. Chem. Eng. Res. Des. 2011, 89, 2566–2574. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Santos-Pereira, G.C.; Corso, C.R.; Forss, J. Evaluation of two different carriers in the biodegradation process of an azo dye. J. Environ. Health Sci. Eng. 2019, 17, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forss, J.; Pinhassi, J.; Lindh, M.; Welander, U. Microbial diversity in a continuous system based on rice husks for biodegradation of the azo dyes Reactive Red 2 and Reactive Black 5. Bioresour. Technol. 2013, 130, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivel, S.P.; Kaleeswari, P.; Mohanapriya, P. Decolorization and adsorption of dyes by consortium of bacteria with agriculture waste. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 865–882. [Google Scholar]
- Jain, D.; Navariya, J.K.; Bhojiya, A.A.; Singh, A.; Mohanty, S.R.; Upadhyay, S.K. Bioprospecting of novel ligninolytic bacteria for effective bioremediation of agricultural by-product and synthetic pollutant dyes. Microbiol. Res. 2023, 270, 127330. [Google Scholar] [CrossRef] [PubMed]



| Degraded Dyes | Bacterial Strains | Carbon Source | Agricultural Waste | Efficiency | Degradation Mechanism | Reference |
|---|---|---|---|---|---|---|
| Reactive Black 5 and Reactive Red 2 | Bacterial community | ND | Un-pretreated rice husks | 80% | Enzymatic pathway | [46] |
| Crystalviolet, Congored, Methyleneblue and Safranin | Consortium | ND | Un-pretreated sawdust | Highest 77.2% MB degradation | Absorption | [47] |
| Reactive Black 5 and Reactive Red 152 | Consortium of Halophilic bacterial strains | Glucose | ND | 87% (Black 5), 85% (Red 152) | Enzymatic pathway | [16] |
| Methylene Blue | Bacillus React3 | Tryptone | ND | 99.50% | Enzymatic pathway | [11] |
| Methyl Orange | Bacterial community | ND | Unpretreated-rice husks | 98% | Absorption | [19] |
| Methylene Blue | Consortium | ND | Pretreated-rice straw | 49.6% | Enzymatic pathway | [48] |
| Methylene Blue and Methyl Orange | Bacillus FW2 | Cellulose | Unpretreated-rice husks | 100% (MO), 99.8% (MB), 98% (MB + MO) | Enzymatic pathways and accumulation | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, V.H.T.; Kim, J.; Chang, S.; Shim, J.; Chung, W.; Bang, D. Rice Husk—Cellulose-Based Agricultural Waste Enhances the Degradation of Synthetic Dyes Using Multiple Enzyme-Producing Extremophiles. Microorganisms 2023, 11, 1974. https://doi.org/10.3390/microorganisms11081974
Pham VHT, Kim J, Chang S, Shim J, Chung W, Bang D. Rice Husk—Cellulose-Based Agricultural Waste Enhances the Degradation of Synthetic Dyes Using Multiple Enzyme-Producing Extremophiles. Microorganisms. 2023; 11(8):1974. https://doi.org/10.3390/microorganisms11081974
Chicago/Turabian StylePham, Van Hong Thi, Jaisoo Kim, Soonwoong Chang, Jeahong Shim, Woojin Chung, and Donggyu Bang. 2023. "Rice Husk—Cellulose-Based Agricultural Waste Enhances the Degradation of Synthetic Dyes Using Multiple Enzyme-Producing Extremophiles" Microorganisms 11, no. 8: 1974. https://doi.org/10.3390/microorganisms11081974







