Monocyte-Derived Chicken Macrophages Exposed to Eimeria tenella Sporozoites Display Reduced Susceptibility to Invasion by Toxoplasma gondii Tachyzoite
Abstract
:1. Introduction
2. Materials and Methods
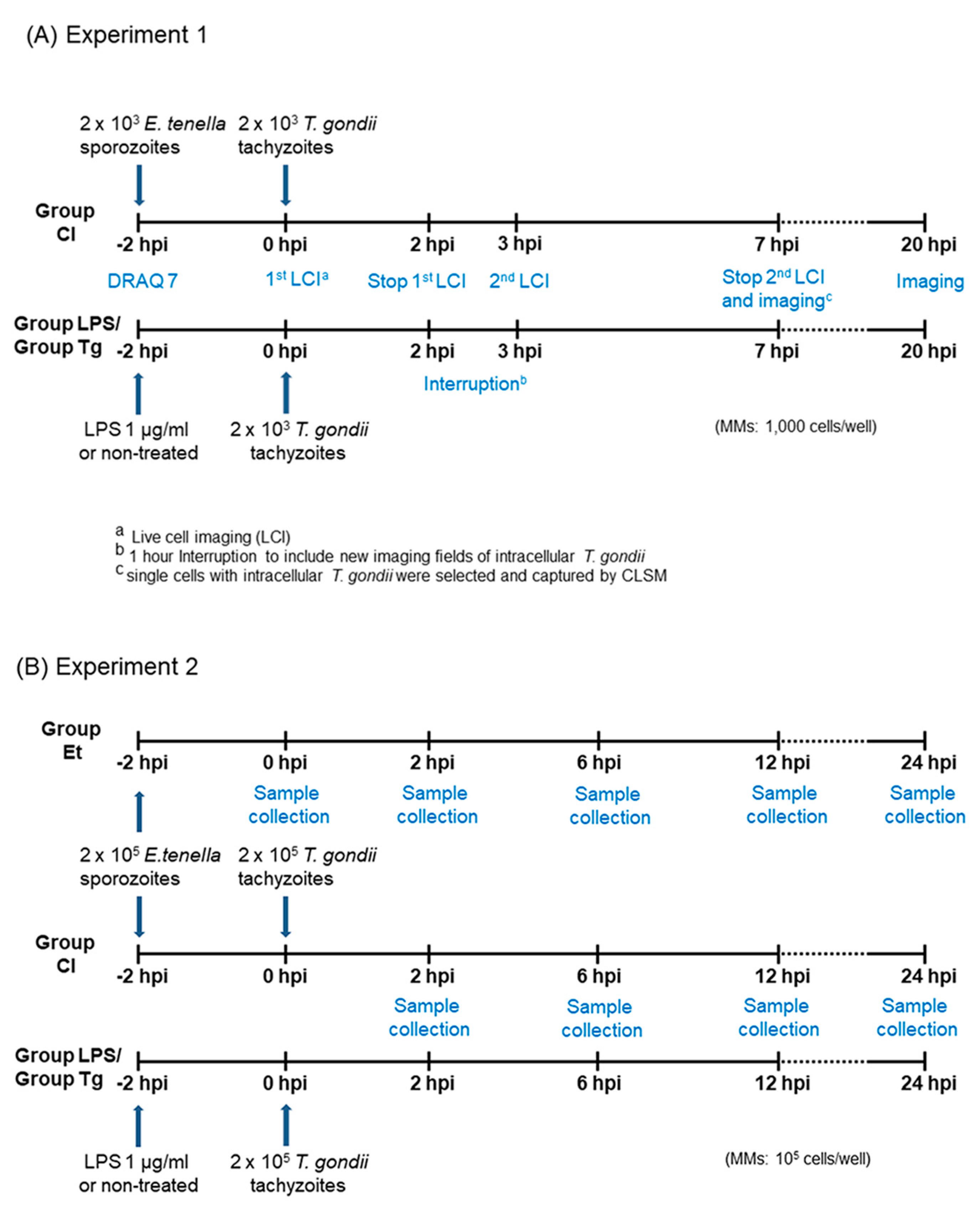
2.1. Parasites and Host Cells
2.2. Infection
2.3. Live-Cell Imaging of T. gondii in MM
2.4. Parasite Quantification by Quantitative Real-Time PCR (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Live Cell Imaging of T. gondii in MM
3.2. Parasite Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohsin, M.; Li, Y.; Zhang, X.; Wang, Y.; Huang, Z.; Yin, G.; Zhang, Z. Development of CRISPR-CAS9 based RNA drugs against Eimeria tenella infection. Genomics 2021, 113, 4126–4135. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; jia Peng, J.; Mohsin, M.; Huang, X.; Lin, X.; Aguilar-Marcelino, L.; Huang, Z.; Yin, G. Construction and evaluation of the Toxoplasma gondii DNA vaccine targeting DEC-205. Pakistan Vet. J. 2022, 42, 256–260. [Google Scholar]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, J.P. Toxoplasma gondii Infections in Chickens (Gallus domesticus): Prevalence, clinical Disease, diagnosis and public health significance. Zoonoses Public Health 2010, 57, 60–73. [Google Scholar] [PubMed]
- Qureshi, M.A.; Heggen, C.L.; Hussain, I. Avian macrophage: Effector functions in health and disease. Dev. Comp. Immunol. 2000, 24, 103–119. [Google Scholar] [CrossRef]
- Meirelles, M.N.; De Souza, W. Killing of Trypanosoma cruzi and Leishmania mexicana, and survival of Toxoplasma gondii, in chicken macrophages in vitro. J. Submicrosc. Cytol. 1985, 17, 327. [Google Scholar]
- Morisaki, J.H.; Heuser, J.E.; Sibley, L.D. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 1995, 108, 2457–2464. [Google Scholar] [CrossRef]
- Vervelde, L.; Vermeulen, A.N.; Jeurissen, S.H.M. In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol. 2010, 18, 247–256. [Google Scholar] [CrossRef]
- Trout, J.M.; Lillehoi, H.S. Evidence of a role for intestinal CD8+ lymphocytes and macrophages in transport of Eimeria acervulina sporozoites. J. Parasitol. 1993, 79, 790–792. [Google Scholar]
- John, R.; Challey, W.M.; Burns, C. The invasion of the cecal mucosa by Eimeria tenella sporozoites and their transport by macrophages. J. Protozool. 1959, 6, 238–241. [Google Scholar]
- Dalloul, R.A.; Lillehoj, H.S.; Shellem, T.A.; Doerr, J.A. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult. Sci. 2003, 82, 62–66. [Google Scholar] [CrossRef]
- Tierney, J.; Gowing, H.; van Sinderen, D.; Flynn, S.; Mulcahy, G. In vitro inhibition of Eimeria tenella invasion by indigenous chicken Lactobacillus species. Vet. Parasitol. 2004, 122, 171–182. [Google Scholar] [CrossRef]
- Marshall, A.J.; Brunet, L.R.; van Gessel, Y.; Alcaraz, A.; Denkers, E.Y. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J. Immunol. 1999, 163, 2089–2097. [Google Scholar]
- Cornelissen, J.B.W.J.; Swinkels, W.J.C.; Boersma, W.A.; Rebel, J.M.J. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: A cumulation of single responses. Vet. Parasitol. 2009, 162, 58–66. [Google Scholar] [CrossRef]
- Guerrero, O.M.; Chinchilla, M.; Abrahams, E. Increasing of Toxoplasma gondii (Coccidia, Sarcocystidae) infections by Trypanosoma lewisi (Kinetoplastida, Trypanosomatidae) in white rats. Rev. Biol. Trop. 1997, 45, 877–882. [Google Scholar]
- Lee, D.H.; Chu, K.B.; Kang, H.J.; Lee, S.H.; Quan, F.S. Previous Infection with Plasmodium berghei Confers Resistance to Toxoplasma gondii Infection in Mice. Korean J. Parasitol. 2019, 57, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Manwell, R.D.; Coulston, F.; Binckley, E.C.; Jones, V.P. Mammalian and avian Toxaplasma. J. Infect. Dis. 1945, 76, 1–14. [Google Scholar] [CrossRef]
- Mason, S.; Dubey, J.P.; Smith, J.E.; Boag, B. Toxoplasma gondii coinfection with diseases and parasites in wild rabbits in Scotland. Parasitology 2015, 142, 1415–1421. [Google Scholar] [CrossRef]
- Hiob, L.; Koethe, M.; Schares, G.; Goroll, T.; Daugschies, A.; Bangoura, B. Experimental Toxoplasma gondii and Eimeria tenella co-infection in chickens. Parasitol. Res. 2017, 116, 3189–3203. [Google Scholar] [CrossRef]
- Zhang, R.; Thabet, A.; Hiob, L.; Zheng, W.; Daugschies, A.; Bangoura, B. Mutual interactions of the apicomplexan parasites Toxoplasma gondii and Eimeria tenella with cultured poultry macrophages. Parasite Vectors 2018, 11, 453. [Google Scholar] [CrossRef]
- Bain, J.; Gow, N.A.R.; Erwig, L.P. Novel insights into host-fungal pathogen interactions derived from live-cell imaging. Semin. Immunopathol. 2015, 37, 131–139. [Google Scholar] [CrossRef] [PubMed]
- McQuate, S.E.; Young, A.M.; Silva-Herzog, E.; Bunker, E.; Hernandez, M.; de Chaumont, F.; Liu, X.; Detweiler, C.S.; Palmer, A.E. Long-term live-cell imaging reveals new roles for Salmonella effector proteins SseG and SteA. Cell. Microbiol. 2016, 19, 12641. [Google Scholar] [CrossRef] [Green Version]
- Mahamed, D.; Boulle, M.; Ganga, Y.; Mc Arthur, C.; Skroch, S.; Oom, L.; Catinas, O.; Pillay, K.; Naicker, M.; Rampersad, S.; et al. Intracellular growth of Mycobacterium tuberculosis after macrophage cell death leads to serial killing of host cells. eLife 2017, 6, e22028. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.C.S.; Gomes, F.; Maciel, L.R.; Seabra, S.H.; Docampo, R.; Moreno, S.; Plattner, H.; Hentschel, J.; Kawazoe, U.; Barrabin, H. Volutin granules of Eimeria parasites are acidic compartments and have physiological and structural characteristics similar to acidocalcisomes. J. Eukaryot. Microbiol. 2011, 58, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, W.; Daugschies, A.; Bangoura, B. Apicomplexan co-infections impair with phagocytic activity in avian macrophages. Parasitol. Res. 2020, 119, 4159–4168. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Zhang, R.; Taha, S.; Daugschies, A. A modified method for purification of Eimeria tenella sporozoites. Parasitol. Res. 2020, 119, 1429–1432. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, B.; Lappalainen, M.; Evengård, B. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin. Microbiol. Infect. 2006, 12, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, F.; Taira, K.; Nagai, S.; Onaga, H.; Onuma, M.; Nunoya, T. Detection of five avian Eimeria species by species-specific real-time polymerase chain reaction assay. Avian Dis. 2008, 52, 652–656. [Google Scholar] [CrossRef]
- Thabet, A.; Alnassan, A.A.; Daugschies, A.; Bangoura, B. Combination of cell culture and qPCR to assess the efficacy of different anticoccidials on Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 2155–2163. [Google Scholar] [CrossRef]
- Long, P.L.; Rose, M.E. Growth of Eimeria tenella in vitro in macrophages from chicken peritoneal exudates. Z. Parasitenk 1976, 48, 291–294. [Google Scholar] [CrossRef]
- Mota, M.M.; Rodriguez, A. Migration through host cells by apicomplexan parasites. Microbes Infect. 2001, 3, 1123–1128. [Google Scholar] [CrossRef]
- Ryning, F.W.; Remington, J.S. Effect of cytochalasin D on Toxoplasma gondii cell entry. Infect. Immun. 1978, 20, 739–743. [Google Scholar] [CrossRef]
- Bumstead, J.; Tomley, F. Induction of secretion and surface capping of microneme proteins in Eimeria tenella. Mol. Biochem. Parasitol. 2000, 110, 311–321. [Google Scholar] [CrossRef]
- Hoff, E.F.; Carruthers, V.B. Is Toxoplasma egress the first step in invasion? Trends Parasitol. 2002, 18, 251–255. [Google Scholar] [CrossRef]
- Dubremetz, J.F.; Garcia-Réguet, N.; Conseil, V.; Fourmaux, M.N. Invited review apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 1998, 28, 1007–1013. [Google Scholar] [CrossRef]
- Mordue, D.G.; Sibley, L.D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 1997, 159, 4452–4459. [Google Scholar] [CrossRef]
- Sibley, L.D.; Weidner, E.; Krahenbuhl, J.L. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature 1985, 315, 416–419. [Google Scholar] [CrossRef]
- Hussain, I.; Qureshi, M.A. The expression and regulation of inducible nitric oxide synthase gene differ in macrophages from chickens of different genetic background. Vet. Immunol. Immunopathol. 1998, 61, 317–329. [Google Scholar] [CrossRef]
- Butcher, B.A.; Denkers, E.Y. Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect. Immun. 2002, 70, 5216–5224. [Google Scholar] [CrossRef] [Green Version]
- Yock-Ping, C.; Kiew-Lian, W.; Blake, D.P.; Fiona, T.; Sheila, N.; Martins, B.E. Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGs) induce inflammatory responses in avian macrophages. PLoS ONE 2011, 6, e25233. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zheng, W.; Daugschies, A.; Bangoura, B. Monocyte-Derived Chicken Macrophages Exposed to Eimeria tenella Sporozoites Display Reduced Susceptibility to Invasion by Toxoplasma gondii Tachyzoite. Microorganisms 2023, 11, 1999. https://doi.org/10.3390/microorganisms11081999
Zhang R, Zheng W, Daugschies A, Bangoura B. Monocyte-Derived Chicken Macrophages Exposed to Eimeria tenella Sporozoites Display Reduced Susceptibility to Invasion by Toxoplasma gondii Tachyzoite. Microorganisms. 2023; 11(8):1999. https://doi.org/10.3390/microorganisms11081999
Chicago/Turabian StyleZhang, Runhui, Wanpeng Zheng, Arwid Daugschies, and Berit Bangoura. 2023. "Monocyte-Derived Chicken Macrophages Exposed to Eimeria tenella Sporozoites Display Reduced Susceptibility to Invasion by Toxoplasma gondii Tachyzoite" Microorganisms 11, no. 8: 1999. https://doi.org/10.3390/microorganisms11081999
APA StyleZhang, R., Zheng, W., Daugschies, A., & Bangoura, B. (2023). Monocyte-Derived Chicken Macrophages Exposed to Eimeria tenella Sporozoites Display Reduced Susceptibility to Invasion by Toxoplasma gondii Tachyzoite. Microorganisms, 11(8), 1999. https://doi.org/10.3390/microorganisms11081999




