Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L.
Abstract
:1. Introduction
2. Methods
2.1. Isolation of Endophytic Fungi from Seeds of Moringa oleifera L.
2.2. ITS Sequencing of Fungal Isolates for Molecular Identification
2.3. Macro- and Microscopic Phenotyping of Seed Endophytic Fungal Isolates
2.4. Metabolic Analysis of Seed Endophytic Fungal Isolates
2.5. Assessment of the Antagonistic Activity by the Dual-Culture Plant Method
2.6. Induction and Evaluation for ACC Deaminase Enzymatic Activity
2.7. Induction and Evaluation for ACC Deaminase Gene Expression Analysis by RT-qPCR
2.8. Root Length Elongation Bioassay with Ethylene Response Mutants
- Col-0 = Control (CK)
- Col-0 = Inoculated with MA1
- Col-0 = Inoculated with MA4
- Col-0 = Inoculated with MA1 and MA4
- eto1 = Control (CK)
- eto1 = Inoculated with MA1
- eto1 = Inoculated with MA4
- eto1 = Inoculated with MA1 and MA4
2.9. Maize-Endophytic Fungal Association Bioassay
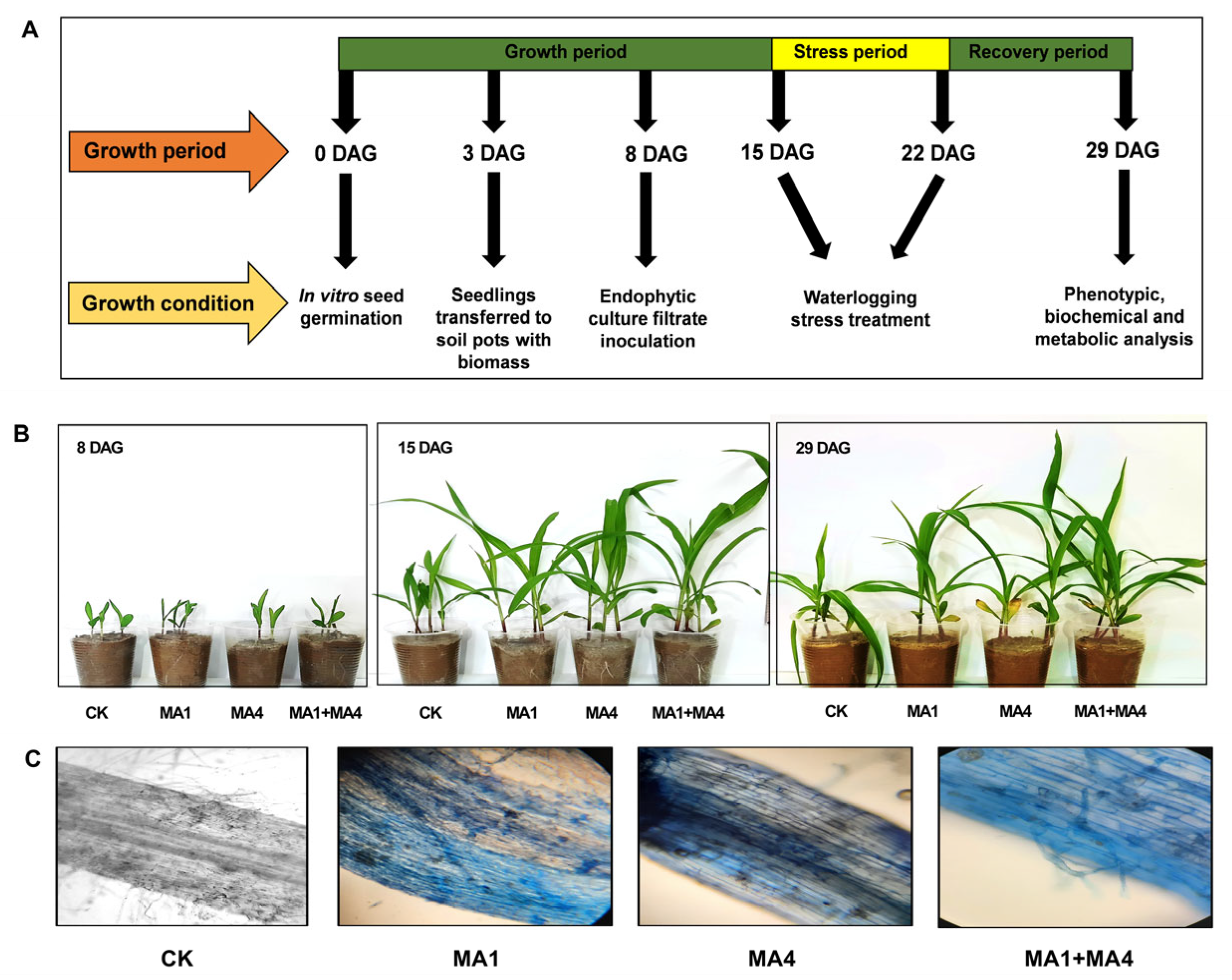
2.9.1. Experimental Design for Plant Bioassay
2.9.2. Colonization Frequency
2.9.3. Waterlogging Treatment
- Control (CK) = no waterlogging stress
- MA1 = endophyte inoculated
- MA4 = endophyte inoculated
- MA1 and MA4 = consortium of endophytes inoculated
- WL = waterlogging stress
- WL + MA1 = endophyte inoculated
- WL + MA4 = endophyte inoculated
- WL + MA1 and MA4 = waterlogging stress and endophyte inoculation
- 0 days of waterlogging treatment and 0 days of endophytic inoculation (8 DAG)
- 0 days of waterlogging treatment and 8 days of endophytic inoculation (15 DAG)
- 7 days of waterlogging treatment and 15 days of endophytic inoculation (22 DAG)
- 7 days after waterlogging treatment (recovery period) and 20 days of endophytic inoculation (29 DAG)
2.9.4. Morphological and Physiological Analysis of Maize Seedlings
2.9.5. Determination of Chlorophyll Content
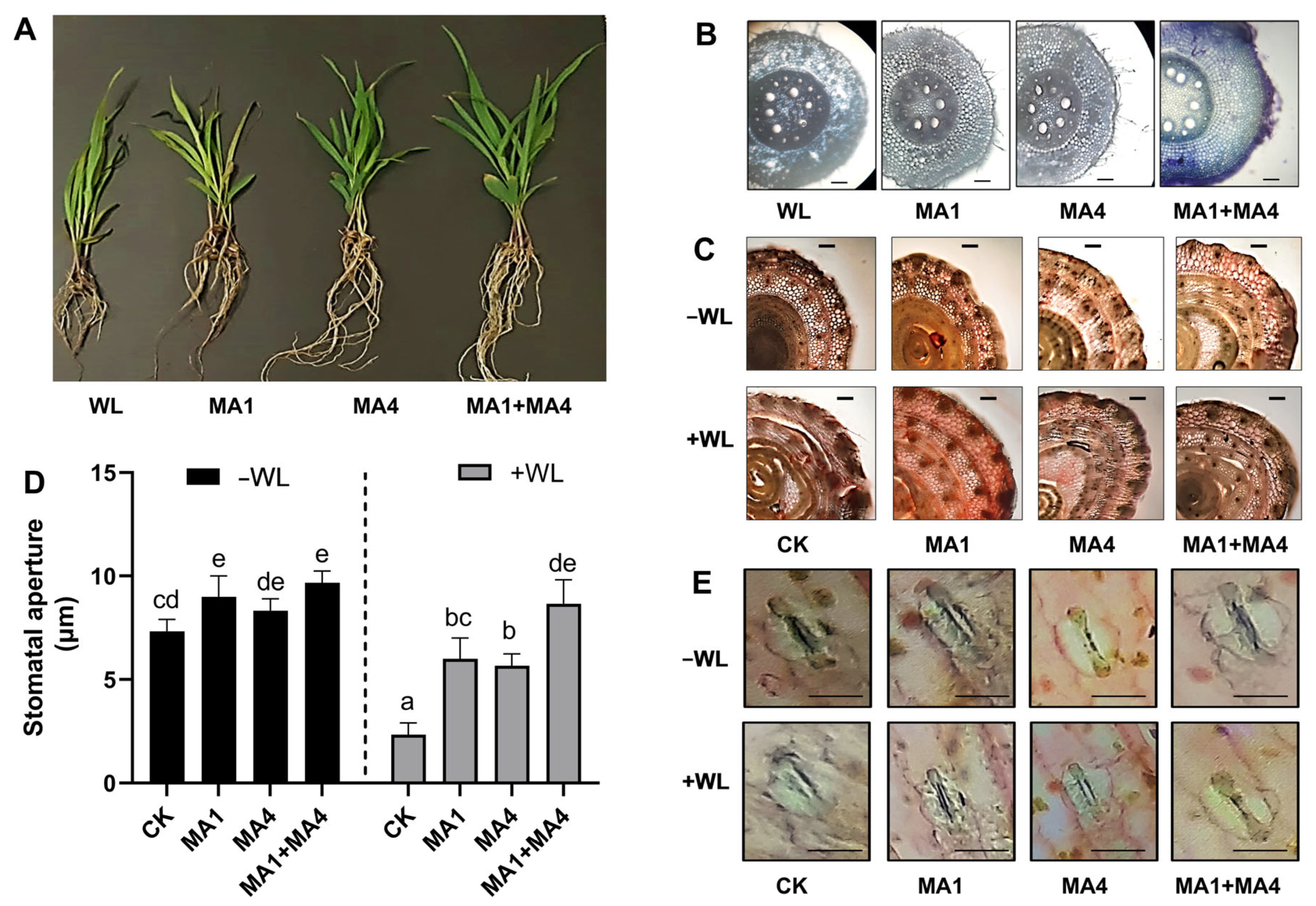
2.9.6. Stem and Stomatal Anatomical Evaluation
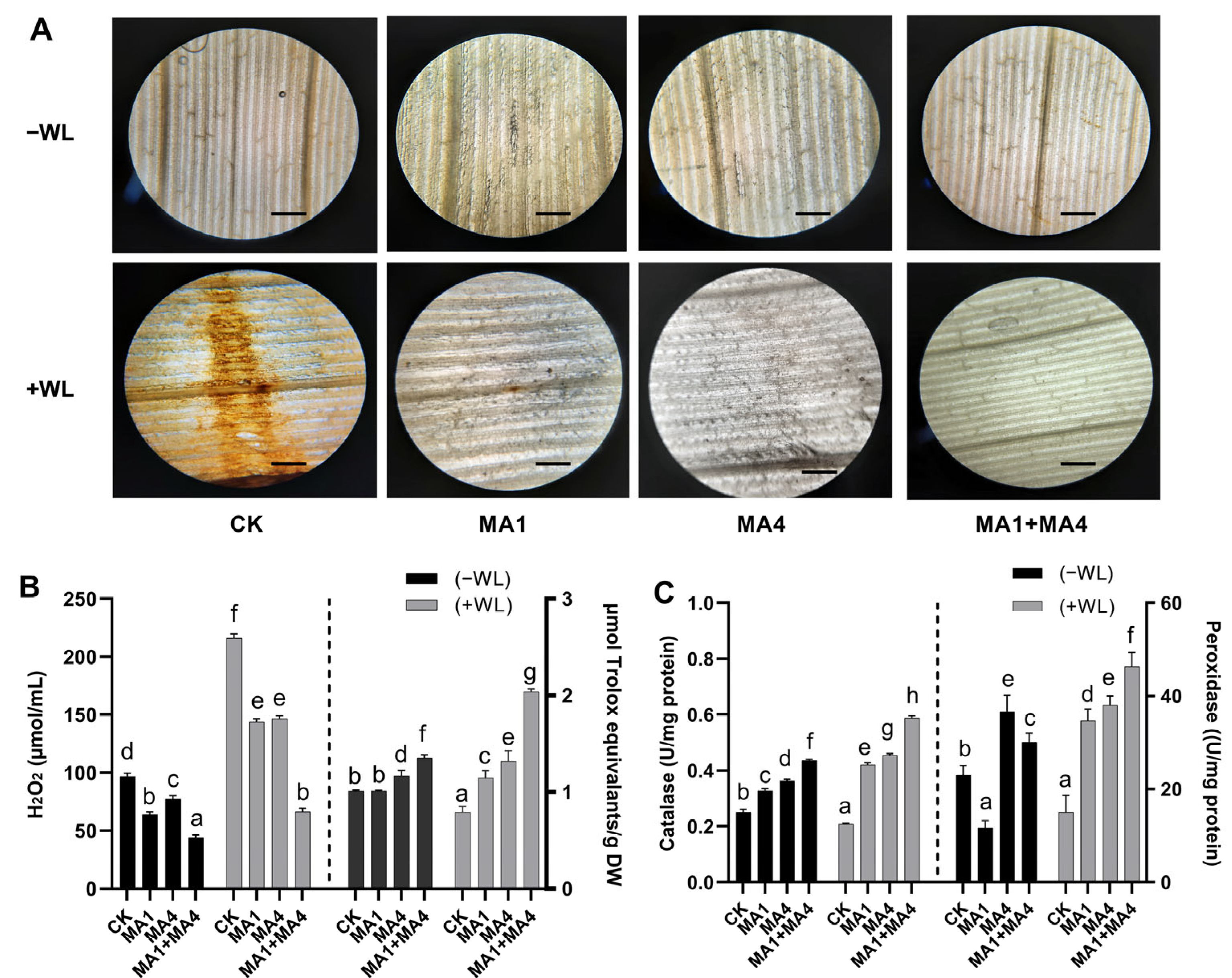
2.9.7. Visualization and Quantification of H2O2
2.9.8. Extraction and Quantification of Antioxidant Enzymes
2.9.9. Metabolic Analysis
2.9.10. Determination of Phytohormones
2.9.11. Waterlogging-Induced Marker Gene Expression Analysis in Maize
2.10. Statistical Analysis
3. Results and Discussion
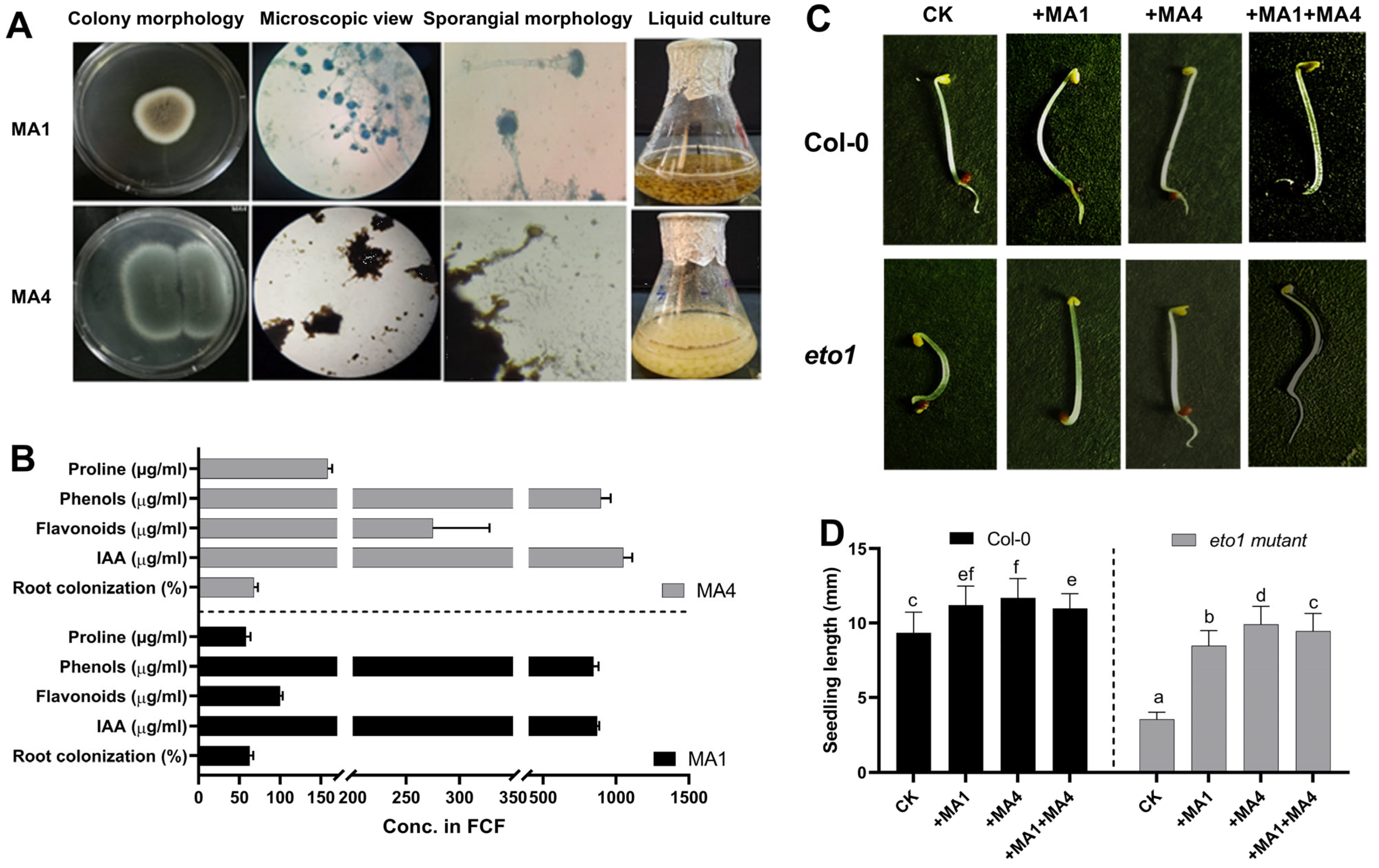
3.1. Endophytic Fungal Isolation, Identification, and Characterization
3.2. Assessment of ACC Deaminase-Producing Fungal Endophytes Using the Ethylene Response 1 Mutant Bioassay
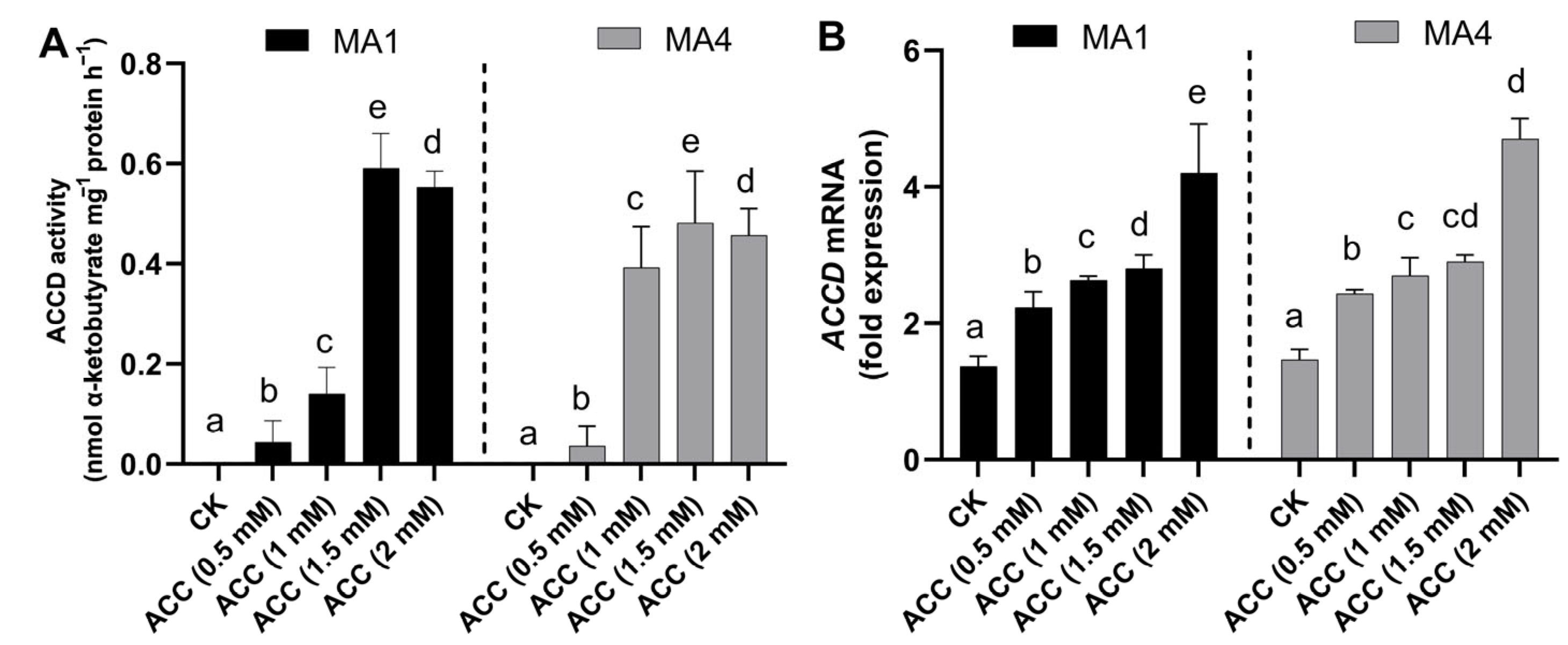
3.3. ACC Deaminase Enzyme Activity and Transcript Abundance in MA1 and MA4
3.4. Effect of ACC Deaminase-Producing MA1 and MA4 on Maize Plants under WS
3.4.1. Phenotypic Response of Maize under WS upon MA1 and MA4 Inoculation
3.4.2. Growth Kinetics and Photosynthetic Activity of Maize Inoculated with MA1 and MA4 under WS
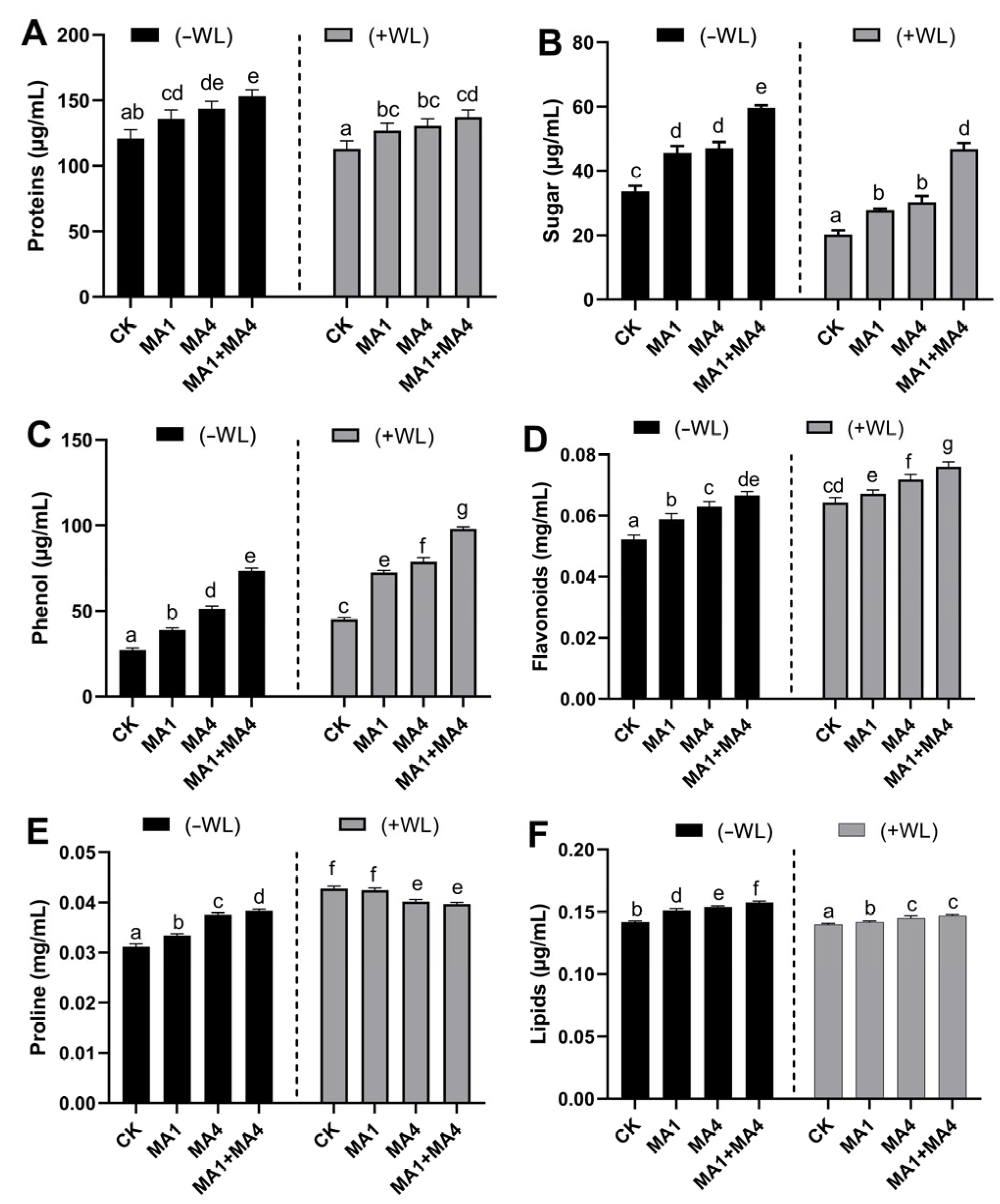
3.4.3. Growth-Related Metabolites in Maize Inoculated with MA1 and MA4 under WS
3.4.4. Endogenous ROS Production in Maize Plants under WS, Inoculated with MA1 and MA4
3.4.5. Antioxidant Potential in Maize Inoculated with MA1 and MA4 under WS
3.4.6. Hormonal Contents in Maize Inoculated with MA1 and MA4 under WS
3.4.7. Ethylene-Responsive gene Expression in Maize Plants under WS, Inoculated with MA1 and MA4
3.4.8. Root and Stem Anatomical Features in Maize Plants under WS, Inoculated with MA1 and MA4
3.4.9. Stomatal Regulation in Maize Plants under WS, Inoculated with MA1 and MA4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bandh, S.A.; Shafi, S.; Peerzada, M.; Rehman, T.; Bashir, S.; Wani, S.A.; Dar, R. Multidimensional analysis of global climate change: A review. Environ. Sci. Pollut. Res. 2021, 28, 24872–24888. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) Under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Christie, K.; Smith, A.; Rawnsley, R.; Harrison, M.; Eckard, R. Simulated seasonal responses of grazed dairy pastures to nitrogen fertilizer in SE Australia: N loss and recovery. Agric. Syst. 2020, 182, 102847. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- De Castro, J.; Hill, R.D.; Stasolla, C.; Badea, A. Waterlogging Stress Physiology in Barley. Agronomy 2022, 12, 780. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-Aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar]
- Ouyang, W.; Yin, X.; Yang, J.; Struik, P.C. Comparisons with wheat reveal root anatomical and histochemical constraints of rice under water-deficit stress. Plant Soil 2020, 452, 547–568. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Liu, Z.; Zou, X.; Jiang, Y.; Qiu, F.; Zheng, Y.; Zhang, Z. Genome-wide identification and analysis of microRNA responding to long-term waterlogging in crown roots of maize seedlings. Physiol. Plant. 2013, 147, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Tuan, P.A.; Mukherjee, S.; Son, S.; Ayele, B.T. Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. J. Exp. Bot. 2018, 69, 4065–4082. [Google Scholar] [CrossRef]
- Hamonts, K.; Clough, T.J.; Stewart, A.; Clinton, P.W.; Richardson, A.E.; Wakelin, S.A.; O’Callaghan, M.; Condron, L.M. Effect of nitrogen and waterlogging on denitrifier gene abundance, community structure and activity in the rhizosphere of wheat. FEMS Microbiol. Ecol. 2013, 83, 568–584. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Xu, Q.; Qi, X.; Weng, Y.; Chen, X. The major-effect quantitative trait locus Cs ARN 6.1 encodes an AAA ATP ase domain-containing protein that is associated with waterlogging stress tolerance by promoting adventitious root formation. Plant J. 2018, 93, 917–930. [Google Scholar] [CrossRef] [Green Version]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Javed, J.; Rauf, M.; Arif, M.; Hamayun, M.; Gul, H.; Ud-Din, A.; Ud-Din, J.; Sohail, M.; Rahman, M.M.; Lee, I.-J. Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors. Antioxidants 2022, 11, 1669. [Google Scholar] [CrossRef]
- Rehman, B.; Javed, J.; Rauf, M.; Khan, S.A.; Arif, M.; Hamayun, M.; Gul, H.; Khilji, S.A.; Sajid, Z.A.; Kim, W.-C.; et al. ACC deaminase-producing endophytic fungal consortia promotes drought stress tolerance in M. oleifera by mitigating ethylene and H2O2. Front. Plant Sci. 2022, 13, 967672. [Google Scholar] [CrossRef]
- Rauf, M.; Ur-Rahman, A.; Arif, M.; Gul, H.; Ud-Din, A.; Hamayun, M.; Lee, I.-J. Immunomodulatory Molecular Mechanisms of Luffa cylindrica for Downy Mildews Resistance Induced by Growth-Promoting Endophytic Fungi. J. Fungi 2022, 8, 689. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Arif, M.; Husssin, A.; Khan, S.A. Endophytic Aspergillus niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ. Exp. Bot. 2021, 186, 104456. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Husssin, A.; Khan, S.A.; Irshad, M.; Lee, I.-J. Aspergillus Flavus reprogrammed morphological and chemical attributes of Solanum lycopersicum through SlGSH1 and SlPCS1 genes modulation under heavy metal stress. J. Plant Interact. 2021, 16, 104–115. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Gul, H.; Arif, M.; Iqbal, A.; Gul, S.; Bibi, H.; Rehman, K.U.; Lee, I.J. Characterization and in vitro screening of different endophytic fungi on rice for growth promotion under heavy metal stress. Fresenius Environ. Bull. 2022, 31, 102–117. [Google Scholar]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Husssin, A.; Khan, S.A.; Shafique, M.; Arif, M.; Ahmad, A.; Rehman, G.; et al. Aspergillus violaceofuscus alleviates cadmium and chromium stress in Okra through biochemical modulation. PLoS ONE 2022, 17, e0273908. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef] [Green Version]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Yim, W.; Seshadri, S.; Kim, K.; Lee, G.; Sa, T. Ethylene emission and PR protein synthesis in ACC deaminase producing Methylobacterium spp. inoculated tomato plants (Lycopersicon esculentum Mill.) challenged with Ralstonia solanacearum under greenhouse conditions. Plant Physiol. Biochem. 2013, 67, 95–104. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Claeys, H.; Broeck, L.V.D.; Inzé, D. Time of day determines Arabidopsis transcriptome and growth dynamics under mild drought. Plant Cell Environ. 2017, 40, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, Y.; Liu, C.; Liu, S. A styrene antioxidant NFA from riparian endophytic fungi enhances flooding tolerance in Arabidopsis. J. Plant Interact. 2020, 15, 111–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Liu, X.; Wen, H.; Wang, M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett. Appl. Microbiol. 2010, 51, 114–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Elsevier Science: Philadelphia, PA, USA, 1990; pp. 315–322. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Gul, H.; Ali, R.; Rauf, M.; Hamayun, M.; Arif, M.; Khan, S.A.; Parveen, Z.; Alrefaei, A.F.; Lee, I.J. Aspergillus welwitschiae BK Isolate Ameliorates the Physicochemical Characteristics and Mineral Profile of Maize under Salt Stress. Plants 2023, 12, 1703. [Google Scholar] [CrossRef]
- Benizri, E.; Courtade, A.; Picard, C.; Guckert, A. Role of maize root exudates in the production of auxins by Pseudomonas fluorescens M.3.1. Soil Biol. Biochem. 1998, 30, 1481–1484. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J. Pharm. Res. 2012, 5, 150–152. [Google Scholar]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Klutts, J.S.; Moye-Rowley, W.S. Analysis of Promoter Function in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1167–1177. [Google Scholar] [CrossRef] [Green Version]
- Yunes, N.B.S.; Oliveira, R.C.; Reis, T.A.; Baquião, A.C.; Rocha, L.O.; Correa, B. Effect of temperature on growth, gene expression, and aflatoxin production by Aspergillus nomius isolated from Brazil nuts. Mycotoxin Res. 2020, 36, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Rothenberg, M.; Roman, G.; Feldmann, K.A.; Ecker, J.R. CTR1, a negative regulator of the ethylene response pathway in arabidopsis, encodes a member of the Raf family of protein kinases. Cell 1993, 72, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hata, K.; Futai, K. Endophytic fungi associated with healthy pine needles and needles infested by the pine needle gall midge, Thecodiplosis japonensis. Can. J. Bot. 1995, 73, 384–390. [Google Scholar] [CrossRef]
- Huang, P.; De-Bashan, L.; Crocker, T.; Kloepper, J.W.; Bashan, Y. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo)bacteria on growth promotion of crop plants. Biol. Fertil. Soils 2017, 53, 199–208. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Thylakoid-Bound Ascorbate Peroxidase in Spinach Chloroplasts and Photoreduction of Its Primary Oxidation Product Monodehydroascorbate Radicals in Thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Bae, H.-H.; Yi, G.; Go, Y.S.; Ha, J.Y.; Choi, Y.; Son, J.-H.; Shin, S.; Jung, T.-W.; Lee, S. Measuring antioxidant activity in yellow corn (Zea mays L.) inbreds from three different geographic regions. Appl. Biol. Chem. 2021, 64, 56. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid Determination of Glycogen and Sugars in Mosquitoes. J. Am. Mosq. Control. Asso-Ciation 1985, 1, 299–301. [Google Scholar]
- Ergün, N.; Topcuoğlu, Ş.F. Auxin (Indole-3-acetic acid), gibberellic acid (GA3), abscisic acid (ABA) and cytokinin (zeatin) production by some species of mosses and lichens. J. Bot. 2002, 26, 13–18. [Google Scholar]
- Wang, T.; Li, Y.; Huang, Y.; Zhao, X.; Dong, Z.; Jin, W.; Huang, W. Amino acid permease 6 regulates grain protein content in maize. Crop. J. 2022, 10, 1536–1544. [Google Scholar] [CrossRef]
- Chae, H.S.; Faure, F.; Kieber, J.J. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003, 15, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Woeste, K.E.; Ye, C.; Kieber, J.J. Two Arabidopsis Mutants That Overproduce Ethylene are Affected in the Posttranscriptional Regulation of 1-Aminocyclopropane-1-Carboxylic Acid Synthase. Plant Physiol. 1999, 119, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Genet. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Minami, R.; Uchiyama, K.; Murakami, T.; Kawai, J.; Mikami, K.; Yamada, T.; Yokoi, D.; Ito, H.; Matsui, H.; Honma, M. Properties, sequence, and synthesis in Escherichia coli of 1-aminocyclopropane-1-carboxylate deaminase from Hansenula saturnus. J. Biochem. 1998, 123, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-J.; Kakuta, Y.; Sugawara, M.; Igarashi, T.; Oki, N.; Kisaki, M.; Shoji, T.; Kanetuna, Y.; Horita, T.; Matsui, H.; et al. Synthesis and Degradation of 1-Aminocyclopropane-1-carboxylic Acid by Penicillium citrinum. Biosci. Biotechnol. Biochem. 1999, 63, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kashyap, S. In silico identification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from Phytophthora sojae. J. Mol. Model. 2012, 18, 4101–4111. [Google Scholar] [CrossRef]
- Palmer, C.; Golden, K.; Danniels, L.; Ahmad, H. ACC Deaminase from Issatchenkia occidentalis. J. Biol. Sci. 2007, 7, 188–193. [Google Scholar] [CrossRef]
- Tian, L.-X.; Zhang, Y.-C.; Chen, P.-L.; Zhang, F.-F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.-L. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B.; Li, H. Nitrapyrin Improves Grain Yield and Nitrogen Use Efficiency of Summer Maize Waterlogged in the Field. Agron. J. 2017, 109, 185–192. [Google Scholar] [CrossRef]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative Physiological, Biochemical, and Genetic Responses to Prolonged Waterlogging Stress in Okra and Maize Given Exogenous Ethylene Priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef]
- Azahar, N.I.; Mokhtar, N.M.; Arifin, M.A. Piper betle: A review on its bioactive compounds, pharmacological properties, and extraction process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 991, p. 012044. [Google Scholar]
- Ren, B.-Z.; Hu, J.; Zhang, J.-W.; Dong, S.-T.; Liu, P.; Zhao, B. Effects of urea mixed with nitrapyrin on leaf photosynthetic and senescence characteristics of summer maize (Zea mays L.) waterlogged in the field. J. Integr. Agric. 2020, 19, 1586–1595. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of spraying exogenous hormone 6-benzyladenine (6-BA) after waterlogging on grain yield and growth of summer maize. Field Crop. Res. 2016a, 188, 96–104. [Google Scholar] [CrossRef]
- Huang, C.; Gao, Y.; Qin, A.; Liu, Z.; Zhao, B.; Ning, D.; Ma, S.; Duan, A.; Liu, Z. Effects of waterlogging at different stages and durations on maize growth and grain yields. Agric. Water Manag. 2022, 261, 107334. [Google Scholar] [CrossRef]
- Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Ridge tillage improves plant growth and grain yield of waterlogged summer maize. Agric. Water Manag. 2016b, 177, 392–399. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.A.; Islam, F.; Yang, C.; Nawaz, A.; Athar, H.-U.; Gill, R.A.; Ali, B.; Song, W.; Zhou, W. Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Ali, R.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Khilji, S.A.; Sajid, Z.A.; Lee, I. Growth-Promoting Endophytic Fungus (Stemphylium lycopersici) Ameliorates Salt Stress Tolerance in Maize by Balancing Ionic and Metabolic Status. Front. Plant Sci. 2022, 13, 890565. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- He, F.; Wang, H.-L.; Li, H.-G.; Su, Y.; Li, S.; Yang, Y.; Feng, C.-H.; Yin, W.; Xia, X. PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus. Plant Biotechnol. J. 2018, 16, 1514–1528. [Google Scholar] [CrossRef] [Green Version]
- Nan, R.; Carman, J.G.; Salisbury, F.B. Water stress, CO2 and photoperiod influence hormone levels in wheat. J. Plant Physiol. 2002, 159, 307–312. [Google Scholar] [CrossRef]
- Komatsu, S.; Han, C.; Nanjo, Y.; Altaf-Un-Nahar, M.; Wang, K.; He, D.; Yang, P. Label-Free Quantitative Proteomic Analysis of Abscisic Acid Effect in Early-Stage Soybean under Flooding. J. Proteome Res. 2013, 12, 4769–4784. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Hasanuzzaman, M.; Dolui, D.; Sikdar, D.; Debnath, S.C.; Adak, M.K. Silver-nanoparticle and abscisic acid modulate sub1A quantitative trait loci functioning towards submergence tolerance in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 181, 104276. [Google Scholar] [CrossRef]
- Dawood, T.; Yang, X.; Visser, E.J.; Beek, T.A.T.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C.; et al. A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Liang, K.; Fang, T.; Zhao, H.; Han, X.; Cai, M.; Qiu, F. A group VII ethylene response factor gene, ZmEREB180, coordinates waterlogging tolerance in maize seedlings. Plant Biotechnol. J. 2019, 17, 2286–2298, Epub 2019 May 14. PMCID:PMC6835127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savada, R.P.; Ozga, J.A.; Jayasinghege, C.P.A.; Waduthanthri, K.D.; Reinecke, D.M. Heat stress differentially modifies ethylene biosynthesis and signaling in pea floral and fruit tissues. Plant Mol. Biol. 2017, 95, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Arif, M.; Fisahn, J.; Xue, G.-P.; Balazadeh, S.; Mueller-Roeber, B. NAC Transcription Factor Speedy Hyponastic Growth Regulates Flooding-Induced Leaf Movement in Arabidopsis. Plant Cell 2013, 25, 4941–4955. [Google Scholar] [CrossRef] [Green Version]
- Giuntoli, B.; Perata, P. Group VII Ethylene Response Factors in Arabidopsis: Regulation and Physiological Roles. Plant Physiol. 2018, 176, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Huber, H.; Beljaars, S.J.M.; Birnbaum, D.; De Best, S.; De Kroon, H.; Visser, E.J.W. Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: Linking plant performance to root functioning. Ann. Bot. 2017, 120, 171–180. [Google Scholar] [CrossRef]
- Yamauchi, T.; Abe, F.; Tsutsumi, N.; Nakazono, M. Root Cortex Provides a Venue for Gas-Space Formation and Is Essential for Plant Adaptation to Waterlogging. Front. Plant Sci. 2019, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef]
- Andrade, C.A.; de Souza, K.R.D.; de Oliveira Santos, M.; da Silva, D.M.; Alves, J.D. Hydrogen peroxide promotes the tolerance of soybeans to waterlogging. Sci. Hortic. 2018, 232, 40–45. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Root Cortical Aerenchyma Enhances the Growth of Maize on Soils with Suboptimal Availability of Nitrogen, Phosphorus, and Potassium. Plant Physiol. 2011, 156, 1190–1201. [Google Scholar] [CrossRef] [Green Version]
- Shimamura, S.; Yoshioka, T.; Yamamoto, R.; Hiraga, S.; Nakamura, T.; Shimada, S.; Komatsu, S. Role of Abscisic Acid in Flood-Induced Secondary Aerenchyma Formation in Soybean (Glycine max) Hypocotyls. Plant Prod. Sci. 2014, 17, 131–137. [Google Scholar] [CrossRef]
- Mignolli, F.; Todaro, J.S.; Vidoz, M.L. Internal aeration and respiration of submerged tomato hypocotyls are enhanced by ethylene-mediated aerenchyma formation and hypertrophy. Physiol. Plant. 2020, 169, 49–63. [Google Scholar] [CrossRef]

| Characteristics | Values |
|---|---|
| Texture | Sandy-loam |
| Silt (%) | 12.9 |
| Sand (%) | 75.8 |
| Clay (%) | 15.2 |
| ECe (dS/m) | 0.8 |
| CEC (dS/cm) | 4.6 |
| pH | 7.8 |
| Chlorides (meq/L) | 1.17 |
| Bicarbonates (meq/L) | 2.8 |
| Carbonates (meq/L) | 1.30 |
| Organic Carbon (%) | 4.21 |
| Organic matter (%) | 1.5 |
| Gene | Forward/Reverse | Sequence of Primers |
|---|---|---|
| ZmActin1 | Forward | TACGAGATGCCTGATGGTCAGGTCA |
| Reverse | TGGAGTTGTACGTGGCCTCATGGAC | |
| ZmEREB180 (GRMZM2G018984) | Forward | AGAGGAAGGAAGGGATCGC |
| Reverse | GAGTCTTCGTCGCATCTCG |
| Parameters | Treatments/ Conditions | CK | MA1 | MA4 | MA1 + MA4 |
|---|---|---|---|---|---|
| Shoot length | −WL | 21.37 ± 0.35 b | 27.5 ± 0.4 d | 30.5 ± 0.3 g | 34.53 ± 0.4 h |
| +WL | 17.5 ± 0.29 a | 23.43 ± 0.29 c | 26.33 ± 0.29 d | 29.07 ± 0.29 f | |
| Root length | −WL | 20.83 ± 0.76 c | 23.2 ± 0.26 e | 25.2 ± 0.3 f | 27.23 ± 0.25 g |
| +WL | 12.27 ± 0.25 a | 19.2 ± 0.16 b | 20.2 ± 0.22 c | 22.03 ± 0.21 d | |
| Fresh weight | −WL | 1.15 ± 0.04 d | 1.28 ± 0.01 e | 1.37 ± 0.05 f | 1.43 ± 0.02 f |
| +WL | 0.19 ± 0.01 a | 0.93 ± 0.02 b | 0.9 ± 0.04 b | 1.05 ± 0.06 c | |
| Dry weight | −WL | 0.14 ± 0.08 d | 0.43 ± 0.06 e | 0.36 ± 0.05 f | 0.73 ± 0.09 f |
| +WL | 0.08 ± 0.01 a | 0.16 ± 0.03 b | 0.23 ± 0.02 b | 0.5 ± 0.11 c | |
| Chlorophyll a | −WL | 4.47 ± 0.04 d | 5.07 ± 0.05 e | 5.24 ± 0.09 f | 6.35 ± 0.05 g |
| +WL | 2.08 ± 0.1 a | 3.47 ± 0.09 b | 4.11 ± 0.11 c | 4.49 ± 0.08 d | |
| Chlorophyll b | −WL | 1.39 ± 0.05 c | 1.59 ± 0.04 d | 2.52 ± 0.05 e | 2.9 ± 0.06 f |
| +WL | 0.69 ± 0.03 a | 1.15 ± 0.04 b | 1.36 ± 0.04 c | 2.55 ± 0.03 e | |
| Total chlorophyll | −WL | 8.87 ± 0.15 e | 9.97 ± 0.08 f | 10.54 ± 0.04 g | 12.23 ± 0.11 h |
| +WL | 4.88 ± 0.03 a | 6.34 ± 0.15 b | 7.66 ± 0.11 c | 8.28 ± 0.24 d | |
| Carotenoids | −WL | 1.39 ± 0.05 c | 1.59 ± 0.04 d | 2.52 ± 0.05 e | 2.9 ± 0.06 f |
| +WL | 0.7 ± 0.03 a | 1.15 ± 0.04 b | 1.36 ± 0.04 c | 2.55 ± 0.03 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, K.U.; Ali, K.; Rauf, M.; Arif, M. Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L. Microorganisms 2023, 11, 2025. https://doi.org/10.3390/microorganisms11082025
Rahman KU, Ali K, Rauf M, Arif M. Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L. Microorganisms. 2023; 11(8):2025. https://doi.org/10.3390/microorganisms11082025
Chicago/Turabian StyleRahman, Khalil Ur, Kashmala Ali, Mamoona Rauf, and Muhammad Arif. 2023. "Aspergillus nomiae and fumigatus Ameliorating the Hypoxic Stress Induced by Waterlogging through Ethylene Metabolism in Zea mays L." Microorganisms 11, no. 8: 2025. https://doi.org/10.3390/microorganisms11082025









