Drinking Warm Water Promotes Performance by Regulating Ruminal Microbial Composition and Serum Metabolites in Yak Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Experiment Management
2.4. Sample Collection
2.5. Analysis of Ruminal Fermentation Parameters
2.6. DNA Extraction and PCR Amplification
2.7. Untargeted Metabolome Detection and Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Rumen Fermentation Parameters
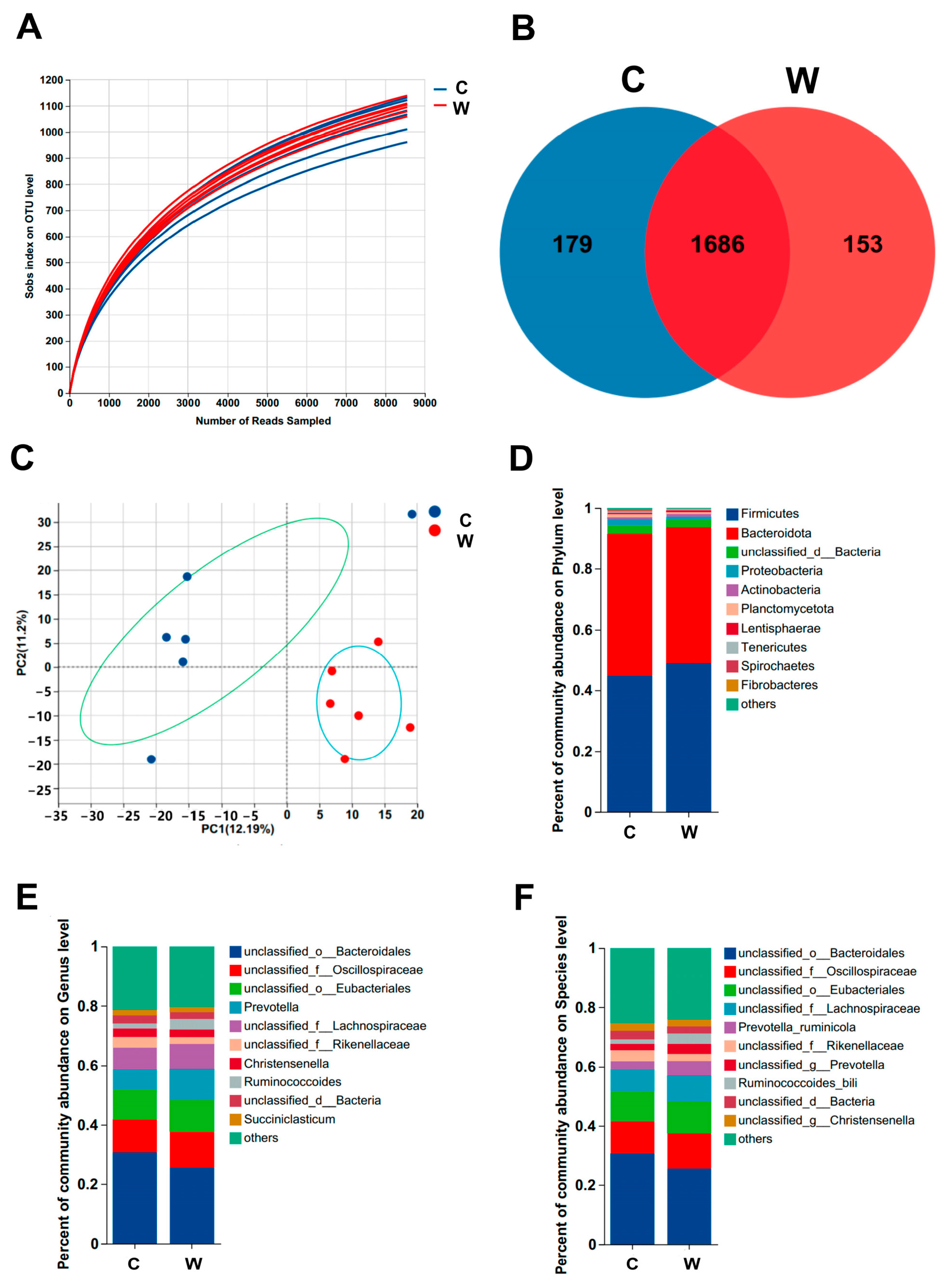
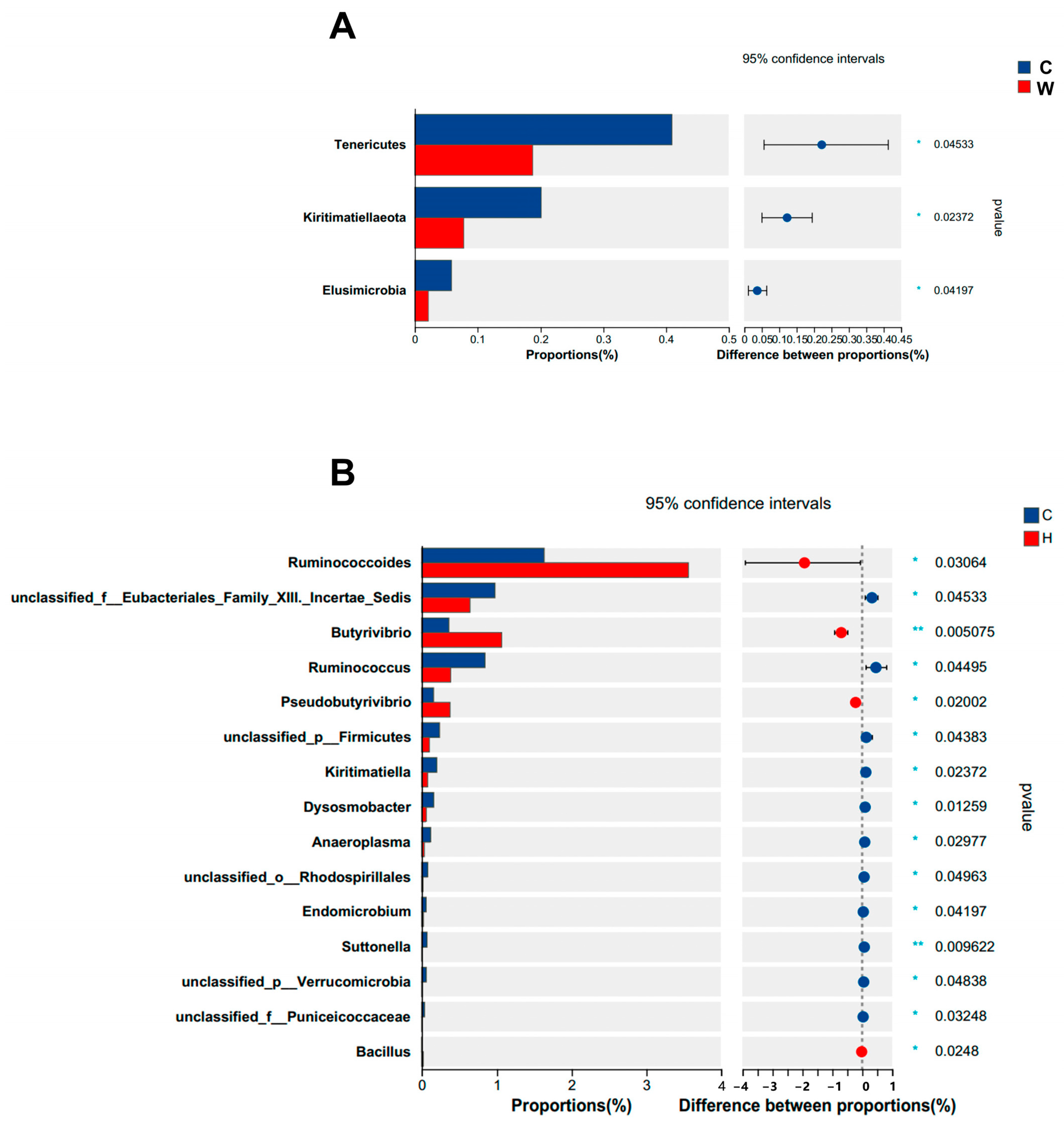
3.3. Ruminal Microbes
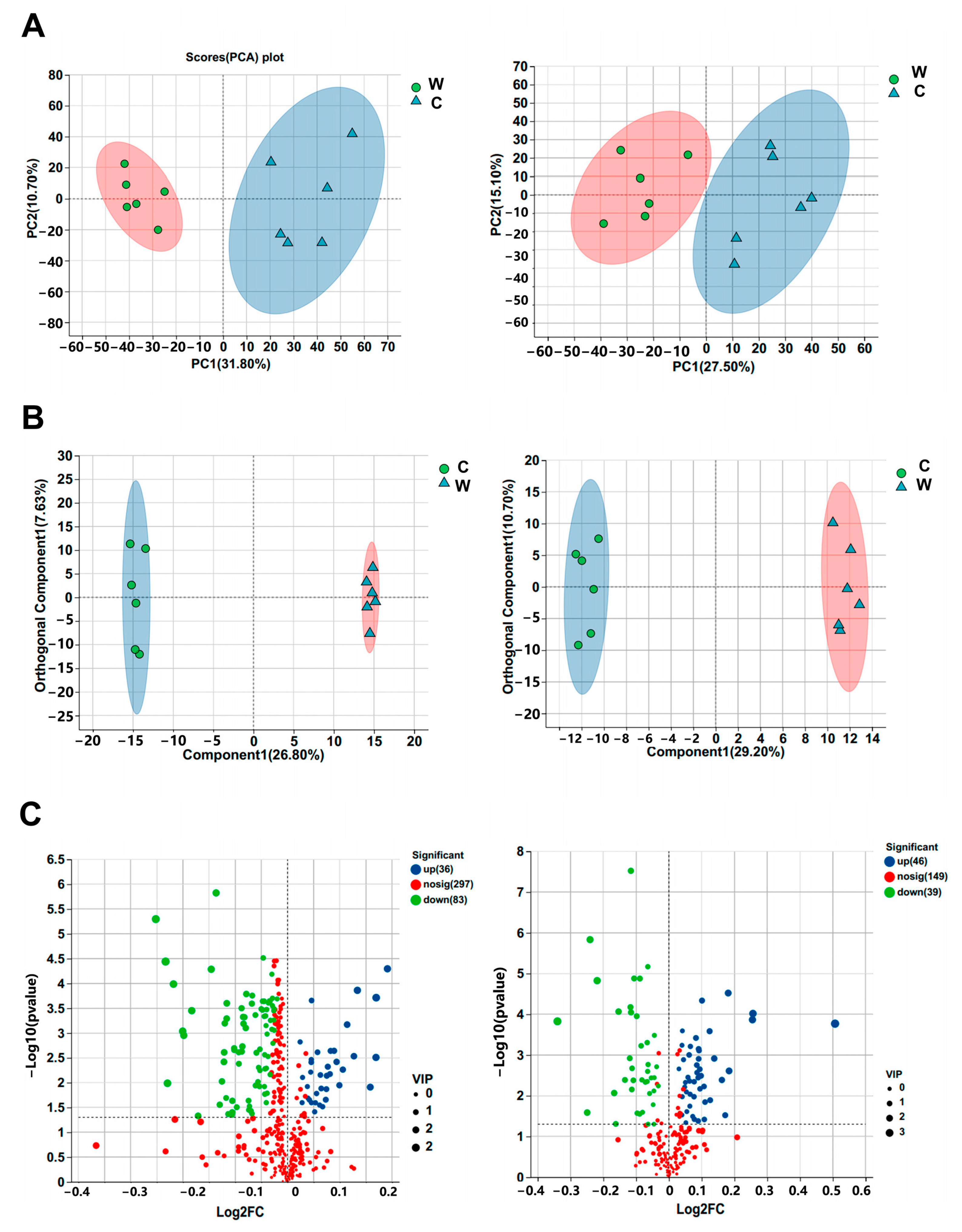
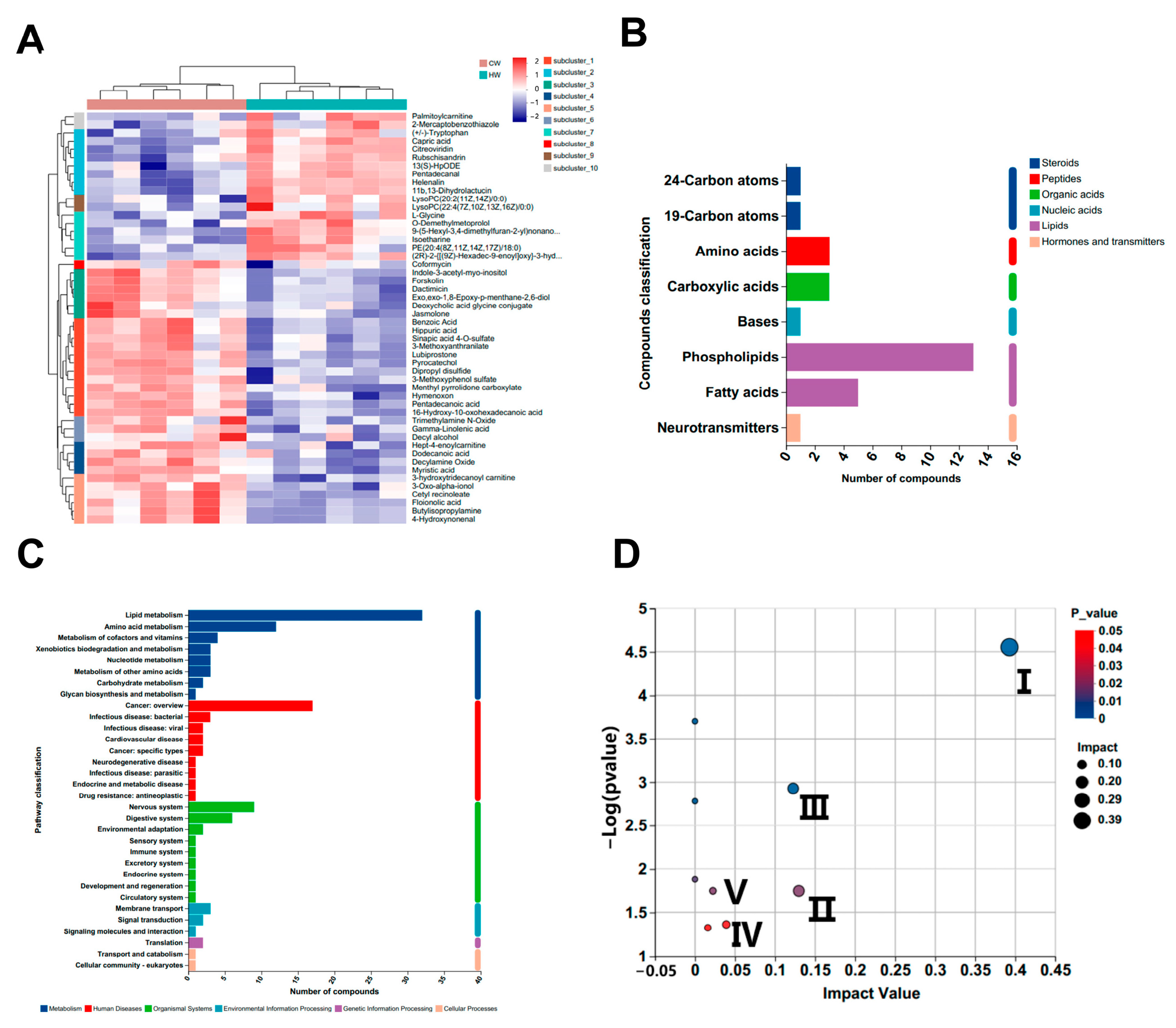
3.4. Untargeted Serum Metabolomics
3.5. Correlation of Microbes and Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, P.; Feng, L.; Zhang, L.; He, J.; An, T.; Fu, X.; Li, C.; Zhao, X.; Zhai, Y.; Li, H.; et al. Antimicrobial resistance, virulence genes, and biofilm formation capacity among enterococcus species from yaks in aba tibetan autonomous prefecture, China. Front. Microbiol. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, M. Metabolomic profiles in yak mammary gland tissue during the lactation cycle. PLoS ONE 2019, 14, e0219220. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hu, R.; Wang, Z.; Shah, A.M.; Zeng, S.; Peng, Q.; Xue, B.; Wang, L.; Zhang, X.; Wang, X.; et al. Effects of nutritional deprivation and re-alimentation on the feed efficiency, blood biochemistry, and rumen microflora in Yaks (Bos grunniens). Animals 2019, 9, 807. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, S.; Yang, X.; Guo, L.; Cai, C.; Yao, J. Effects of dietary leucine and phenylalanine on gastrointestinal development and small intestinal enzyme activities in milk-fed holstein dairy calves. Biosci. Rep. 2019, 39, BSR20181733. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Jiang, X.; Zhang, H.; Mehmood, K.; Zhang, L.; Jiang, J.; Waqas, M.; Iqbal, M.; Li, J. Probiotic potential of leuconostoc pseudomesenteroides and lactobacillus strains isolated from yaks. Front. Microbiol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- Chen, Z.; Pang, C.; Jin, W.; Liu, J.; Zhu, Y. Optimization of water supply system with constant temperature in winter based on effect of water temperature on growth performance of beef cattle. Trans. CSAE 2015, 31, 212–218. [Google Scholar] [CrossRef]
- Golher, D.M.; Thirumurugan, P.; Patel, B.H.; Upadhyay, V.K.; Sahu, S.; Gaur, G.K.; Bhoite, S.H. Effect of drinking water temperature on physiological variables of crossbred dairy cattle at high altitude temperate region of Himalayas. Vet. World 2015, 8, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sha, Y.Z.; Dingkao, R.; Zhang, W.; Lv, W.; Wei, H.; Shi, H.; Hu, J.; Wang, J.; Li, S.; et al. Interactions between rumen microbes, vfas, and host genes regulate nutrient absorption and epithelial barrier function during cold season nutritional stress in tibetan sheep. Front. Microbiol. 2020, 11, 593062. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Khonkhaeng, B.; Cherdthong, A. Pleurotus ostreatus and volvariella volvacea can enhance the quality of purple field corn stover and modulate ruminal fermentation and feed utilization in tropical beef cattle. Animals 2019, 9, 1084. [Google Scholar] [CrossRef]
- Grossi, S.; Rossi, L.; Dell’Anno, M.; Biffani, S.; Sgoifo, R.C. Effects of heated drinking water on the growth performance and rumen functionality of fattening charolaise beef cattle in winter. Animals 2021, 11, 2218. [Google Scholar] [CrossRef]
- Nakamura, S.; Haga, S.; Kimura, K.; Matsuyama, S. Propionate and butyrate induce gene expression of monocarboxylate transporter 4 and cluster of differentiation 147 in cultured rumen epithelial cells derived from preweaning dairy calves1. J. Anim. Sci. 2018, 96, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Tsukamoto, Y.; Nagasawa, H.; Nagata, S. Imbalanced hemolymph lipid levels affect feeding motivation in the two-spotted cricket, gryllus bimaculatus. PLoS ONE 2016, 11, e0154841. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Xu, X.; Wang, J.; Li, X.; Meng, K.; Yang, P. Serum metabolomics analysis for biomarkers of Lactobacillus plantarum frt4 in high-fat diet-induced obese mice. Foods 2022, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Kultschar, B.; Dudley, E.; Wilson, S.; Llewellyn, C.A. Intracellular and extracellular metabolites from the cyanobacterium Chlorogloeopsis fritschii, PCC 6912, during 48 hours of uv-b exposure. Metabolites 2019, 9, 74. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, W.; Guan, X.; Liu, Y.; Bian, G.; He, D.; Fan, Q.; Cai, X.; Zhang, Y.; Wang, G.; et al. A diet-microbial metabolism feedforward loop modulates intestinal stem cell renewal in the stressed gut. Nat. Commun. 2021, 12, 271. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver nanoparticles alter soil microbial community compositions and metabolite profiles in unplanted and cucumber-planted soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, C.; Long, S.; Wang, Q.; He, T.; Wu, Z.; Chen, Z. Drinking heated water improves performance via increasing nutrient digestibility and ruminal fermentation function in yak calves. Animals 2023, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liu, T.; Long, S.; Zhang, X.; Liu, R.; Ling, X.; Liu, J.; Chen, Z. Feasibility analysis of the drinking heated water under fencing fattening mode of beef cattle in winter. Trans. CSAE 2022, 38, 182–188. [Google Scholar] [CrossRef]
- Mao, K.; Lu, G.; Li, Y.; Zang, Y.; Zhao, X.; Qiu, Q.; Qu, M.; Ouyang, K. Effects of rumen-protected creatine pyruvate on blood biochemical parameters and rumen fluid characteristics in transported beef cattle. BMC Vet. Res. 2022, 18, 35. [Google Scholar] [CrossRef]
- McGovern, E.; McGee, M.; Byrne, C.J.; Kenny, D.A.; Kelly, A.K.; Waters, S.M. Investigation into the effect of divergent feed efficiency phenotype on the bovine rumen microbiota across diet and breed. Sci. Rep. 2020, 10, 15317. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chai, J.; Diao, Q.; Huang, W.; Zhuang, Y.; Zhang, N. The signature microbiota drive rumen function shifts in goat kids introduced to solid diet regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef]
- Brod, D.L.; Bolsen, K.K.; Brent, B.E. Effect of water temperature on rumen temperature, digestion and rumen fermentation in sheep. J. Anim. Sci. 1982, 54, 179–182. [Google Scholar] [CrossRef]
- Gengler, W.R.; Martz, F.A.; Johnson, H.D.; Krause, G.F.; Hahn, L. Effect of Temperature on Food and Water Intake and Rumen Fermentation. J. Dairy Sci. 1970, 53, 434–437. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.; Tong, J.; Zhao, G.; Jiang, L.; Xiong, B. Steam explosion pretreatment changes ruminal fermentation in vitro of corn stover by shifting archaeal and bacterial community structure. Front. Microbiol. 2020, 11, 2027. [Google Scholar] [CrossRef]
- Patel, V.; Patel, A.K.; Parmar, N.R.; Patel, A.B.; Reddy, B.; Joshi, C.G. Characterization of the rumen microbiome of Indian Kankrej cattle (Bos indicus) adapted to different forage diet. Appl. Microbiol. Biotechnol. 2014, 98, 9749–9761. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Cao, Z.; Yang, H.; Wang, Y.; Li, S. Effects of altitude on the gut microbiome and metabolomics of Sanhe heifers. Front. Microbiol. 2023, 14, 1076011. [Google Scholar] [CrossRef] [PubMed]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A Structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef]
- Terry, S.A.; Ribeiro, G.O.; Gruninger, R.J.; Chaves, A.V.; Beauchemin, K.A.; Okine, E.; McAllister, T.A. A pine enhanced biochar does not decrease enteric ch(4) emissions, but alters the rumen microbiota. Front. Vet. Sci. 2019, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.; Dong, C.; Li, F.; Wang, W.; Yuan, Z.; Mo, F.; Weng, X. Rumen bacteria communities and performances of fattening lambs with a lower or greater subacute ruminal acidosis risk. Front. Microbiol. 2017, 8, 2506. [Google Scholar] [CrossRef]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus subtilis delivery route: Effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021, 100, 100809. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Jiao, J.; Padmanabha, J.; Denman, S.E.; McSweeney, C.S. Seasonal and nutrient supplement responses in rumen microbiota structure and metabolites of tropical rangeland cattle. Microorganisms 2020, 8, 1550. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.; Li, X.; Zhao, Y.; Wang, W. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Molinero, N.; Conti, E.; Walker, A.W.; Margolles, A.; Duncan, S.H.; Delgado, S. Survival strategies and metabolic interactions between Ruminococcus gauvreauii and Ruminococcoides bili, isolated from human bile. Microbiol. Spectr. 2022, 10, e0277621. [Google Scholar] [CrossRef]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- La Reau, A.J.; Suen, G. The ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Paßlack, N.; van Bömmel-Wegmann, S.; Vahjen, W.; Zentek, J. Impact of dietary zinc chloride hydroxide and zinc methionine on the faecal microbiota of healthy adult horses and ponies. J. Equine Vet. Sci. 2021, 110, 103804. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Mizrahi, I. Islands in the stream: From individual to communal fiber degradation in the rumen ecosystem. FEMS Microbiol. Rev. 2019, 43, 362–379. [Google Scholar] [CrossRef]
- Valentini, J.; Da Silva, A.; Fortuoso, B.F.; Reis, J.H.; Gebert, R.R.; Griss, L.G.; Boiago, M.; Lopes, L.; Christ-Santos, R.; Wagner, R.; et al. Chemical composition, lipid peroxidation, and fatty acid profile in meat of broilers fed with glycerol monolaurate additive. Food Chem. 2020, 330, 127187. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, F.; Zhang, H.; Xu, C.; Wu, L.; Xia, C. Follicular fluid metabolite changes in dairy cows with inactive ovary identified using untargeted metabolomics. BioMed Res. Int. 2020, 2020, 9837543. [Google Scholar] [CrossRef]
- Gaebler, A.; Milan, R.; Straub, L.; Hoelper, D.; Kuerschner, L.; Thiele, C. Alkyne lipids as substrates for click chemistry-based in vitro enzymatic assays. J. Lipid Res. 2013, 54, 2282–2290. [Google Scholar] [CrossRef]
- Gibb, A.J. Choline and acetylcholine: What a difference an acetate makes! J. Physiol. 2017, 595, 1021–1022. [Google Scholar] [CrossRef]
- Jain, A.; Li, X.H.; Chen, W.N. An untargeted fecal and urine metabolomics analysis of the interplay between the gut microbiome, diet and human metabolism in Indian and Chinese adults. Sci. Rep. 2019, 9, 9191. [Google Scholar] [CrossRef]
- Mariz, L.D.S.; Amaral, P.M.; Filho, S.C.V.; Santos, S.A.; Detmann, E.; Marcondes, M.I.; Pereira, J.M.V.; Júnior, J.M.S.; Prados, L.F.; Faciola, A.P. Dietary protein reduction on microbial protein, amino acid digestibility, and body retention in beef cattle: 2. Amino acid intestinal absorption and their efficiency for whole-body deposition. J. Anim. Sci. 2018, 96, 670–683. [Google Scholar] [CrossRef]
- Hartwich, K.; Poehlein, A.; Daniel, R. The purine-utilizing bacterium clostridium acidurici 9a: A genome-guided metabolic reconsideration. PLoS ONE 2012, 7, e51662. [Google Scholar] [CrossRef]
- Drake, K.J.; Sidorov, V.Y.; Mcguinness, O.P.; Wasserman, D.H.; Wikswo, J.P. Amino acids as metabolic substrates during cardiac ischemia. Exp. Biol. Med. 2012, 237, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Prahl, M.C.; Müller, C.; Albrecht, D.; Koch, F.; Wimmers, K.; Kuhla, B. Hepatic urea, creatinine and uric acid metabolism in dairy cows with divergent milk urea concentrations. Sci. Rep. 2022, 12, 17593. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Li, J.; Yang, Y.; Wang, P.; Yan, F.; Yao, J.; Wu, S. Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis. NPJ Biofilms Microbiomes 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]


| Item | Amount |
|---|---|
| Ingredients (%) | |
| Dried wheat grass | 37.0 |
| Dried oat grass | 32.0 |
| Corn silage | 17.0 |
| Wheat | 4.0 |
| Wheat bran | 2.5 |
| Rapeseed meal | 4.0 |
| Soybean meal | 1.5 |
| NaCl | 0.5 |
| Premix 1 | 1.5 |
| Chemical composition (% of dry matter (DM)) | |
| DM | 86.32 |
| Crude protein (CP) | 6.25 |
| Ether extract (EE) | 1.28 |
| Neutral detergent fiber (NDF) | 54.26 |
| Acid detergent fiber (ADF) | 31.57 |
| Coarse ash (CA) | 9.50 |
| Items | Group C | Group W | p-Value |
|---|---|---|---|
| Body weight day 0 (kg) | 58.03 ± 3.11 | 59.62 ± 2.77 | 0.200 |
| Body weight day 60 (kg) | 61.32 ± 3.59 | 64.96 ± 3.69 | 0.023 |
| Total weight gain (kg) | 3.28 ± 1.13 | 5.34 ± 1.88 | 0.004 |
| Average daily gain (g) | 109.40 ± 37.58 | 177.90 ± 62.74 | 0.004 |
| Items | Group C | Group W | p-Value |
|---|---|---|---|
| pH | 6.97 ± 0.10 | 6.86 ± 0.28 | 0.402 |
| NH3-N (mg/kg) | 68.33 ± 71.95 | 186.70 ± 122.40 | 0.069 |
| VFA (mg/kg) | |||
| Acetic acid (A) | 2373.00 ± 189.50 | 2849.00 ± 241.90 | 0.004 |
| Propionic acid (P) | 848.20 ± 93.66 | 1141.00 ± 112.80 | 0.009 |
| Butyric acid | 681.00 ± 73.85 | 766.80 ± 96.80 | 0.115 |
| Isobutyric acid | 41.47 ± 13.19 | 59.95 ± 6.06 | 0.011 |
| Valeric acid | 64.05 ± 17.90 | 86.45 ± 13.58 | 0.035 |
| Isovaleric acid | 57.75 ± 21.53 | 79.23 ± 8.19 | 0.045 |
| Hexanoic acid | 20.32 ± 9.06 | 16.85 ± 7.99 | 0.498 |
| A/P | 2.81 ± 0.25 | 2.50 ± 0.13 | 0.020 |
| Index | Group C | Group W | p-Value |
|---|---|---|---|
| ACE | 1377 ± 84.77 | 1416 ± 29.38 | 0.318 |
| Chao | 1373 ± 82.97 | 1417 ± 33.59 | 0.256 |
| Shannon | 5.818 ± 0.1278 | 5.962 ± 0.0857 | 0.045 |
| Simpson | 0.008914 ± 0.002306 | 0.006136 ± 0.0007554 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wang, Q.; Gao, C.; Long, S.; He, T.; Wu, Z.; Chen, Z. Drinking Warm Water Promotes Performance by Regulating Ruminal Microbial Composition and Serum Metabolites in Yak Calves. Microorganisms 2023, 11, 2092. https://doi.org/10.3390/microorganisms11082092
Liu T, Wang Q, Gao C, Long S, He T, Wu Z, Chen Z. Drinking Warm Water Promotes Performance by Regulating Ruminal Microbial Composition and Serum Metabolites in Yak Calves. Microorganisms. 2023; 11(8):2092. https://doi.org/10.3390/microorganisms11082092
Chicago/Turabian StyleLiu, Tianxu, Qianqian Wang, Chenxi Gao, Shenfei Long, Tengfei He, Zhenlong Wu, and Zhaohui Chen. 2023. "Drinking Warm Water Promotes Performance by Regulating Ruminal Microbial Composition and Serum Metabolites in Yak Calves" Microorganisms 11, no. 8: 2092. https://doi.org/10.3390/microorganisms11082092
APA StyleLiu, T., Wang, Q., Gao, C., Long, S., He, T., Wu, Z., & Chen, Z. (2023). Drinking Warm Water Promotes Performance by Regulating Ruminal Microbial Composition and Serum Metabolites in Yak Calves. Microorganisms, 11(8), 2092. https://doi.org/10.3390/microorganisms11082092






