Differences in Juniperus przewalskii Rhizosphere Microbiomes across Age Classes: Community Diversity and Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Rhizosphere Soil Sampling
2.2. DNA Extraction and High-Throughput Amplicon Sequencing
2.3. Statistical Analysis
3. Results
3.1. Vegetation and Soil Chemical Characteristics
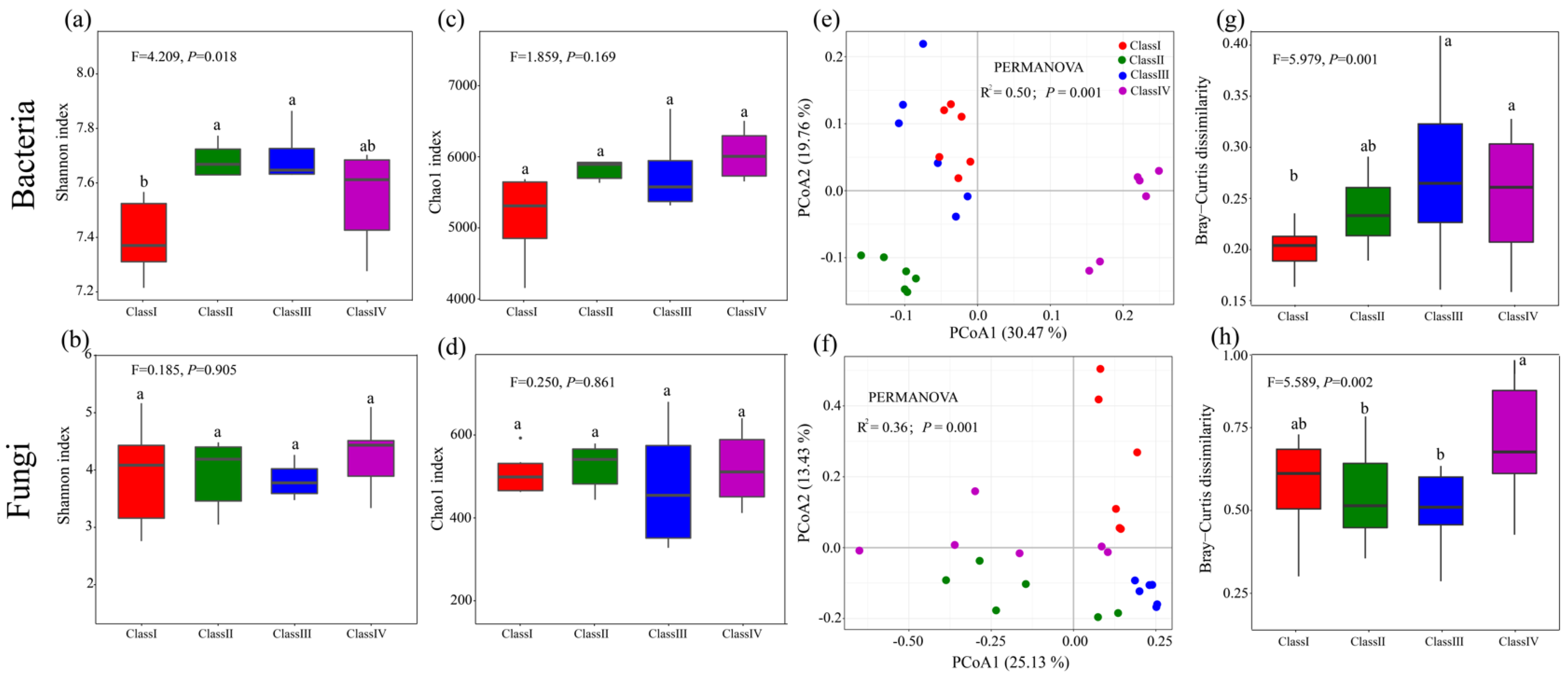
3.2. Microbial Community Diversity and Composition
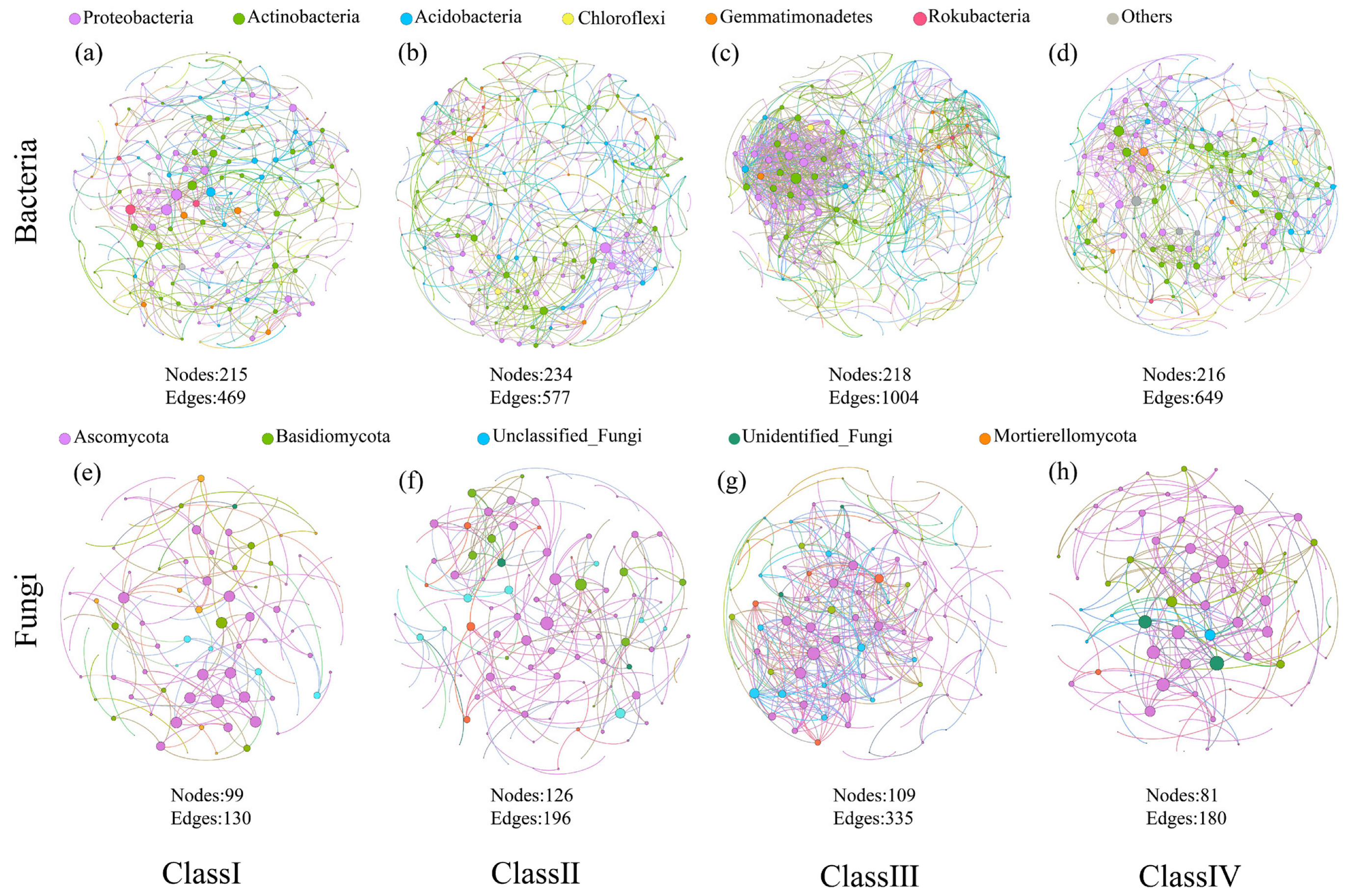
3.3. Microbial Co-Occurrence Patterns
3.4. Quantifying Community Assembly Processes
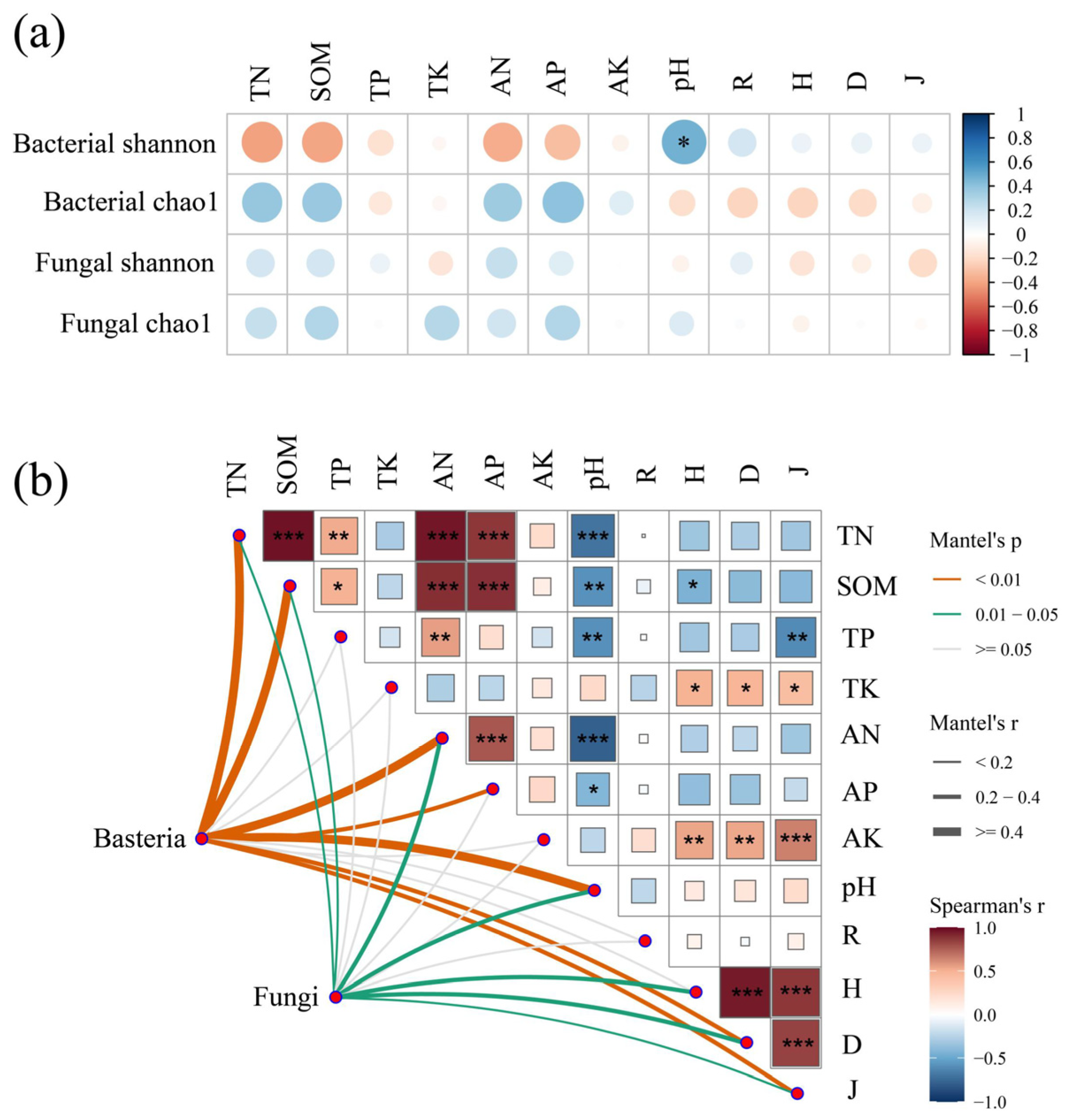
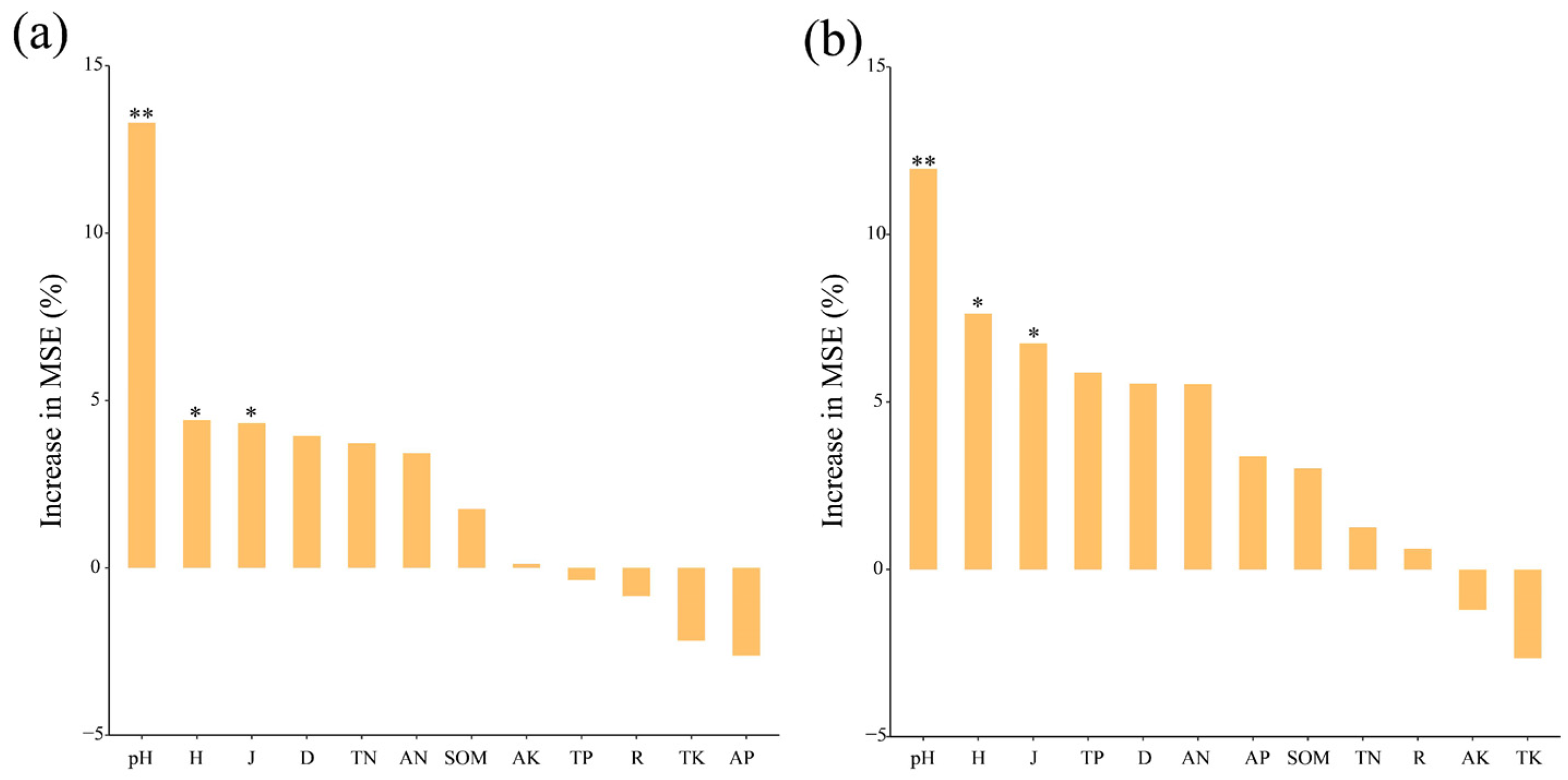
3.5. Factors Influencing the Microbial Community
4. Discussion
4.1. Microbial Community Diversity and Composition
4.2. Microbial Co-Occurrence Patterns
4.3. Microbial Assembly Processes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.P.; Guo, X.Y.; Tao, Q.; Li, J.X.; Liu, Y.K.; Du, Y.L.; Liu, Y.Y.; Liang, Y.C.; Li, T.Q. Succession of the composition and co-occurrence networks of rhizosphere microbiota is linked to Cd/Zn hyperaccumulation. Soil Biol. Biochem. 2021, 153, 108120. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.J.; Cho, H.J.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Zhang, M.J.; Chai, L.W.; Huang, M.K.; Jia, W.Q.; Guo, J.B.; Huang, Y. Deciphering the archaeal communities in tree rhizosphere of the Qinghai-Tibetan plateau. BMC Microbiol. 2020, 20, 235. [Google Scholar] [CrossRef]
- Fan, K.K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H.Y. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Atauri, J.A.; de Pablo, C.L.; de Agar, P.M.; Schmitz, M.F.; Pineda, F.D. Effects of management on understory diversity in the forest ecosystems of Northern Spain. Environ. Manag. 2004, 34, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Bessler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S.; et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Su, B.Q.; Shangguan, Z.P. Response of water use efficiency and plant-soil C:N:P stoichiometry to stand quality in Robinia pseudoacacia on the Loess Plateau of China. Catena 2021, 206, 105571. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Al-Soud, W.A.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef]
- Min, S.; Chen, X.; Zhang, X.; Hamel, C.; Cui, X.; Jie, C.; Hui, C.; Ming, T. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2018, 599–600, 273–283. [Google Scholar]
- Zheng, C.Y.; Kong, K.P.; Zhang, Y.; Yang, W.H.; Wu, L.Q.; Munir, M.Z.; Ji, B.M.; Muneer, M.A. Differential response of bacterial diversity and community composition to different tree ages of pomelo under red and paddy soils. Front. Microbiol. 2022, 13, 958788. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, F.D.; Manzano, M.; Marquet, P.A.; Gaxiola, A. Microbial communities in soil chronosequences with distinct parent material: The effect of soil pH and litter quality. J. Ecol. 2017, 105, 1709–1722. [Google Scholar] [CrossRef]
- Ma, Y.N.; McCormick, M.K.; Szlavecz, K.; Filley, T.R. Controls on soil organic carbon stability and temperature sensitivity with increased aboveground litter input in deciduous forests of different forest ages. Soil Biol. Biochem. 2019, 134, 90–99. [Google Scholar] [CrossRef]
- Cai, X.W.; Zhang, D.; Wang, Y.Q.; Diao, L.F.; Cheng, X.L.; Luo, Y.Q.; An, S.Q.; Yang, W. Shift in soil microbial communities along ~160 years of natural vegetation restoration on the Loess Plateau of China. Appl. Soil Ecol. 2022, 173, 104394. [Google Scholar] [CrossRef]
- Qu, Z.L.; Liu, B.; Ma, Y.; Sun, H. Differences in bacterial community structure and potential functions among Eucalyptus plantations with different ages and species of trees. Appl. Soil Ecol. 2020, 149, 103515. [Google Scholar] [CrossRef]
- Wan, P.; Peng, H.; Ji, X.L.; Chen, X.L.; Zhou, H.M. Effect of stand age on soil microbial communities of a plantation Ormosia hosiei forest in southern China. Ecol. Inform. 2021, 62, 101282. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.G.; Zhang, M.; Cui, Y.X.; Bai, X.X.; Song, B.; Zhang, J.W.; Yu, X. Dynamic microbial community composition, co-occurrence pattern and assembly in rhizosphere and bulk soils along a coniferous plantation chronosequence. Catena 2023, 223, 106914. [Google Scholar] [CrossRef]
- Yue, Y.H.; Tang, Y.; Cai, L.; Yang, Z.H.; Chen, X.P.; Ouyang, Y.R.; Dai, J.J.; Yang, M. Co-occurrence relationship and stochastic processes affect sedimentary archaeal and bacterial community assembly in Estuarine-coastal margins. Microorganisms 2022, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Liu, Z.S.; Lin, Y.B.; Yang, J.; Chen, W.M.; Wei, G.H. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Freilich, S.; Kreimer, A.; Meilijson, I.; Gophna, U.; Sharan, R.; Ruppin, E. The large-scale organization of the bacterial network of ecological co-occurrence interactions. Nucleic Acids Res. 2010, 38, 3857–3868. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef]
- Das, P.; Ji, B.; Kovatcheva-Datchary, P.; Bäckhed, F.; Nielsen, J. In vitro co-cultures of human gut bacterial species as predicted from co-occurrence network analysis. PLoS ONE 2021, 13, e0195161. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; Countway, P.D.; Xia, L.; Vigil, P.D.; Beman, J.M.; Kim, D.Y.; Chow, C.T.; Sachdeva, R.; Jones, A.C.; Schwalbach, M.S.; et al. Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J. 2011, 5, 1414–1425. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.X.; Fei, H.Y.; Wan, C.Y.; Han, F.P. Changes in diversity, composition and assembly processes of soil microbial communities during Robinia pseudoacacia L. restoration on the Loess Plateau, China. J. Arid Land 2022, 14, 561–575. [Google Scholar] [CrossRef]
- Ku, Y.L.; Han, X.T.; Lei, Y.T.; Zhang, M.; Zhao, Z. Different sensitivities and assembly mechanisms of the root-associated microbial communities of Robinia pseudoacacia to spatial variation at the regional scale. Plant Soil 2023, 486, 621–637. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.J.; Liu, G.C.; Hu, C.Y.; Wang, X.C.; Yan, G.Y.; Wang, Q.G. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Goberna, M.; Verdú, M. Cautionary notes on the use of co-occurrence networks in soil ecology. Soil Biol. Biochem. 2022, 166, 108534. [Google Scholar] [CrossRef]
- Ji, L.; Shen, F.Y.; Liu, Y.; Yang, Y.C.; Wang, J.; Purahong, W.; Yang, L.X. Contrasting altitudinal patterns and co-occurrence networks of soil bacterial and fungal communities along soil depths in the cold-temperate montane forests of China. Catena 2022, 209, 105844. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, Y.Q.; Zhang, J.; Hao, X.; Lu, Y.H. Core microbiota in agricultural soils and their potential associations with nutrient cycling. mSystems 2019, 4, e00313-18. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.E.; Youngblut, N.D.; Buckley, D.H. Soil characteristics and land-use drive bacterial community assembly patterns. FEMS Microbiol. Ecol. 2020, 96, fiz194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Ning, D.L. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Chen, Q.L.; Hu, H.W.; Yan, Z.Z.; Li, C.Y.; Nguyen, B.A.T.; Sun, A.Q.; Zhu, Y.G.; He, J.Z. Deterministic selection dominates microbial community assembly in termite mounds. Soil Biol. Biochem. 2021, 152, 108073. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.J.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.J.; Fredrickson, J.K.; Chen, X.Y.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Na, X.F.; Li, X.R.; Zhang, Z.Y.; Li, M.; Kardol, P.; Xu, T.T.; Wang, M.; Cao, X.N.; Ma, F. Bacterial community dynamics in the rhizosphere of a long-lived, leguminous shrub across a 40-year age sequence. J. Soils Sediments 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the loess plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhang, Z.J.; Liu, H.Y.; Liu, Y.Y.; Feng, Y.Z.; Yang, G.H.; Ren, C.J.; Han, X.H. Linking soil bacterial community assembly with the composition of organic carbon during forest succession. Soil Biol. Biochem. 2022, 173, 108790. [Google Scholar] [CrossRef]
- Ning, D.L.; Yuan, M.T.; Wu, L.W.; Zhang, Y.; Guo, X.; Zhou, X.S.; Yang, Y.F.; Arkin, A.P.; Firestone, M.K.; Zhou, J.Z. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Tian, L.X.; Feng, Y.; Gao, Z.J.; Li, H.Q.; Wang, B.S.; Huang, Y.; Gao, X.L.; Feng, B.L. Co-occurrence pattern and community assembly of broomcorn millet rhizosphere microbiomes in a typical agricultural ecosystem. Appl. Soil Ecol. 2022, 176, 104478. [Google Scholar] [CrossRef]
- Wang, F.; Gou, X.H.; Zhang, F.; Wang, Y.F.; Yu, A.L.; Zhang, J.Z.; Fonti, P.; Liu, J.G. Variations in leaf traits of Juniperus przewalskii from an extremely arid and cold environment. Sci. Total Environ. 2019, 689, 434–443. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Alexander, M.R.; Xia, J.Q.; Wang, F.; Zhang, F.; Man, Z.H.; Pederson, N. Drought limits wood production of Juniperus przewalskii even as growing seasons lengthens in a cold and arid environment. Catena 2021, 196, 104936. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Pederson, N.; Zhang, F.; Niu, H.G.; Zhao, S.D.; Wang, F. Cambial phenology in Juniperus przewalskii along different altitudinal gradients in a cold and arid region. Tree Physiol. 2018, 38, 840–852. [Google Scholar] [CrossRef]
- Qian, D.W.; Cao, G.M.; Du, Y.G.; Li, Q.; Guo, X.W. Impacts of climate change and human factors on land cover change in inland mountain protected areas: A case study of the Qilian Mountain National Nature Reserve in China. Environ. Monit. Assess. 2019, 191, 486. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, X.X.; Lo, K.; Dang, Q.W.; Wen, R.Y. Vegetation responses to climate change in the Qilian Mountain Nature Reserve, Northwest China. Glob. Ecol. Conserv. 2021, 28, e01698. [Google Scholar] [CrossRef]
- Qiao, J.J.; Sun, Y.J.; Pan, L.; Luo, M.; Ding, Z.D.; Sun, Z. Variability in the climate-radial growth correlation of Pinus massoniana of different diameter classes. J. For. Res. 2022, 33, 1781–1792. [Google Scholar] [CrossRef]
- Tian, X.P.; Ma, L.; Zhan, Y.F. Community characteristics of sabina przewalskii in northern slope of Qilian Mountains. J. Northwest For. Univ. 2015, 30, 77–83. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis Method; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the Third International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; Volume 3, pp. 361–362. [Google Scholar]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Luo, D.; Liu, S.; Shi, Z.M.; Feng, Q.H.; Liu, Q.L.; Zhang, L.; Huang, Q.; He, J.S. Soil microbial community structure in Picea asperata plantations with different ages in subal-pine of western Sichuan, Southwest China. Chin. J. Appl. Ecol. 2017, 28, 519–527. [Google Scholar]
- Zhou, Y.J.; Li, J.H.; Friedman, C.R.; Wang, H.F. Variation of soil bacterial communities in a chronosequence of rubber tree (Hevea brasiliensis) plantations. Front. Plant Sci. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.J.; Lin, J.; Guo, X.P.; Liu, X.; Wu, J.S.; Zhao, Y.P.; Zhang, J.C. Impacts of forest conversion on soil bacterial community composition and diversity in subtropical forests. Catena 2019, 175, 167–173. [Google Scholar] [CrossRef]
- Shade, A.; Read, J.S.; Welkie, D.G.; Kratz, T.K.; Wu, C.H.; McMahon, K.D. Resistance, resilience and recovery: Aquatic bacterial dynamics after water column disturbance. Environ. Microbiol. 2011, 13, 2752–2767. [Google Scholar] [CrossRef]
- Liu, J.L.; Ha, V.N.; Shen, Z.; Zhu, H.L.; Zhao, F.; Zhao, Z. Characteristics of bulk and rhizosphere soil microbial community in an ancient Platycladus orientalis forest. Appl. Soil Ecol. 2018, 132, 91–98. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Shi, C.; Zhao, M.Y.; Wang, K.F.; Zhang, M.; Wang, T.T.; Shi, F.C. Improving effects of afforestation with different forest types on soil nutrients and bacterial community in barren hills of North China. Sustainability 2022, 14, 1202. [Google Scholar] [CrossRef]
- Wei, H.; Peng, C.H.; Yang, B.; Song, H.X.; Li, Q.; Jiang, L.; Wei, G.; Wang, K.F.; Wang, H.; Liu, S.R.; et al. Contrasting soil bacterial community, diversity, and function in two forests in China. Front. Microbiol. 2018, 9, 1693. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Rojas, C.; Gutierrez, R.M.; Bruns, M.A. Bacterial and eukaryal diversity in soils forming from acid mine drainage precipitates under reclaimed vegetation and biological crusts. Appl. Soil Ecol. 2016, 105, 57–66. [Google Scholar] [CrossRef]
- Ren, C.J.; Liu, W.C.; Zhao, F.Z.; Zhong, Z.K.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil bacterial and fungal diversity and compositions respond differently to forest development. Catena 2019, 181, 104071. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.F.; Huang, X.B.; Tang, R.; Zhang, R.; Li, C.; Xu, C.H.; Su, J.R. Plant diversity and soil properties regulate the microbial community of monsoon evergreen broad-leaved forest under different intensities of woodland use. Sci. Total Environ. 2022, 821, 153565. [Google Scholar] [CrossRef]
- Santolini, M.; Barabási, A.L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, J.-M.; Liu, X.-D.; Jiang, Y.-Y.; Zheng, C.-C.; Zhao, R.-H.; Deng, Z.-S. Environmental filtering drives the establishment of the distinctive rhizosphere, bulk, and root nodule bacterial communities of Sophora davidii in hilly and gully regions of the Loess Plateau of China. Front. Microbiol. 2022, 13, 945127. [Google Scholar]
- Hu, A.Y.; Wang, H.J.; Cao, M.X.; Rashid, A.; Li, M.F.; Yu, C.P. Environmental filtering drives the assembly of habitat generalists and specialists in the coastal sand microbial communities of southern China. Microorganisms 2019, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Farjalla, V.F.; Srivastava, D.S.; Marino, N.A.C.; Azevedo, F.D.; Dib, V.; Lopes, P.M.; Rosado, A.S.; Bozelli, R.L.; Esteves, F.A. Ecological determinism increases with organism size. Ecology 2012, 93, 1752–1759. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.; Li, S.P.; Wang, X.; Zhang, Z.C.; Shen, M.W.; Yang, Q.S.; Lian, J.Y.; Wang, X.H.; et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Bao, J.B.; Wang, X.; Liu, Y.; Li, C.; Chai, B.F. Deterministic processes dominate soil microbial community assembly in subalpine coniferous forests on the Loess Plateau. Peer J. 2019, 7, e6746. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, L.; Li, W.J.; Li, E.; Lv, G.H. Structural characteristics and assembly mechanisms of soil microbial communities under water-salt gradients in arid regions. Microorganisms 2023, 11, 1060. [Google Scholar] [CrossRef] [PubMed]


| Age Class | Average Diameter (cm) | Longitude (° E) | Latitude (° N) | Altitude (m) |
|---|---|---|---|---|
| Class I | 10 | 102.4325 | 37.1336 | 2730 |
| Class II | 18.5 | 102.4344 | 37.1338 | 2770 |
| Class III | 25.2 | 102.1519 | 37.1425 | 2700 |
| Class IV | 35.4 | 102.4611 | 37.1325 | 2820 |
| Properties | Class I | Class II | Class III | Class IV |
|---|---|---|---|---|
| Species richness | 11.25 ± 1.25 a | 10.83 ± 0.85 a | 13.17 ± 2.49 a | 11.25 ± 0.75 a |
| Shannon–Weiner index | 1.36 ± 0.16 b | 1.45 ± 0.05 b | 1.95 ± 0.23 a | 1.21 ± 0.17 b |
| Simpson diversity index | 0.56 ± 0.14 b | 0.64 ± 0.03 ab | 0.76 ± 0.07 a | 0.44 ± 0.07 b |
| Pielou’s evenness index | 0.53 ± 0.15 bc | 0.75 ± 0.02 ab | 0.88 ± 0.05 a | 0.45 ± 0.15 c |
| TN (g/kg) | 2.47 ± 0.50 b | 2.48 ± 0.92 b | 2.69 ± 0.68 b | 7.35 ± 1.05 a |
| SOM (g/kg) | 54.07 ± 14.01 b | 71.68 ± 23.12 b | 57.82 ± 16.54 b | 112.83 ± 21.30 a |
| TP (g/kg) | 0.73 ± 0.07 a | 0.49 ± 0.12 b | 0.63 ± 0.02 a | 0.74 ± 0.05 a |
| TK (g/kg) | 20.59 ± 3.66 a | 18.97 ± 1.11 a | 18.80 ± 1.08 a | 17.54 ± 0.86 a |
| AN (mg/kg) | 69.95 ± 14.19 b | 62.84 ± 15.33 b | 75.71 ± 17.63 b | 159.94 ± 13.92 a |
| AP (mg/kg) | 2.74 ± 0.61 b | 4.19 ± 1.20 ab | 3.31 ± 1.28 b | 5.39 ± 0.58 a |
| AK (mg/kg) | 94.83 ± 20.93 b | 143.17 ± 34.65 b | 205.75 ± 39.24 a | 130.17 ± 16.55 b |
| pH | 8.04 ± 0.05 ab | 8.19 ± 0.10 a | 7.89 ± 0.04 b | 7.62 ± 0.12 c |
| Topological Properties | Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| Class I | Class II | Class III | Class IV | Class I | Class II | Class III | Class IV | |
| Nodes | 215 | 234 | 218 | 216 | 99 | 126 | 109 | 81 |
| Edges | 469 | 577 | 1004 | 649 | 130 | 196 | 335 | 180 |
| Average degree | 2.18 | 2.47 | 4.61 | 3.01 | 1.31 | 1.56 | 3.07 | 2.22 |
| Average path length | 2.28 | 2.26 | 2.55 | 2.42 | 1.47 | 1.44 | 2.16 | 1.71 |
| Network diameter | 9 | 8 | 9 | 7 | 4 | 4 | 6 | 5 |
| Graph density | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 |
| Average clusteringcoefficient | 0.07 | 0.06 | 0.09 | 0.11 | 0.06 | 0.13 | 0.05 | 0.09 |
| Modularity | 0.76 | 0.73 | 0.58 | 0.68 | 0.82 | 0.86 | 0.54 | 0.64 |
| Positive edges (%) | 53.09 | 53.20 | 52.39 | 53.06 | 56.15 | 68.37 | 67.76 | 88.33 |
| Negative edges (%) | 46.91 | 46.80 | 47.61 | 46.94 | 43.85 | 31.63 | 32.24 | 11.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, D.; Luo, N.; Yang, J. Differences in Juniperus przewalskii Rhizosphere Microbiomes across Age Classes: Community Diversity and Assembly. Microorganisms 2023, 11, 2094. https://doi.org/10.3390/microorganisms11082094
Chen Q, Li D, Luo N, Yang J. Differences in Juniperus przewalskii Rhizosphere Microbiomes across Age Classes: Community Diversity and Assembly. Microorganisms. 2023; 11(8):2094. https://doi.org/10.3390/microorganisms11082094
Chicago/Turabian StyleChen, Qian, Dengwu Li, Na Luo, and Jinyan Yang. 2023. "Differences in Juniperus przewalskii Rhizosphere Microbiomes across Age Classes: Community Diversity and Assembly" Microorganisms 11, no. 8: 2094. https://doi.org/10.3390/microorganisms11082094




