Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment
Abstract
1. Introduction
2. Virulence Factors
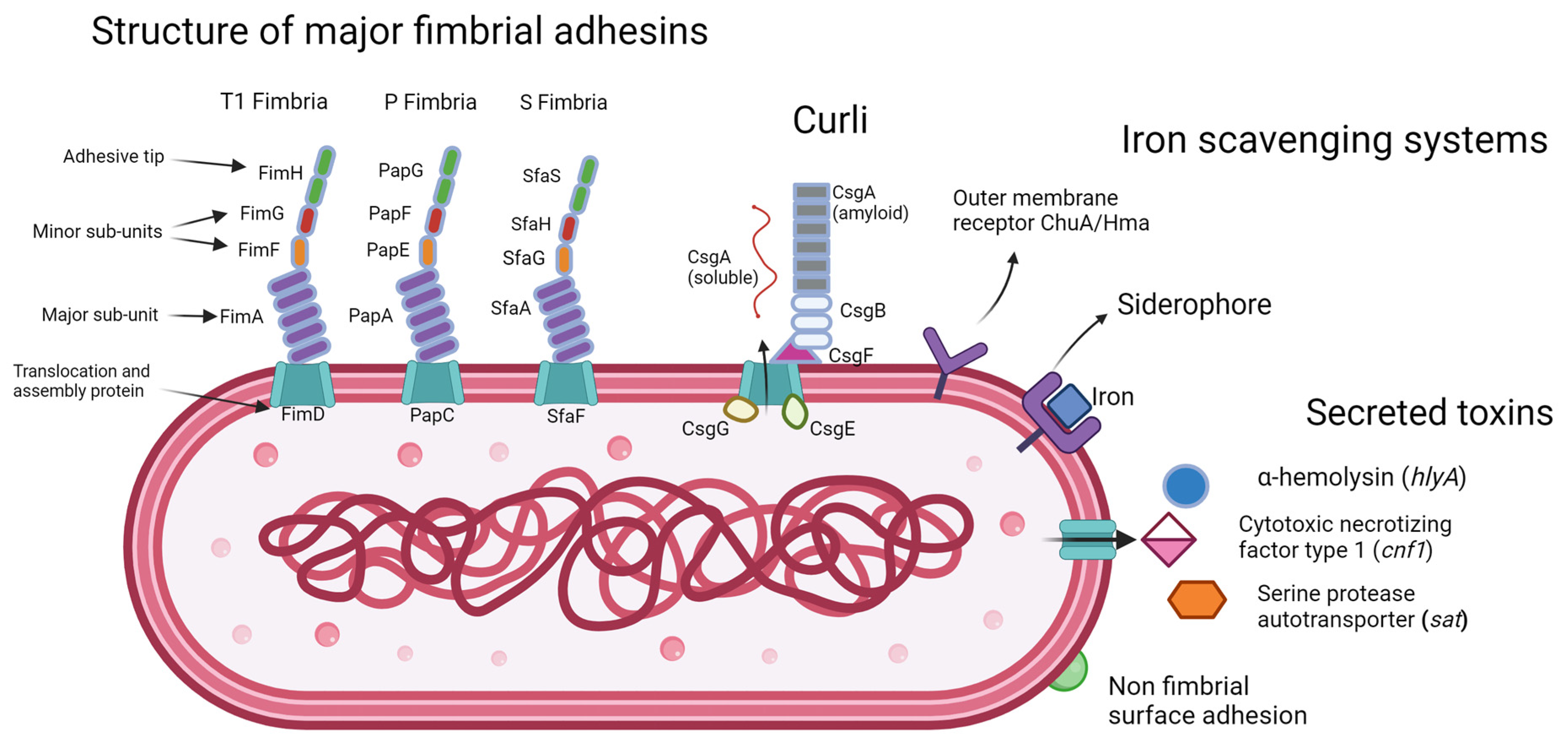
2.1. Adhesions
2.1.1. Type 1 Fimbriae
2.1.2. P Fimbriae
2.1.3. S Fimbriae
2.1.4. The Afa/Dr Family of Fimbrial Surface Adhesins
2.1.5. Non-Fimbrial Adhesins
2.2. Curli
2.3. Biofilm Formation
2.4. Toxin Production and Cytotoxic Effects
2.4.1. Hemolysin
2.4.2. Cytotoxic Necrotizing Factor Type 1 (CNF1)
2.4.3. Serine Protease Autotransporter
2.5. Iron Acquisition Systems
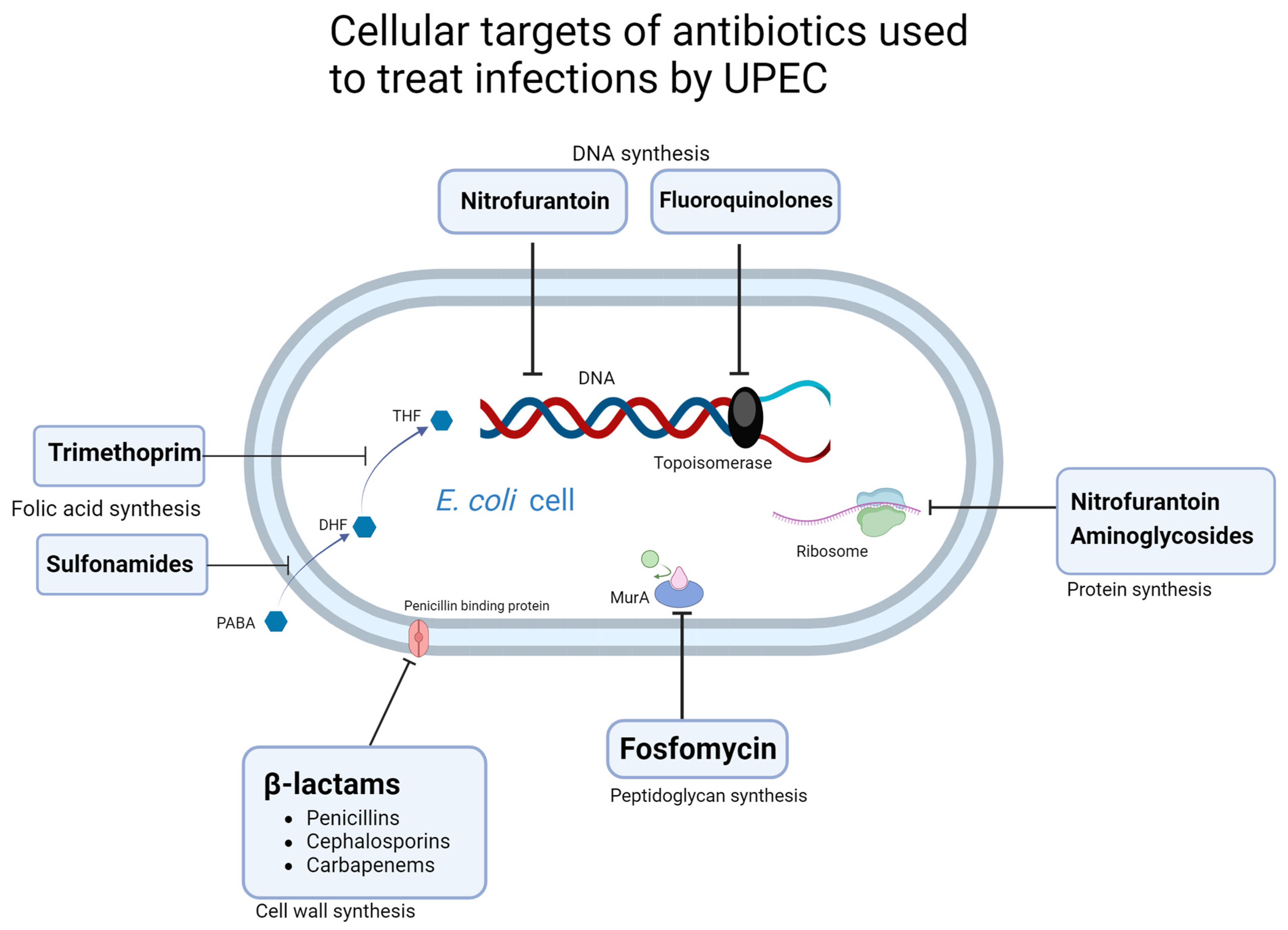
3. Antibiotic Resistance Mechanisms
3.1. β-Lactams
3.2. Nitrofurantoin
3.3. Fosfomycin
3.4. Trimethoprim-Sulfamethoxazole
3.5. Fluoroquinolones
| Chromosomal Mutations | Enzymatic Mechanism (Plasmid Encoded) | Efflux Mediated | |
|---|---|---|---|
| Nitrofurantoin | nfsA, nfsB [182] ribE [183] | CTX-M-14 (mutated) [187] | oqxAB [185] |
| Fosfomycin | murA, [192] glpT, uhpT [194] | fosA, fosL [195,196,197,225] (Various alleles) | |
| Trimethoprim | mgrB [214] | dfrA1, dfrA5, dfrA7, dfrA12, dfrA17 [226] dfrA8, dfrA14, dfr2d, dfrA3, dfrA9, dfrA10, dfrA24, dfrA26 [227] | acrAB-tolC [213] |
| Sulfamethoxazole | sul1, sul2, sul3 [228] | ||
| Fluoroquinolones | gryA, gyrB, parC [217] | qnrA, qnrB, qnrC, qnrS, qnrD, qnrE, qnrVC [219] aac(6′)-Ib-cr [223] | acrB, [218] qepA, oqxAB [219] |
| β-lactams | mrdA [164] ampC [92] | blaCTX-M, blaTEM, blaSHV [163], blaOXA, blaCMY [229] pAmpC [91] | acrAB-tolC, acrAD-tolC [165] |
4. The Mobilome
4.1. Plasmids
4.2. Integrons
4.3. Transposons and Insertion Sequences
5. Perspective Alternative Treatments for UTI
5.1. Vaccines and Immunomodulation Therapies
5.2. Phage-Based Therapeutics
5.3. Antipathogenic Agents
5.4. Nano Formulations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [Google Scholar] [CrossRef]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, 519–528. [Google Scholar] [CrossRef]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef]
- Vallejo-Torres, L.; Pujol, M.; Shaw, E.; Wiegand, I.; Vigo, J.M.; Stoddart, M.; Grier, S.; Gibbs, J.; Vank, C.; Cuperus, N.; et al. Cost of hospitalised patients due to complicated urinary tract infections: A retrospective observational study in countries with high prevalence of multidrug-resistant Gram-negative bacteria: The combacte-magnet, rescuing study. BMJ Open 2018, 8, e020251. [Google Scholar] [CrossRef]
- Callan, A.; O’Shea, E.; Galvin, S.; Duane, S.; Corry, O.; SIMPle Team, T.; Vellinga, A. The Economic Cost Of Urinary Tract Infections In The Community: Results From Ireland. Value Health 2014, 17, A468. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. EAU Guidelines on Urological Infections. Eur. Assoc. Urol. 2023, 182, 237–257. [Google Scholar]
- Aggarwal, N.; Lotfollahzadeh, S. Recurrent Urinary Tract Infections; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nazarko, L. Recurrent urinary tract infection in older women. NursePrescribing 2014, 12, 608–613. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47 (Suppl. 1), 3–30. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, P.; Hang, L.; Wullt, B.; Irjala, H.; Svanborg, C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 2004, 72, 3179–3186. [Google Scholar] [CrossRef]
- Liu, M.; Zen, K. Toll-Like Receptors Regulate the Development and Progression of Renal Diseases. Kidney Dis. 2021, 7, 14–23. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Muñoz, I.; Compte, M.; Álvarez-Cienfuegos, A.; Álvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef]
- Pu, D.; Wang, W. Toll-like receptor 4 agonist, lipopolysaccharide, increases the expression levels of cytokines and chemokines in human peripheral blood mononuclear cells. Exp. Ther. Med. 2014, 8, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chen, Q.; Zhu, W.; Wu, H.; Wang, Q.; Shi, L.; Zhao, X.; Han, D. Toll-like Receptors 4 and 5 Cooperatively Initiate the Innate Immune Responses to Uropathogenic Escherichia coli Infection in Mouse Epididymal Epithelial Cells1. Biol. Reprod. 2016, 94, 58. [Google Scholar] [CrossRef]
- Song, J.; Bishop, B.L.; Li, G.; Grady, R.; Stapleton, A.; Abraham, S.N. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14966–14971. [Google Scholar] [CrossRef]
- Krogfelt, K.A.; Bergmans, H.; Klemm, P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 1990, 58, 1995–1998. [Google Scholar] [CrossRef]
- Legros, N.; Ptascheck, S.; Pohlentz, G.; Karch, H.; Dobrindt, U.; Müthing, J. PapG subtype-specific binding characteristics of Escherichia coli towards globo-series glycosphingolipids of human kidney and bladder uroepithelial cells. Glycobiology 2019, 29, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.C.; Mobley, H.L.T. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef]
- Justice, S.S.; Hung, C.; Theriot, J.A.; Fletcher, D.A.; Anderson, G.G.; Footer, M.J.; Hultgren, S.J. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.; McKinney, J.D. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. eLife 2021, 10, e66481. [Google Scholar] [CrossRef]
- Behzadi, P.; Urbán, E.; Gajdács, M. Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Tewawong, N.; Kowaboot, S.; Pimainog, Y.; Watanagul, N.; Thongmee, T.; Poovorawan, Y. Distribution of phylogenetic groups, adhesin genes, biofilm formation, and antimicrobial resistance of uropathogenic Escherichia coli isolated from hospitalized patients in Thailand. PeerJ 2020, 8, e10453. [Google Scholar] [CrossRef]
- Qasemi, A.; Rahimi, F.; Katouli, M. Genetic diversity and virulence characteristics of biofilm-producing uropathogenic Escherichia coli. Int. Microbiol. 2022, 25, 297–307. [Google Scholar] [CrossRef]
- Król, J.E.; Wojtowicz, A.J.; Rogers, L.M.; Heuer, H.; Smalla, K.; Krone, S.M.; Top, E.M. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid 2013, 70, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Massip, C.; Oswald, E. Siderophore-Microcins in Escherichia coli: Determinants of Digestive Colonization, the First Step Toward Virulence. Front. Cell. Infect. Microbiol. 2020, 10, 381. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Lv, J.; Sun, X.; Cao, Y.; Yu, K.; Miao, C.; Zhang, Z.S.; Yao, Z.; Wang, Q. Alpha-hemolysin of uropathogenic Escherichia coli induces GM-CSF-mediated acute kidney injury. Mucosal Immunol. 2020, 13, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Carlini, F.; Maroccia, Z.; Fiorentini, C.; Travaglione, S.; Fabbri, A. Effects of the Escherichia coli Bacterial Toxin Cytotoxic Necrotizing Factor 1 on Different Human and Animal Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12610. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.A.; Silva, R.M.; Ruiz, R.C.; Pimenta, D.C.; Bryant, J.A.; Henderson, I.R.; Barbosa, A.S.; Elias, W.P. Secreted Autotransporter Toxin (Sat) Mediates Innate Immune System Evasion. Front. Immunol. 2022, 13, 844878. [Google Scholar] [CrossRef]
- Dobrindt, U.; Agerer, F.; Michaelis, K.; Janka, A.; Buchrieser, C.; Samuelson, M.; Svanborg, C.; Gottschalk, G.; Karch, H.; Hacker, J. Analysis of genome plasticity in pathogenic and commensal Escherichia coli isolates by use of DNA arrays. J. Bacteriol. 2003, 185, 1831–1840. [Google Scholar] [CrossRef]
- Cusumano, C.K.; Hung, C.S.; Chen, S.L.; Hultgren, S.J. Virulence plasmid harbored by uropathogenic Escherichia coli functions in acute stages of pathogenesis. Infect. Immun. 2010, 78, 1457–1467. [Google Scholar] [CrossRef]
- Valiatti, T.B.; Santos, F.F.; Santos, A.C.M.; Nascimento, J.A.S.; Silva, R.M.; Carvalho, E.; Sinigaglia, R.; Gomes, T.A.T. Genetic and Virulence Characteristics of a Hybrid Atypical Enteropathogenic and Uropathogenic Escherichia coli (aEPEC/UPEC) Strain. Front. Cell. Infect. Microbiol. 2020, 10, 492. [Google Scholar] [CrossRef]
- Nascimento, J.A.S.; Santos, F.F.; Valiatti, T.B.; Santos-Neto, J.F.; M Santos, A.C.; Cayô, R.; Gales, A.C.; Gomes, T.A.T. Frequency and Diversity of Hybrid Escherichia coli Strains Isolated from Urinary Tract Infections. Microorganisms 2021, 9, 693. [Google Scholar] [CrossRef]
- Tanabe, R.H.S.; Dias, R.C.B.; Orsi, H.; de Lira, D.R.P.; Vieira, M.A.; Dos Santos, L.F.; Ferreira, A.M.; Rall, V.L.M.; Mondelli, A.L.; Gomes, T.A.T.; et al. Characterization of Uropathogenic Escherichia coli Reveals Hybrid Isolates of Uropathogenic and Diarrheagenic (UPEC/DEC) E. coli. Microorganisms 2022, 10, 645. [Google Scholar] [CrossRef]
- Donnenberg, M.S.; Tzipori, S.; McKee, M.L.; O’Brien, A.D.; Alroy, J.; Kaper, J.B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 1993, 92, 1418–1424. [Google Scholar] [CrossRef]
- Morin, N.; Santiago, A.E.; Ernst, R.K.; Guillot, S.J.; Nataro, J.P. Characterization of the AggR regulon in enteroaggregative Escherichia coli. Infect. Immun. 2013, 81, 122–132. [Google Scholar] [CrossRef]
- Yousefipour, M.; Rezatofighi, S.E.; Ardakani, M.R. Detection and characterization of hybrid uropathogenic Escherichia coli strains among E. coli isolates causing community-acquired urinary tract infection. J. Med. Microbiol. 2023, 72, 001660. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- de Castro Stoppe, N.; Silva, J.S.; Carlos, C.; Sato, M.I.Z.; Saraiva, A.M.; Ottoboni, L.M.M.; Torres, T.T. Worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Saralaya, V.; Adhikari, P.; Shenoy, S.; Baliga, S.; Hegde, A. Characterization of Escherichia coli Phylogenetic Groups Associated with Extraintestinal Infections in South Indian Population. Ann. Med. Health Sci. Res. 2015, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Halaji, M.; Fayyazi, A.; Rajabnia, M.; Zare, D.; Pournajaf, A.; Ranjbar, R. Phylogenetic Group Distribution of Uropathogenic Escherichia coli and Related Antimicrobial Resistance Pattern: A Meta-Analysis and Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 790184. [Google Scholar] [CrossRef]
- Fratamico, P.M.; DebRoy, C.; Liu, Y.; Needleman, D.S.; Baranzoni, G.M.; Feng, P. Advances in Molecular Serotyping and Subtyping of Escherichia coli. Front. Microbiol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Abe, C.M.; Salvador, F.A.; Falsetti, I.N.; Vieira, M.A.M.; Blanco, J.; Blanco, J.E.; Blanco, M.; Machado, A.M.O.; Elias, W.P.; Hernandes, R.T.; et al. Uropathogenic Escherichia coli (UPEC) strains may carry virulence properties of diarrhoeagenic E. coli. FEMS Immunol. Med. Microbiol. 2008, 52, 397–406. [Google Scholar] [CrossRef]
- Yamamoto, S. Molecular epidemiology of uropathogenic Escherichia coli. J. Infect. Chemother. 2007, 13, 68–73. [Google Scholar] [CrossRef]
- Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Rodríguez-Moctezuma, J.R.; Domínguez-Trejo, P.; Vaca-Paniagua, F.; Vaca, S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community-acquired urinary tract infection patients in Mexico. J. Microbiol. Immunol. Infect. 2017, 50, 478–485. [Google Scholar] [CrossRef]
- Mohammed, E.J.; Hasan, K.C.; Allami, M. Phylogenetic groups, serogroups and virulence factors of uropathogenic Escherichia coli isolated from patients with urinary tract infection in Baghdad, Iraq. Iran. J. Microbiol. 2022, 14, 445–457. [Google Scholar] [CrossRef]
- Prakapaite, R.; Saab, F.; Planciuniene, R.; Petraitis, V.; Walsh, T.J.; Petraitiene, R.; Semoskaite, R.; Baneviciene, R.; Kalediene, L.; Kavaliauskas, P. Molecular Characterization of Uropathogenic Escherichia coli Reveals Emergence of Drug Resistant O15, O22 and O25 Serogroups. Medicina 2019, 55, 733. [Google Scholar] [CrossRef]
- Noie Oskouie, A.; Hasani, A.; Ahangarzadeh Rezaee, M.; Soroush Bar Haghi, M.H.; Hasani, A.; Soltani, E. A Relationship between O-Serotype, Antibiotic Susceptibility and Biofilm Formation in Uropathogenic Escherichia coli. Microb. Drug Resist. 2019, 25, 951–958. [Google Scholar] [CrossRef]
- Dormanesh, B.; Safarpoor Dehkordi, F.; Hosseini, S.; Momtaz, H.; Mirnejad, R.; Hoseini, M.J.; Yahaghi, E.; Tarhriz, V.; Khodaverdi Darian, E. Virulence factors and o-serogroups profiles of uropathogenic Escherichia coli isolated from Iranian pediatric patients. Iran. Red Crescent Med. J. 2014, 16, e14627. [Google Scholar] [CrossRef]
- O’Grady, M.C.; Barry, L.; Corcoran, G.D.; Hooton, C.; Sleator, R.D.; Lucey, B. Empirical treatment of urinary tract infections: How rational are our guidelines? J. Antimicrob. Chemother. 2018, 74, 214–217. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control 2016, 5, 5. [Google Scholar] [CrossRef]
- Eberly, A.R.; Floyd, K.A.; Beebout, C.J.; Colling, S.J.; Fitzgerald, M.J.; Stratton, C.W.; Schmitz, J.E.; Hadjifrangiskou, M. Biofilm Formation by Uropathogenic Escherichia coli Is Favored under Oxygen Conditions That Mimic the Bladder Environment. Int. J. Mol. Sci. 2017, 18, 2077. [Google Scholar] [CrossRef]
- Rahdar, M.; Rashki, A.; Miri, H.R.; Ghalehnoo, M.R. Detection of pap, sfa, afa, foc, and fim adhesin-encoding operons in uropathogenic Escherichia coli isolates collected from patients with urinary tract infection. Jundishapur J. Microbiol. 2015, 8, e22647. [Google Scholar] [CrossRef]
- Kadry, A.A.; Al-Kashef, N.M.; El-Ganiny, A.M. Distribution of genes encoding adhesins and biofilm formation capacity among Uropathogenic Escherichia coli isolates in relation to the antimicrobial resistance. Afr. Health Sci. 2020, 20, 238. [Google Scholar] [CrossRef]
- Schüroff, P.A.; Abe, C.M.; Silva, J.W.; de Paula Coelho, C.; Andrade, F.B.; Hernandes, R.T.; Dobrindt, U.; Gomes, T.A.T.; Elias, W.P. Role of aggregate-forming pilus (AFP) in adherence and colonization of both intestinal and urinary tracts. Virulence 2022, 13, 1423–1433. [Google Scholar] [CrossRef]
- Wojciuk, B.; Majewska, K.; Grygorcewicz, B.; Krukowska, Ż.; Kwiatkowska, E.; Ciechanowski, K.; Dołęgowska, B. The role of uropathogenic Escherichia coli adhesive molecules in inflammatory response-comparative study on immunocompetent hosts and kidney recipients. PLoS ONE 2022, 17, e0268243. [Google Scholar] [CrossRef]
- Nhu, N.T.K.; Phan, M.D.; Peters, K.M.; Lo, A.W.; Forde, B.M.; Chong, T.M.; Yin, W.F.; Chan, K.G.; Chromek, M.; Brauner, A.; et al. Discovery of new genes involved in curli production by a uropathogenic Escherichia coli strain from the highly virulent O45:K1:H7 lineage. MBio 2018, 9, 1462–1480. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Chapman, M.R. Curli Biogenesis and Function. Annu. Rev. Microbiol. 2006, 60, 131. [Google Scholar] [CrossRef]
- Das Mitra, S.; Irshad, P.; Anusree, M.; Rekha, I.; Shailaja, S.; Suresh, J.; Aishwarya, G.; Shrestha, S.; Shome, B.R. Whole genome global insight of antibiotic resistance gene repertoire and virulome of high-risk multidrug-resistant Uropathogenic Escherichia coli. Microb. Pathog. 2021, 161, 105256. [Google Scholar] [CrossRef]
- Zamani, H.; Salehzadeh, A. Biofilm formation in uropathogenic Escherichia coli: Association with adhesion factor genes. Turkish J. Med. Sci. 2018, 48, 162–167. [Google Scholar] [CrossRef]
- Yazdi, M.; Bouzari, M.; Ghaemi, E.A. Detection of fim, pap, sfa and afa Adhesin-Encoding Operons in Escherichia coli Strains Isolated from Urinary Tract Infections. Med. Lab. J. 2018, 12, 10–15. [Google Scholar] [CrossRef][Green Version]
- Kerrn, M.B.; Struve, C.; Blom, J.; Frimodt-Møller, N.; Krogfelt, K.A. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J. Antimicrob. Chemother. 2005, 55, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Mysorekar, I.U.; Hultgren, S.J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA 2006, 103, 14170–14175. [Google Scholar] [CrossRef]
- Sivick, K.E.; Mobley, H.L.T. Waging war against uropathogenic Escherichia coli: Winning back the urinary tract. Infect. Immun. 2010, 78, 568–585. [Google Scholar] [CrossRef]
- Schwan, W.R. Regulation of fim genes in uropathogenic Escherichia coli. World J. Clin. Infect. Dis. 2011, 1, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Melican, K.; Sandoval, R.M.; Kader, A.; Josefsson, L.; Tanner, G.A.; Molitoris, B.A.; Richter-Dahlfors, A. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog. 2011, 7, e1001298. [Google Scholar] [CrossRef]
- Blomfield, I.C. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv. Microb. Physiol. 2001, 45, 1–49. [Google Scholar] [CrossRef]
- van der Woude, M.W.; Bäumler, A.J. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 2004, 17, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Totsika, M.; Beatson, S.A.; Holden, N.; Gally, D.L. Regulatory interplay between pap operons in uropathogenic Escherichia coli. Mol. Microbiol. 2008, 67, 996–1011. [Google Scholar] [CrossRef]
- Abraham, J.M.; Freitag, C.S.; Clements, J.R.; Eisenstein, B.I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 1985, 82, 5724–5727. [Google Scholar] [CrossRef]
- Bessaiah, H.; Anamalé, C.; Sung, J.; Dozois, C.M. What Flips the Switch? Signals and Stress Regulating Extraintestinal Pathogenic Escherichia coli Type 1 Fimbriae (Pili). Microorganisms 2022, 10, 5. [Google Scholar] [CrossRef]
- Dalebroux, Z.D.; Svensson, S.L.; Gaynor, E.C.; Swanson, M.S. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 2010, 74, 171–199. [Google Scholar] [CrossRef]
- Yang, J.; Anderson, B.W.; Turdiev, A.; Turdiev, H.; Stevenson, D.M.; Amador-Noguez, D.; Lee, V.T.; Wang, J.D. The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p) ppGpp. Nat. Commun. 2020, 11, 5388. [Google Scholar] [CrossRef] [PubMed]
- Karczewska, M.; Strzelecki, P.; Bogucka, K.; Potrykus, K.; Szalewska-Pałasz, A.; Nowicki, D. Increased Levels of (p)ppGpp Correlate with Virulence and Biofilm Formation, but Not with Growth, in Strains of Uropathogenic Escherichia coli. Int. J. Mol. Sci. 2023, 24, 3315. [Google Scholar] [CrossRef]
- Qin, X.; Hu, F.; Wu, S.; Ye, X.; Zhu, D.; Zhang, Y.; Wang, M. Comparison of Adhesin Genes and Antimicrobial Susceptibilities between Uropathogenic and Intestinal Commensal Escherichia coli Strains. PLoS ONE 2013, 8, e61169. [Google Scholar] [CrossRef]
- Källenius, G.; Möllby, R.; Svenson, S.B.; Helin, I.; Hultberg, H.; Cedergren, B.; Winberg, J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet 1981, 2, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Kudinha, T.; Kong, F. Distribution of papG alleles among uropathogenic Escherichia coli from reproductive age women. J. Biomed. Sci. 2022, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Russo, T.A.; Brown, J.J.; Stapleton, A. papG alleles of Escherichia coli strains causing first-episode or recurrent acute cystitis in adult women. J. Infect. Dis. 1998, 177, 97–101. [Google Scholar] [CrossRef][Green Version]
- Nou, X.; Skinner, B.; Braaten, B.; Blyn, L.; Hirsch, D.; Low, D. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: Binding of the PapI and the Lrp regulatory proteins is controlled by DNA methylation. Mol. Microbiol. 1993, 7, 545–553. [Google Scholar] [CrossRef]
- Hernday, A.D.; Braaten, B.A.; Low, D.A. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol. Cell 2003, 12, 947–957. [Google Scholar] [CrossRef]
- Forsman, K.; Göransson, M.; Uhlin, B.E. Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E. coli pili biogenesis. EMBO J. 1989, 8, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P. Uropathogenic Escherichia coli and Fimbrial Adhesins Virulome. In Urinary Tract Infection; Jarzembowski, T., Daca, A., Dębska-Ślizień, M.A., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Balsalobre, C.; Morschhäuser, J.; Hacker, J.; Uhlin, B.E. Transcriptional analysis of the SFA and PAP determinants of uropathogenic Escherichia coli strains. Adv. Exp. Med. Biol. 2000, 485, 119–122. [Google Scholar]
- Sabitha, B.; Vimal, K.; Geetha, R. Adhesins of Uropathogenic Escherichia coli (UPEC). IP Int. J. Med. Microbiol. Trop. Dis. 2016, 2, 10–18. [Google Scholar]
- Korhonen, T.K.; Väisänen-Rhen, V.; Rhen, M.; Pere, A.; Parkkinen, J.; Finne, J. Escherichia coli fimbriae recognizing sialyl galactosides. J. Bacteriol. 1984, 159, 762–766. [Google Scholar] [CrossRef]
- Prasadarao, N.V.; Wass, C.A.; Kim, K.S. Identification and characterization of S fimbria-binding sialoglycoproteins on brain microvascular endothelial cells. Infect. Immun. 1997, 65, 2852–2860. [Google Scholar] [CrossRef]
- Tajbakhsh, E.; Ahmadi, P.; Abedpour-Dehkordi, E.; Arbab-Soleimani, N.; Khamesipour, F. Biofilm formation, antimicrobial susceptibility, serogroups and virulence genes of uropathogenic E. coli isolated from clinical samples in Iran. Antimicrob. Resist. Infect. Control 2016, 5, 11. [Google Scholar] [CrossRef]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Dahbi, G.; Blanco, M.; del Carmen Ponte, M.; Soriano, F. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef]
- Servin, A.L. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin. Microbiol. Rev. 2005, 18, 264–292. [Google Scholar] [CrossRef] [PubMed]
- Archambaud, M.; Courcoux, P.; Labigne-Roussel, A. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann. Inst. Pasteur. Microbiol. 1988, 139, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Zhang, L.; Tallman, P.; Palin, K.; Rode, C.; Bloch, C.; Gillespie, B.; Marrs, C.F. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J. Infect. Dis. 1995, 172, 1536–1541. [Google Scholar] [CrossRef]
- Alvarez-Fraga, L.; Phan, M.-D.; Goh, K.G.K.; Nhu, N.T.K.; Hancock, S.J.; Allsopp, L.P.; Peters, K.M.; Forde, B.M.; Roberts, L.W.; Sullivan, M.J.; et al. Differential Afa/Dr Fimbriae Expression in the Multidrug-Resistant Escherichia coli ST131 Clone. MBio 2022, 13, e0351921. [Google Scholar] [CrossRef]
- Nowicki, B.; Selvarangan, R.; Nowicki, S. Family of Escherichia coli Dr adhesins: Decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 2001, 183 (Suppl. 1), S24–S27. [Google Scholar] [CrossRef]
- Korotkova, N.; Yarova-Yarovaya, Y.; Tchesnokova, V.; Yazvenko, N.; Carl, M.A.; Stapleton, A.E.; Moseley, S.L. Escherichia coli DraE adhesin-associated bacterial internalization by epithelial cells is promoted independently by decay-accelerating factor and carcinoembryonic antigen-related cell adhesion molecule binding and does not require the DraD invasin. Infect. Immun. 2008, 76, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Hart-Van Tassell, A.; Urvil, P.T.; Lea, S.; Pettigrew, D.; Anderson, K.L.; Samet, A.; Kur, J.; Matthews, S.; Nowicki, S.; et al. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect. Immun. 2005, 73, 6119–6126. [Google Scholar] [CrossRef]
- van der Woude, M.W.; Low, D.A. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol. Microbiol. 1994, 11, 605–618. [Google Scholar] [CrossRef]
- Benz, R. RTX-Toxins. Toxins 2020, 12, 359. [Google Scholar] [CrossRef]
- Guo, S.; Vance, T.D.R.; Stevens, C.A.; Voets, I.; Davies, P.L. RTX Adhesins are Key Bacterial Surface Megaproteins in the Formation of Biofilms. Trends Microbiol. 2019, 27, 453–467. [Google Scholar] [CrossRef]
- Vigil, P.D.; Wiles, T.J.; Engstrom, M.D.; Prasov, L.; Mulvey, M.A.; Mobley, H.L.T. The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia. Infect. Immun. 2012, 80, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Luterbach, C.L.; Forsyth, V.S.; Engstrom, M.D.; Mobley, H.L.T. TosR-Mediated Regulation of Adhesins and Biofilm Formation in Uropathogenic Escherichia coli. mSphere 2018, 3, e00222-18. [Google Scholar] [CrossRef]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, Ç. Microbiome or Infections: Amyloid-Containing Biofilms as a Trigger for Complex Human Diseases. Front. Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef]
- Bhoite, S.; van Gerven, N.; Chapman, M.R.; Remaut, H. Curli Biogenesis: Bacterial Amyloid Assembly by the Type VIII Secretion Pathway. EcoSal Plus 2019, 8, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Chapman, M.R. Curli biogenesis: Order out of disorder. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Homma, M.; Kojima, S. Roles of the second messenger c-di-GMP in bacteria: Focusing on the topics of flagellar regulation and Vibrio spp. Genes Cells 2022, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Weinhouse, H.; Amikam, D.; Huggirat, Y.; Benziman, M.; de Vroom, E.; Fidder, A.; de Paus, P.; Sliedregt, L.A. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J. Biol. Chem. 1990, 265, 18933–18943. [Google Scholar] [CrossRef]
- Cepas, V.; Ballén, V.; Gabasa, Y.; Ramírez, M.; López, Y.; Soto, S.M. Transposon Insertion in the purL Gene Induces Biofilm Depletion in Escherichia coli ATCC 25922. Pathogens 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Morar, M.; Hoskins, A.A.; Stubbe, J.; Ealick, S.E. Formylglycinamide ribonucleotide amidotransferase from Thermotoga maritima: Structural insights into complex formation. Biochemistry 2008, 47, 7816–7830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín-Rodríguez, A.J.; Rhen, M.; Melican, K.; Richter-Dahlfors, A. Nitrate Metabolism Modulates Biosynthesis of Biofilm Components in Uropathogenic Escherichia coli and Acts as a Fitness Factor During Experimental Urinary Tract Infection. Front. Microbiol. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Holden, E.R.; Yasir, M.; Turner, A.K.; Wain, J.; Charles, I.G.; Webber, M.A. Massively parallel transposon mutagenesis identifies temporally essential genes for biofilm formation in Escherichia coli. Microb. Genom. 2021, 7, 000673. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Mirani, Z.A.; Pirzada, Z.A. Phylogenetic Group B2 Expressed Significant Biofilm Formation among Drug Resistant Uropathogenic Escherichia coli. Libyan J. Med. 2021, 16. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Cruz-Córdova, A.; Luna-Pineda, V.M.; Reyes-Grajeda, J.P.; Cázares-Domínguez, V.; Escalona, G.; Sepúlveda-González, M.E.; López-Montiel, F.; Arellano-Galindo, J.; López-Martínez, B.; et al. Multidrug- and Extensively Drug-Resistant Uropathogenic Escherichia coli Clinical Strains: Phylogenetic Groups Widely Associated with Integrons Maintain High Genetic Diversity. Front. Microbiol. 2016, 7, 2042. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.C.; Corcoran, D.; Finn, K.; Lucey, B. Uropathogenic Escherichia coli Biofilm-Forming Capabilities are not Predictable from Clinical Details or from Colonial Morphology. Diseases 2020, 8, 11. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.C.; Corcoran, G.D.; Finn, K.; Lucey, B. Effect of Sub-Inhibitory Concentrations of Nitrofurantoin, Ciprofloxacin, and Trimethoprim on In Vitro Biofilm Formation in Uropathogenic Escherichia coli (UPEC). Med. Sci. 2022, 11, 1. [Google Scholar] [CrossRef]
- Yasmeen, K.; Chunchanur, S.K.; Nadagir, S.D.; Halesh, L.H.; Chandrasekhar, M.R. Virulence factors, serotypes and antimicrobial suspectibility pattern of Escherichia coli in urinary tract infections. Al Ameen J. Med. Sci. 2009, 2, 47–51. [Google Scholar]
- Karam, M.R.A.; Habibi, M.; Bouzari, S. Relationships between Virulence Factors and Antimicrobial Resistance among Escherichia coli Isolated from Urinary Tract Infections and Commensal Isolates in Tehran, Iran. Osong Public Health Res. Perspect. 2018, 9, 217–224. [Google Scholar] [CrossRef]
- Shah, C.; Baral, R.; Bartaula, B.; Shrestha, L.B. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019, 19, 204. [Google Scholar] [CrossRef]
- Radera, S.; Srivastava, S.; Agarwal, J. Virulence Genotyping and Multidrug Resistance Pattern of Escherichia coli Isolated From Community-Acquired and Hospital-Acquired Urinary Tract Infections. Cureus 2022, 14, e29404. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.; Koronakis, V.; Hughes, C. Acylation of Escherichia coli hemolysin: A unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. 1998, 62, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, T.; Pellett, S.; Welch, R.A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J. Bacteriol. 1985, 163, 94–105. [Google Scholar] [CrossRef]
- Wiles, T.J.; Dhakal, B.K.; Eto, D.S.; Mulvey, M.A. Inactivation of host Akt/protein kinase B signaling by bacterial pore-forming toxins. Mol. Biol. Cell 2008, 19, 1427–1438. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Zhang, Y.; Zhang, Y.; Bartkuhn, M.; Markmann, M.; Hossain, H.; Chakraborty, T.; Hake, S.B.; Jia, Z.; et al. Uropathogenic Escherichia coli Virulence Factor α-Hemolysin Reduces Histone Acetylation to Inhibit Expression of Proinflammatory Cytokine Genes. J. Infect. Dis. 2021, 223, 1040–1051. [Google Scholar] [CrossRef]
- Doye, A.; Mettouchi, A.; Bossis, G.; Clément, R.; Buisson-Touati, C.; Flatau, G.; Gagnoux, L.; Piechaczyk, M.; Boquet, P.; Lemichez, E. CNF1 Exploits the Ubiquitin-Proteasome Machinery to Restrict Rho GTPase Activation for Bacterial Host Cell Invasion. Cell 2002, 111, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Lerm, M.; Schmidt, G.; Aktories, K. Bacterial protein toxins targeting rho GTPases. FEMS Microbiol. Lett. 2000, 188, 1–6. [Google Scholar] [CrossRef]
- Villalonga, P.; Ridley, A.J. Rho GTPases and cell cycle control. Growth Factors 2006, 24, 159–164. [Google Scholar] [CrossRef]
- Mao, Y.; Finnemann, S.C. Regulation of phagocytosis by Rho GTPases. Small GTPases 2015, 6, 89–99. [Google Scholar] [CrossRef]
- Oswald, E.; Sugai, M.; Labigne, A.; Wu, H.C.; Fiorentini, C.; Boquet, P.; O’Brien, A.D. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc. Natl. Acad. Sci. USA 1994, 91, 3814–3818. [Google Scholar] [CrossRef]
- Sugai, M.; Hatazaki, K.; Mogami, A.; Ohta, H.; Pérès, S.Y.; Hérault, F.; Horiguchi, Y.; Masuda, M.; Ueno, Y.; Komatsuzawa, H.; et al. Cytotoxic necrotizing factor type 2 produced by pathogenic Escherichia coli deamidates a Gln residue in the conserved G-3 domain of the rho family and preferentially inhibits the GTPase activity of RhoA and Rac1. Infect. Immun. 1999, 67, 6550–6557. [Google Scholar] [CrossRef]
- Oswald, E.; De Rycke, J. A single protein of 110 kDa is associated with the multinucleating and necrotizing activity coded by the Vir plasmid of Escherichia coli. FEMS Microbiol. Lett. 1990, 56, 279–284. [Google Scholar]
- Blum, G.; Falbo, V.; Caprioli, A.; Hacker, J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 1995, 126, 189–195. [Google Scholar] [CrossRef]
- McNichol, B.A.; Rasmussen, S.B.; Carvalho, H.M.; Meysick, K.C.; O’Brien, A.D. Two domains of cytotoxic necrotizing factor type 1 bind the cellular receptor, laminin receptor precursor protein. Infect. Immun. 2007, 75, 5095–5104. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Hong, S.J.; Kim, K.J.; Goti, D.; Stins, M.F.; Shin, S.; Dawson, V.L.; Dawson, T.M.; Kim, K.S. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003, 278, 16857–16862. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsukamoto, T.; Terai, A.; Kurazono, H.; Takeda, Y.; Yoshida, O. Distribution of virulence factors in Escherichia coli isolated from urine of cystitis patients. Microbiol. Immunol. 1995, 39, 401–404. [Google Scholar] [CrossRef]
- Rippere-Lampe, K.E.; O’Brien, A.D.; Conran, R.; Lockman, H.A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf(1)) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 2001, 69, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.J.; Srinivas, S.; Albert, M.J.; Alam, K.; Robins-Browne, R.M.; Gunzburg, S.T.; Mee, B.J.; Chang, B.J. Characterization of the roles of hemolysin and other toxins in enteropathy caused by alpha-hemolytic Escherichia coli linked to human diarrhea. Infect. Immun. 1998, 66, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Landraud, L.; Gauthier, M.; Fosse, T.; Boquet, P. Frequency of Escherichia coli strains producing the cytotoxic necrotizing factor (CNF1) in nosocomial urinary tract infections. Lett. Appl. Microbiol. 2000, 30, 213–216. [Google Scholar] [CrossRef]
- Wang, M.-H.; Kim, K.S. Cytotoxic necrotizing factor 1 contributes to Escherichia coli meningitis. Toxins 2013, 5, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C.; Krishnan, S.; Prasadarao, N.V. The effects of cytotoxic necrotizing factor 1 expression in the uptake of Escherichia coli K1 by macrophages and the onset of meningitis in newborn mice. Virulence 2016, 7, 806–818. [Google Scholar] [CrossRef]
- Pokharel, P.; Habouria, H.; Bessaiah, H.; Dozois, C.M. Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up. Microorganisms 2019, 7, 594. [Google Scholar] [CrossRef]
- Guyer, D.M.; Henderson, I.R.; Nataro, J.P.; Mobley, H.L.T. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 2000, 38, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.M.; Radulovic, S.; Jones, F.-E.; Mobley, H.L.T. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 2002, 70, 4539–4546. [Google Scholar] [CrossRef]
- Cover, T.L. The vacuolating cytotoxin of Helicobacter pylori. Mol. Microbiol. 1996, 20, 241–246. [Google Scholar] [CrossRef]
- Balsalobre, C.; Morschhäuser, J.; Jass, J.; Hacker, J.; Uhlin, B.E. Transcriptional analysis of the sfa determinant revealing mmRNA processing events in the biogenesis of S fimbriae in pathogenic Escherichia coli. J. Bacteriol. 2003, 185, 620–629. [Google Scholar] [CrossRef][Green Version]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef]
- Nairz, M.; Dichtl, S.; Schroll, A.; Haschka, D.; Tymoszuk, P.; Theurl, I.; Weiss, G. Iron and innate antimicrobial immunity—Depriving the pathogen, defending the host. J. Trace Elem. Med. Biol. 2018, 48, 118–133. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhao, T.; Zhang, C.; Li, P.; Wang, J.; Han, J.; Zhang, N.; Pang, B.; Liu, S. Ferritinophagy-mediated iron competition in RUTIs: Tug-of-war between UPEC and host. Biomed. Pharmacother. 2023, 163, 114859. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.P.; Crowley, J.R.; Pinkner, J.S.; Walker, J.N.; Tsukayama, P.; Stamm, W.E.; Hooton, T.M.; Hultgren, S.J. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009, 5, e1000305. [Google Scholar] [CrossRef]
- Robinson, A.E.; Heffernan, J.R.; Henderson, J.P. The iron hand of uropathogenic Escherichia coli: The role of transition metal control in virulence. Future Microbiol. 2018, 13, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Rezatofighi, S.E.; Mirzarazi, M.; Salehi, M. Virulence genes and phylogenetic groups of uropathogenic Escherichia coli isolates from patients with urinary tract infection and uninfected control subjects: A case-control study. BMC Infect. Dis. 2021, 21, 361. [Google Scholar] [CrossRef]
- Torres, A.G.; Payne, S.M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 1997, 23, 825–833. [Google Scholar] [CrossRef]
- Hagan, E.C.; Mobley, H.L.T. Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia coli for kidney infection. Mol. Microbiol. 2009, 71, 79–91. [Google Scholar] [CrossRef]
- Roos, V.; Ulett, G.C.; Schembri, M.A.; Klemm, P. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect. Immun. 2006, 74, 615–624. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Halaji, M.; Shahidi, S.; Atapour, A.; Ataei, B.; Feizi, A.; Havaei, S.A. Characterization of Extended-Spectrum β-Lactamase-Producing Uropathogenic Escherichia coli Among Iranian Kidney Transplant Patients. Infect. Drug Resist. 2020, 13, 1429–1437. [Google Scholar] [CrossRef]
- Lange, F.; Pfennigwerth, N.; Höfken, L.-M.; Gatermann, S.G.; Kaase, M. Characterization of mutations in Escherichia coli PBP2 leading to increased carbapenem MICs. J. Antimicrob. Chemother. 2019, 74, 571–576. [Google Scholar] [CrossRef]
- Chetri, S.; Bhowmik, D.; Paul, D.; Pandey, P.; Chanda, D.D.; Chakravarty, A.; Bora, D.; Bhattacharjee, A. AcrAB-TolC efflux pump system plays a role in carbapenem non-susceptibility in Escherichia coli. BMC Microbiol. 2019, 19, 210. [Google Scholar] [CrossRef]
- Rossolini, G.M.; D’Andrea, M.M.; Mugnaioli, C. The Spread of CTX-M-Type Extended-Spectrum β-Lactamases. Clin. Microbiol. Infect. 2008, 14, 33–41. [Google Scholar] [CrossRef]
- Shrestha, R.; Khanal, S.; Poudel, P.; Khadayat, K.; Ghaju, S.; Bhandari, A.; Lekhak, S.; Pant, N.D.; Sharma, M.; Marasini, B.P. Extended spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.C.; Upreti, M.K.; Sah, A.K.; Ansari, M.; Nepal, K.; Dhungel, B.; Adhikari, N.; Lekhak, B.; Rijal, K.R. Plasmid-Mediated AmpC β-Lactamase CITM and DHAM Genes Among Gram-Negative Clinical Isolates. Infect. Drug Resist. 2020, 13, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Tracz, D.M.; Boyd, D.A.; Hizon, R.; Bryce, E.; McGeer, A.; Ofner-Agostini, M.; Simor, A.E.; Paton, S.; Mulvey, M.R.; Program, C.N.I.S. ampC gene expression in promoter mutants of cefoxitin-resistant Escherichia coli clinical isolates. FEMS Microbiol. Lett. 2007, 270, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zangane Matin, F.; Rezatofighi, S.E.; Roayaei Ardakani, M.; Akhoond, M.R.; Mahmoodi, F. Virulence characterization and clonal analysis of uropathogenic Escherichia coli metallo-beta-lactamase-producing isolates. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 50. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Reck, F.; Ke, X.; Zhu, Q.; McEnroe, G.; Lopez, S.L.; Dean, C.R. Identification of Mutations in the mrdA Gene Encoding PBP2 That Reduce Carbapenem and Diazabicyclooctane Susceptibility of Escherichia coli Clinical Isolates with Mutations in ftsI (PBP3) and Which Carry bla(NDM-1). mSphere 2019, 4, e00074-19. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Huang, P.; Lin, Q.; Sun, C.; Zeng, H.; Deng, Y. Novel β-lactam/β-lactamase inhibitors versus alternative antibiotics for the treatment of complicated intra-abdominal infection and complicated urinary tract infection: A meta-analysis of randomized controlled trials. Expert Rev. Anti-Infect. Ther. 2018, 16, 111–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Kashikar, A.; Brown, C.A.; Denys, G.; Bush, K. Unusual Escherichia coli PBP 3 Insertion Sequence Identified from a Collection of Carbapenem-Resistant Enterobacteriaceae Tested In Vitro with a Combination of Ceftazidime-, Ceftaroline-, or Aztreonam-Avibactam. Antimicrob. Agents Chemother. 2017, 61, 10–128. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Ogurek, S.; Li, Z.; Wang, P.; Xie, Q.; Sheng, Z.; Wang, M. Efflux Pump AcrAB Confers Decreased Susceptibility to Piperacillin-Tazobactam and Ceftolozane-Tazobactam in Tigecycline-Non-Susceptible Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 4309–4319. [Google Scholar] [CrossRef] [PubMed]
- Tutone, M.; Bjerklund Johansen, T.E.; Cai, T.; Mushtaq, S.; Livermore, D.M. SUsceptibility and Resistance to Fosfomycin and other antimicrobial agents among pathogens causing lower urinary tract infections: Findings of the SURF study. Int. J. Antimicrob. Agents 2022, 59, 106574. [Google Scholar] [CrossRef] [PubMed]
- McOsker, C.C.; Fitzpatrick, P.M. Nitrofurantoin: Mechanism of action and implications for resistance development in common uropathogens. J. Antimicrob. Chemother. 1994, 33, 23–30. [Google Scholar] [CrossRef]
- Whiteway, J.; Koziarz, P.; Veall, J.; Sandhu, N.; Kumar, P.; Hoecher, B.; Lambert, I.B. Oxygen-insensitive nitroreductases: Analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 1998, 180, 5529–5539. [Google Scholar] [CrossRef]
- Wan, Y.; Mills, E.; Leung, R.C.Y.; Vieira, A.; Zhi, X.; Croucher, N.J.; Woodford, N.; Jauneikaite, E.; Ellington, M.J.; Sriskandan, S. Alterations in chromosomal genes nfsA, nfsB, and ribE are associated with nitrofurantoin resistance in Escherichia coli from the United Kingdom. Microb. Genom. 2021, 7, 702. [Google Scholar] [CrossRef]
- Vervoort, J.; Xavier, B.B.; Stewardson, A.; Coenen, S.; Godycki-Cwirko, M.; Adriaenssens, N.; Kowalczyk, A.; Lammens, C.; Harbarth, S.; Goossens, H.; et al. An In Vitro Deletion in ribE Encoding Lumazine Synthase Contributes to Nitrofurantoin Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 7225. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ning, J.; Sajid, A.; Cheng, G.; Yuan, Z.; Hao, H. The nature and epidemiology of OqxAB, a multidrug efflux pump. Antimicrob. Resist. Infect. Control. 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.L.; Ng, K.Y.; Lo, W.U.; Law, P.Y.; Lai, E.L.Y.; Wang, Y.; Chow, K.H. Plasmid-Mediated OqxAB Is an Important Mechanism for Nitrofurantoin Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 537. [Google Scholar] [CrossRef]
- Khamari, B.; Kumar, P.; Pradeep, B.E. Resistance to nitrofurantoin is an indicator of extensive drug-resistant (XDR) Enterobacteriaceae. J. Med. Microbiol. 2021, 70, 001347. [Google Scholar] [CrossRef] [PubMed]
- Edowik, Y.; Caspari, T.; Williams, H.M. The amino acid changes t55a, a273p and r277c in the beta-lactamase CTX-M-14 render E. coli resistant to the antibiotic nitrofurantoin, a first-line treatment of urinary tract infections. Microorganisms 2020, 8, 1983. [Google Scholar] [CrossRef]
- Dzib-Baak, H.E.; Uc-Cachón, A.H.; de Jesús Dzul-Beh, A.; Rosado-Manzano, R.F.; Gracida-Osorno, C.; Molina-Salinas, G.M. Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates. Trop. Med. Infect. Dis. 2022, 7, 235. [Google Scholar] [CrossRef]
- Falagas, M.E.; Athanasaki, F.; Voulgaris, G.L.; Triarides, N.A.; Vardakas, K.Z. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int. J. Antimicrob. Agents 2019, 53, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E.; del Carmen López Diaz, M.; Culebras, E.; Rodríguez-Avial, I.; Rodríguez-Avial, C. Resistance to fosfomycin is increasing and is significantly associated with extended-spectrum β-lactamase-production in urinary isolates of Escherichia coli. Med. Microbiol. Immunol. 2022, 211, 269–272. [Google Scholar] [CrossRef]
- Silver, L.L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb. Perspect. Med. 2017, 7, a025262. [Google Scholar] [CrossRef]
- Fu, Z.; Ma, Y.; Chen, C.; Guo, Y.; Hu, F.; Liu, Y.; Xu, X.; Wang, M. Prevalence of Fosfomycin Resistance and Mutations in murA, glpT, and uhpT in Methicillin-Resistant Staphylococcus aureus Strains Isolated from Blood and Cerebrospinal Fluid Samples. Front. Microbiol. 2015, 6, 1544. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Larson, T.J.; Maloney, P.C. Reconstitution of sugar phosphate transport systems of Escherichia coli. J. Biol. Chem. 1986, 261, 9083–9086. [Google Scholar] [CrossRef] [PubMed]
- Ballestero-Téllez, M.; Docobo-Pérez, F.; Portillo-Calderón, I.; Rodríguez-Martínez, J.M.; Racero, L.; Ramos-Guelfo, M.S.; Blázquez, J.; Rodríguez-Baño, J.; Pascual, A. Molecular insights into fosfomycin resistance in Escherichia coli. J. Antimicrob. Chemother. 2017, 72, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Méndez, M.; Navarrete-Salazar, H.; Baltazar-Jiménez, F.; Muñoz-de la Paz, E.; Sánchez-Mawcinitt, M.F.; Gómez-Pardo, A.; Garza-González, E.; Ponce-de-León-Garduño, L.A.; Franco-Cendejas, R.; Morfín-Otero, R.; et al. Emergence of Fosfomycin Resistance by Plasmid-Mediated fos Genes in Uropathogenic ESBL-Producing E. coli Isolates in Mexico. Antibiotics 2022, 11, 1383. [Google Scholar] [CrossRef]
- Poirel, L.; Vuillemin, X.; Kieffer, N.; Mueller, L.; Descombes, M.C.; Nordmann, P. Identification of FosA8, a Plasmid-Encoded Fosfomycin Resistance Determinant from Escherichia coli, and Its Origin in Leclercia adecarboxylata. Antimicrob. Agents Chemother. 2019, 63, e01403-19. [Google Scholar] [CrossRef]
- Kieffer, N.; Poirel, L.; Descombes, M.C.; Nordmann, P. Characterization of FosL1, a Plasmid-Encoded Fosfomycin Resistance Protein Identified in Escherichia coli. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- López-Montesinos, I.; Horcajada, J.P. Oral and intravenous fosfomycin in complicated urinary tract infections. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 37–44. [Google Scholar] [PubMed]
- Bergan, T.; Thorsteinsson, S.B.; Albini, E. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy 1993, 39, 297–301. [Google Scholar] [CrossRef]
- Marino, A.; Stracquadanio, S.; Ceccarelli, M.; Zagami, A.; Nunnari, G.; Cacopardo, B. Oral fosfomycin formulation for acute bacterial prostatitis; a new role for an old molecule: A case report and brief literature review. World Acad. Sci. J. 2022, 4, 1–6. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Mahony, A.A.; Ellis, A.G.; Lawrentschuk, N.; Bolton, D.M.; Zeglinski, P.T.; Frauman, A.G.; Grayson, M.L. Is Fosfomycin a Potential Treatment Alternative for Multidrug-Resistant Gram-Negative Prostatitis? Clin. Infect. Dis. 2013, 58, e101–e105. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, Z.; Farhadi, T. Fosfomycin: The characteristics, activity, and use in critical care. Ther. Clin. Risk Manag. 2019, 15, 525–530. [Google Scholar] [CrossRef]
- Marino, A.; Stracquadanio, S.; Campanella, E.; Munafò, A.; Gussio, M.; Ceccarelli, M.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Intravenous Fosfomycin: A Potential Good Partner for Cefiderocol. Clinical Experience and Considerations. Antibiotics 2023, 12, 49. [Google Scholar] [CrossRef]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-Sulfamethoxazole Revisited. Arch. Intern. Med. 2003, 163, 402–410. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Ibrahim, A.H. Investigation of the Gene Expression of Class I Integron and Its Relationship to Antibiotic Resistance in Isolates of Escherichia coli Causing Urinary Tract Diseases. HIV Nurs. 2023, 23, 705–710. [Google Scholar]
- Stephens, C.; Arismendi, T.; Wright, M.; Hartman, A.; Gonzalez, A.; Gill, M.; Pandori, M.; Hess, D. F Plasmids Are the Major Carriers of Antibiotic Resistance Genes in Human-Associated Commensal Escherichia coli. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Osuna, M.; Cortés, P.; Llagostera, M.; Barbé, J.; Erill, I. Exploration into the origins and mobilization of di-hydrofolate reductase genes and the emergence of clinical resistance to trimethoprim. Microb. Genom. 2020, 6, mgen000440. [Google Scholar] [CrossRef] [PubMed]
- Al-Assil, B.; Mahfoud, M.; Hamzeh, A.R. First report on class 1 integrons and Trimethoprim-resistance genes from dfrA group in uropathogenic E. coli (UPEC) from the Aleppo area in Syria. Mob. Genet. Elements 2013, 3, e25204. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Reid, C.J.; Kudinha, T.; Jarocki, V.M.; Djordjevic, S.P. Genomic analysis of trimethoprim-resistant extraintestinal pathogenic Escherichia coli and recurrent urinary tract infections. Microb. Genom. 2020, 6, mgen000475. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.M.K.; Rubaya, R.; Halder, J.; Karim, M.E.; Bhuiya, A.A.; Khatun, A.; Alam, J. Association of Antibiotic Resistance Traits in Uropathogenic Escherichia coli (UPEC) Isolates. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 4251486. [Google Scholar] [CrossRef]
- Zeng, Q.; Xiao, S.; Gu, F.; He, W.; Xie, Q.; Yu, F.; Han, L. Antimicrobial Resistance and Molecular Epidemiology of Uropathogenic Escherichia coli Isolated From Female Patients in Shanghai, China. Front. Cell. Infect. Microbiol. 2021, 11, 653983. [Google Scholar] [CrossRef]
- Mulder, M.; Verbon, A.; Lous, J.; Goessens, W.; Stricker, B.H. Use of other antimicrobial drugs is associated with trimethoprim resistance in patients with urinary tract infections caused by E. coli. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2283. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, Z.; Gu, J.; Yang, J.; Deng, J. Reducing the Periplasmic Glutathione Content Makes Escherichia coli Resistant to Trimethoprim and Other Antimicrobial Drugs. Microbiol. Spectr. 2021, 9, e00743-21. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, T.; Xu, J.; Yu, J.; Yang, S.; Zhang, X.E.; Tao, S.; Gu, J.; Deng, J.Y. MgrB Inactivation Confers Trimethoprim Resistance in Escherichia coli. Front. Microbiol. 2021, 12, 682205. [Google Scholar] [CrossRef]
- Chao, Y.-S.; Farrah, K. Fluoroquinolones for the Treatment of Urinary Tract Infection 2; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, N.E.; Gerges, M.A.; Hosny, T.A.; Ali, A.R.; Gebriel, M.G. Detection of Chromosomal and Plasmid-Mediated Quinolone Resistance Among Escherichia coli Isolated from Urinary Tract Infection Cases; Zagazig University Hospitals, Egypt. Infect. Drug Resist. 2020, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Pensec, J.; Soumet, C. Resistance in Escherichia coli: Variable contribution of efflux pumps with respect to different fluoroquinolones. J. Appl. Microbiol. 2013, 114, 1294–1299. [Google Scholar] [CrossRef]
- Sato, T.; Yokota, S.-I.; Uchida, I.; Okubo, T.; Usui, M.; Kusumoto, M.; Akiba, M.; Fujii, N.; Tamura, Y. Fluoroquinolone resistance mechanisms in an Escherichia coli isolate, HUE1, without quinolone resistance-determining region mutations. Front. Microbiol. 2013, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Salah, F.D.; Soubeiga, S.T.; Ouattara, A.K.; Sadji, A.Y.; Metuor-Dabire, A.; Obiri-Yeboah, D.; Banla-Kere, A.; Karou, S.; Simpore, J. Distribution of quinolone resistance gene (qnr) in ESBL-producing Escherichia coli and Klebsiella spp. in Lomé, Togo. Antimicrob. Resist. Infect. Control 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Deekshit, V.K.; Jazeela, K.; Vittal, R.; Rohit, A.; Chakraborty, A.; Karunasagar, I. Plasmid-mediated fluoroquinolone resistance associated with extra-intestinal Escherichia coli isolates from hospital samples. Indian J. Med. Res. 2019, 149, 192. [Google Scholar] [CrossRef]
- Kuo, P.Y.; Lo, Y.T.; Chiou, Y.J.; Chen, C.A.; Hidrosollo, J.H.; Thuy, T.T.D.; Zhang, Y.Z.; Wang, M.C.; Lin, T.P.; Lin, W.H.; et al. Plasmid-mediated quinolone resistance determinants in fluoroquinolone-nonsusceptible Escherichia coli isolated from patients with urinary tract infections in a university hospital, 2009–2010 and 2020. J. Glob. Antimicrob. Resist. 2022, 30, 241–248. [Google Scholar] [CrossRef]
- Phan, M.D.; Peters, K.M.; Fraga, L.A.; Wallis, S.C.; Hancock, S.J.; Nhu, N.T.K.; Forde, B.M.; Bauer, M.J.; Paterson, D.L.; Beatson, S.A.; et al. Plasmid-Mediated Ciprofloxacin Resistance Imparts a Selective Advantage on Escherichia coli ST131. Antimicrob. Agents Chemother. 2022, 66, e02146-21. [Google Scholar] [CrossRef]
- Machuca, J.; Ortiz, M.; Recacha, E.; Díaz-De-Alba, P.; Docobo-Perez, F.; Rodríguez-Martínez, J.-M.; Pascual, Á. Impact of AAC(6′)-Ib-cr in combination with chromosomal-mediated mechanisms on clinical quinolone resistance in Escherichia coli. J. Antimicrob. Chemother. 2016, 71, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Treier, A.; Schmitt, K.; Stephan, R. Mobile fosfomycin resistance genes in Enterobacteriaceae—An increasing threat. Microbiologyopen 2020, 9, e1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Oh, J.Y.; Cho, J.W.; Park, J.C.; Kim, J.M.; Seol, S.Y.; Cho, D.T. The prevalence of trimethoprim-resistance-conferring dihydrofolate reductase genes in urinary isolates of Escherichia coli in Korea. J. Antimicrob. Chemother. 2001, 47, 599–604. [Google Scholar] [CrossRef]
- Brolund, A.; Sundqvist, M.; Kahlmeter, G.; Grape, M. Molecular characterisation of trimethoprim resistance in Escherichia coli and Klebsiella pneumoniae during a two year intervention on trimethoprim use. PLoS ONE 2010, 5, e9233. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, M.; Starcic Erjavec, M.; Ambrozic Avgustin, J.; Reissbrodt, R.; Fruth, A.; Krizan-Hergouth, V.; Zgur-Bertok, D. High prevalence of multidrug resistance and random distribution of mobile genetic elements among uropathogenic Escherichia coli (UPEC) of the four major phylogenetic groups. Curr. Microbiol. 2006, 53, 158–162. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Gizatullina, J.S. Epidemiological characteristics of uropatogenic isolates of Escherichia coli in hospitals. Klin. Lab. Diagn. 2021, 66, 248–256. [Google Scholar] [CrossRef]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- Bousquet, A.; Henquet, S.; Compain, F.; Genel, N.; Arlet, G.; Decré, D. Partition locus-based classification of selected plasmids in Klebsiella pneumoniae, Escherichia coli and Salmonella enterica spp.: An additional tool. J. Microbiol. Methods 2015, 110, 85–91. [Google Scholar] [CrossRef]
- Garcillán-Barcia, M.P.; Francia, M.V.; de La Cruz, F. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 2009, 33, 657–687. [Google Scholar] [CrossRef]
- García-Fernández, A.; Carattoli, A. Plasmid double locus sequence typing for IncHI2 plasmids, a subtyping scheme for the characterization of IncHI2 plasmids carrying extended-spectrum β-lactamase and quinolone resistance genes. J. Antimicrob. Chemother. 2010, 65, 1155–1161. [Google Scholar] [CrossRef]
- Johnson, T.J.; Nolan, L.K. Plasmid replicon typing. Methods Mol. Biol. 2009, 551, 27–35. [Google Scholar] [PubMed]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.M.; Johnson, S.J.; Logue, C.M.; White, D.G.; Doetkott, C.; Nolan, L.K. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 2007, 73, 1976–1983. [Google Scholar] [CrossRef]
- Smith, H.; Bossers, A.; Harders, F.; Wu, G.; Woodford, N.; Schwarz, S.; Guerra, B.; Rodríguez, I.; van Essen-Zandbergen, A.; Brouwer, M.; et al. Characterization of epidemic IncI1-Iγ plasmids harboring ambler class A and C genes in Escherichia coli and Salmonella enterica from animals and humans. Antimicrob. Agents Chemother. 2015, 59, 5357–5365. [Google Scholar] [CrossRef]
- Tijet, N.; Faccone, D.; Rapoport, M.; Seah, C.; Pasterán, F.; Ceriana, P.; Albornoz, E.; Corso, A.; Petroni, A.; Melano, R.G. Molecular characteristics of mcr-1-carrying plasmids and new mcr-1 variant recovered from polyclonal clinical Escherichia coli from Argentina and Canada. PLoS ONE 2017, 12, e0180347. [Google Scholar] [CrossRef]
- Yang, Q.-E.; Sun, J.; Li, L.; Deng, H.; Liu, B.-T.; Fang, L.-X.; Liao, X.-P.; Liu, Y.-H. IncF plasmid diversity in multi-drug resistant Escherichia coli strains from animals in China. Front. Microbiol. 2015, 6, 964. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Ganaie, F.; Venugopal, N.; Mitra, S.; Shome, R.; Shome, B.R. Occurrence and characterization of genetic determinants of β-lactam-resistance in Escherichia coli clinical isolates. Infect. Genet. Evol. 2022, 100, 105257. [Google Scholar] [CrossRef]
- Soleimani, N.; Derakhshan, S.; Memariani, M. Plasmid Profile Analysis of Aminoglycoside-Resistant Escherichia coli Isolated From Urinary Tract Infections. Int. J. Enteric Pathog. 2016, 4, e33806. [Google Scholar] [CrossRef]
- Bean, D.C.; Livermore, D.M.; Hall, L.M.C. Plasmids imparting sulfonamide resistance in Escherichia coli: Implications for persistence. Antimicrob. Agents Chemother. 2009, 53, 1088–1093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.; Chen, C.; Cui, C.-Y.; Zhang, Y.; Liu, X.; Cui, Z.-H.; Ma, X.-Y.; Feng, Y.; Fang, L.-X.; Lian, X.-L.; et al. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019, 4, 1457–1464. [Google Scholar] [CrossRef]
- Basu, S.; Mukherjee, M. Incidence and risk of co-transmission of plasmid-mediated quinolone resistance and extended-spectrum β-lactamase genes in fluoroquinolone-resistant uropathogenic Escherichia coli: A first study from Kolkata, India. J. Glob. Antimicrob. Resist. 2018, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Król, J.E.; Nguyen, H.D.; Rogers, L.M.; Beyenal, H.; Krone, S.M.; Top, E.M. Increased transfer of a multidrug resistance plasmid in Escherichia coli biofilms at the air-liquid interface. Appl. Environ. Microbiol. 2011, 77, 5079–5088. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Maslennikova, I.L.; Pospelova, J.S.; Žgur Bertok, D.; Starčič Erjavec, M. Differences in recipient ability of uropathogenic Escherichia coli strains in relation with their pathogenic potential. Infect. Genet. Evol. 2022, 97, 105160. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Sun, X.; Liu, M.; Wang, H. Antimicrobial Resistance and Molecular Characterization of Gene Cassettes from Class 1 Integrons in Escherichia coli Strains. Microb. Drug Resist. 2022, 28, 413. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, C.; Diamantino, C.; Oliveira, P.L.; Reis, M.P.; Costa, P.S.; Paiva, M.C.; Nardi, R.M.D.; Magalhães, P.P.; Chartone-Souza, E.; Nascimento, A.M.A. Occurrence and characterization of class 1 integrons in Escherichia coli from healthy individuals and those with urinary infection. J. Med. Microbiol. 2017, 66, 577–583. [Google Scholar] [CrossRef]
- Javed, S.; Mirani, Z.A.; Pirzada, Z.A. Study of class 1 integrons and plasmid profile among multiple drug resistant uropathogenic Escherichia coli. Pak. J. Pharm. Sci. 2020, 33, 2643–2649. [Google Scholar] [CrossRef]
- González-Villalobos, E.; Ribas-Aparicio, R.M.; Belmont-Monroy, L.; Aparicio-Ozores, G.; Manjarrez-Hernández, H.Á.; Gavilanes-Parra, S.; Balcázar, J.L.; Molina-López, J. Identification and characterization of class 1 integrons among multidrug-resistant uropathogenic Escherichia coli strains in Mexico. Microb. Pathog. 2022, 162, 105348. [Google Scholar] [CrossRef]
- Fallah, F.; Karimi, A.; Goudarzi, M.; Shiva, F.; Navidinia, M.; Hadipour Jahromi, M.; Sajadi Nia, R.S. Determination of integron frequency by a polymerase chain reaction-restriction fragment length polymorphism method in multidrug-resistant Escherichia coli, which causes urinary tract infections. Microb. Drug Resist. 2012, 18, 546–549. [Google Scholar] [CrossRef]
- de los Santos, E.; Laviña, M.; Poey, M.E. Strict relationship between class 1 integrons and resistance to sulfamethoxazole in Escherichia coli. Microb. Pathog. 2021, 161, 105206. [Google Scholar] [CrossRef]
- Rozwadowski, M.; Gawel, D. Molecular Factors and Mechanisms Driving Multidrug Resistance in Uropathogenic Escherichia coli—An Update. Genes 2022, 13, 1397. [Google Scholar] [CrossRef]
- Pérez-Etayo, L.; Berzosa, M.; González, D.; Vitas, A.I. Prevalence of Integrons and Insertion Sequences in ESBL-Producing E. coli Isolated from Different Sources in Navarra, Spain. Int. J. Environ. Res. Public Health 2018, 15, 2308. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Aburjaile, F.; Canario, M.; Nascimento, A.M.A.; Chartone-Souza, E.; de Jesus, L.; Zamyatnin, A.A.; Brenig, B.; Barh, D.; Ghosh, P.; et al. Genomic Characterization of Multidrug-Resistant Escherichia coli BH100 Sub-strains. Front. Microbiol. 2021, 11, 549254. [Google Scholar] [CrossRef] [PubMed]
- Schink, A.K.; Kadlec, K.; Schwarz, S. Detection of qnr genes among Escherichia coli isolates of animal origin and complete sequence of the conjugative qnrB19-carrying plasmid pQNR2078. J. Antimicrob. Chemother. 2012, 67, 1099–1102. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, T.; Yu, L.; Zong, Z.; Zhao, S.; Li, R.; Wang, Q.; Yuan, L.; Hu, G.; He, D. IS 1294 Reorganizes Plasmids in a Multidrug-Resistant Escherichia coli Strain. Microbiol. Spectr. 2021, 9, e00503-21. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, A.R.; Smith, S.N.; Mobley, H.L.T. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect. Immun. 2013, 81, 3309–3316. [Google Scholar] [CrossRef]
- Mike, L.A.; Smith, S.N.; Sumner, C.A.; Eaton, K.A.; Mobley, H.L.T. Siderophore vaccine conjugates protect against Uropathogenic Escherichia coli urinary tract infection. Proc. Natl. Acad. Sci. USA 2016, 113, 13468–13473. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Alteri, C.J. Development of a Vaccine against Escherichia coli Urinary Tract Infections. Pathogens 2015, 5, 1. [Google Scholar] [CrossRef]
- Forsyth, V.S.; Himpsl, S.D.; Smith, S.N.; Sarkissian, C.A.; Mike, L.A.; Stocki, J.A.; Sintsova, A.; Alteri, C.J.; Mobley, H.L.T. Optimization of an experimental vaccine to prevent Escherichia coli urinary tract infection. MBio 2020, 11, e00555-20. [Google Scholar] [CrossRef]
- Wu, J.; Bao, C.; Lee Reinhardt, R.; Abraham, S.N. Local induction of bladder Th1 responses to combat urinary tract infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2026461118. [Google Scholar] [CrossRef]
- Lorenzo-Gómez, M.-F.; Foley, S.; Nickel, J.C.; García-Cenador, M.-B.; Padilla-Fernández, B.-Y.; González-Casado, I.; Martínez-Huélamo, M.; Yang, B.; Blick, C.; Ferreira, F.; et al. Sublingual MV140 for Prevention of Recurrent Urinary Tract Infections. NEJM Evid. 2022, 1, EVIDoa2100018. [Google Scholar] [CrossRef]
- Kelly, S.H.; Votaw, N.L.; Cossette, B.J.; Wu, Y.; Shetty, S.; Shores, L.S.; Issah, L.A.; Collier, J.H. A sublingual nanofiber vaccine to prevent urinary tract infections. Sci. Adv. 2022, 8, eabq4120. [Google Scholar] [CrossRef]
- Butler, D.; Ambite, I.; Wan, M.L.Y.; Tran, T.H.; Wullt, B.; Svanborg, C. Immunomodulation Therapy Offers New Molecular Strategies to Treat UTI. Nat. Rev. Urol. 2022, 19, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Puthia, M.; Ambite, I.; Cafaro, C.; Butler, D.; Huang, Y.; Lutay, N.; Rydström, G.; Gullstrand, B.; Swaminathan, B.; Nadeem, A.; et al. IRF7 inhibition prevents destructive innate immunity-A target for nonantibiotic therapy of bacterial infections. Sci. Transl. Med. 2016, 8, 336ra59. [Google Scholar] [CrossRef]
- Ambite, I.; Puthia, M.; Nagy, K.; Cafaro, C.; Nadeem, A.; Butler, D.S.C.; Rydström, G.; Filenko, N.A.; Wullt, B.; Miethke, T.; et al. Molecular Basis of Acute Cystitis Reveals Susceptibility Genes and Immunotherapeutic Targets. PLoS Pathog. 2016, 12, e1005848. [Google Scholar] [CrossRef]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and Evolutionary Determinants of Bacteriophage Host Range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zulk, J.J.; Clark, J.R.; Ottinger, S.; Ballard, M.B.; Mejia, M.E.; Mercado-Evans, V.; Heckmann, E.R.; Sanchez, B.C.; Trautner, B.W.; Maresso, A.W.; et al. Phage Resistance Accompanies Reduced Fitness of Uropathogenic Escherichia coli in the Urinary Environment. mSphere 2022, 7, e0034522. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Grant, R.; Chan, B.K.; Turner, P.E. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef]
- Pagnout, C.; Sohm, B.; Razafitianamaharavo, A.; Caillet, C.; Offroy, M.; Leduc, M.; Gendre, H.; Jomini, S.; Beaussart, A.; Bauda, P.; et al. Pleiotropic effects of rfa-gene mutations on Escherichia coli envelope properties. Sci. Rep. 2019, 9, 9696. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.S.; Lehman, S.M.; Tweardy, D.J.; Donlan, R.M.; Trautner, B.W. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J. Appl. Microbiol. 2012, 113, 1530–1539. [Google Scholar] [CrossRef]
- Sillankorva, S.; Oliveira, D.; Moura, A.; Henriques, M.; Faustino, A.; Nicolau, A.; Azeredo, J. Efficacy of a broad host range lytic bacteriophage against E. coli adhered to urothelium. Curr. Microbiol. 2011, 62, 1128–1132. [Google Scholar] [CrossRef]
- Valério, N.; Oliveira, C.; Jesus, V.; Branco, T.; Pereira, C.; Moreirinha, C.; Almeida, A. Effects of single and combined use of bacteriophages and antibiotics to inactivate Escherichia coli. Virus Res. 2017, 240, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Nath, G.; Dhameja, N.; Kumar, R.; Aseri, G.K.; Jain, N. Bacteriophage therapy for Escherichia coli-induced urinary tract infection in rats. Future Microbiol. 2023, 18, 323–334. [Google Scholar] [CrossRef]
- Mijbel Ali, B.; Gatea Kaabi, S.A.; Al-Bayati, M.A.; Musafer, H.K. A Novel Phage Cocktail Therapy of the Urinary Tract Infection in a Mouse Model. Arch. Razi Inst. 2021, 76, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Nang, S.C.; Zhao, J.; Yu, H.H.; Li, J.; Gill, J.J.; Liu, M.; Aslam, S. Therapeutic Potential of Intravenous Phage as Standalone Therapy for Recurrent Drug-Resistant Urinary Tract Infections. Antimicrob. Agents Chemother. 2023, 67, e00037-23. [Google Scholar] [CrossRef]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef]
- Loubet, P.; Ranfaing, J.; Dinh, A.; Dunyach-Remy, C.; Bernard, L.; Bruyère, F.; Lavigne, J.P.; Sotto, A. Alternative Therapeutic Options to Antibiotics for the Treatment of Urinary Tract Infections. Front. Microbiol. 2020, 11, 1509. [Google Scholar] [CrossRef]
- Greene, S.E.; Pinkner, J.S.; Chorell, E.; Dodson, K.W.; Shaffer, C.L.; Conover, M.S.; Livny, J.; Hadjifrangiskou, M.; Almqvist, F.; Hultgren, S.J. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. MBio 2014, 5. [Google Scholar] [CrossRef]
- Aberg, V.; Almqvist, F. Pilicides-small molecules targeting bacterial virulence. Org. Biomol. Chem. 2007, 5, 1827–1834. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Aberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef]
- Baloyi, I.T.; Cosa, S.; Combrinck, S.; Leonard, C.M.; Viljoen, A.M. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. S. Afr. J. Bot. 2019, 122, 484–491. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-formulations of drugs: Recent developments, impact and challenges. Biochimie 2016, 128–129, 99–112. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing--oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Sil, P.C. Role of nanostructures in improvising oral medicine. Toxicol. Rep. 2019, 6, 358–368. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Zhao, J.; Xu, R. Recent Advances in Oral Nano-Antibiotics for Bacterial Infection Therapy. Int. J. Nanomed. 2020, 15, 9587–9610. [Google Scholar] [CrossRef]
- Khare, T.; Mahalunkar, S.; Shriram, V.; Gosavi, S.; Kumar, V. Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ. Res. 2021, 199, 111321. [Google Scholar] [CrossRef]
- Mukherjee, R.; Patra, M.; Dutta, D.; Banik, M.; Basu, T. Tetracycline-loaded calcium phosphate nanoparticle (Tet-CPNP): Rejuvenation of an obsolete antibiotic to further action. Biochim. Biophys. Acta 2016, 1860, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Urinary Tract Infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Huwaimel, B.; Alobaida, A.; Hussain, T.; Rafi, Z.; Mehmood, K.; Abdallah, M.H.; Al Hagbani, T.; Rizvi, S.M.D.; Moin, A.; et al. Delafloxacin-Capped Gold Nanoparticles (DFX-AuNPs): An Effective Antibacterial Nano-Formulation of Fluoroquinolone Antibiotic. Materials 2022, 15, 5709. [Google Scholar] [CrossRef] [PubMed]
| Recommended First-Line Treatment | Recommended Empirical Treatments | |
|---|---|---|
| Uncomplicated cystitis | Fosfomycin trometamol (Oral) | Trimethoprim |
| Nitrofurantoin | Trimethoprim/sulfamethoxazole | |
| Cephalosporins (alternative) | ||
| Pivmecillinam | ||
| Uncomplicated pyelonephritis | Fluoroquinolones | Ciprofloxacin |
| Levofloxacin | ||
| Trimethoprim/sulfamethoxazole | ||
| Cefpodoxime or Ceftibuten | ||
| Complicated UTIs | Amoxicillin plus an aminoglycoside | 3rd generation cephalosporin used intravenously |
| 2nd generation cephalosporin plus an aminoglycoside |
| Adhesions | Genes | Host Receptor |
|---|---|---|
| Type 1 Fimbriae | fimABCDEFGHI [72] | D-mannose-containing receptors, uroplakins [23] |
| P Fimbriae | papABCDEFG [84] | mannose-resistant binding to globoseries glycosphingolipids [24] |
| Afa/Dr | afaABCDEF [96] | decay-accelerating factor, carcinoembryonic antigens [100,101] |
| S Fimbriae | sfaABCDEFGHS [149] | Sialo-glycoproteins [89,92] |
| Type one secretion A | tosRCBDAEF [106] | human kidney and epithelial cells [106] |
| Toxins | Genes | Effect in host |
| α-hemolysin | hlyCABD [125,126] | PBK and ACLY inhibition, GM-CSF upregulation [34,127,128] |
| Cytotoxic necrotizing factor | cnf1 [136] | Inhibits Rho family of GTPases [129] |
| Serine protease autotransporter | sat [146] | Induces vacuolation in glomeruli and proximal tubules, degrades complement proteins [36,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whelan, S.; Lucey, B.; Finn, K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms 2023, 11, 2169. https://doi.org/10.3390/microorganisms11092169
Whelan S, Lucey B, Finn K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms. 2023; 11(9):2169. https://doi.org/10.3390/microorganisms11092169
Chicago/Turabian StyleWhelan, Shane, Brigid Lucey, and Karen Finn. 2023. "Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment" Microorganisms 11, no. 9: 2169. https://doi.org/10.3390/microorganisms11092169
APA StyleWhelan, S., Lucey, B., & Finn, K. (2023). Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms, 11(9), 2169. https://doi.org/10.3390/microorganisms11092169






