Prokaryote Distribution Patterns along a Dissolved Oxygen Gradient Section in the Tropical Pacific Ocean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Station Locations and Sampling Process
2.2. Physical and Chemical Analyses
2.3. DNA Extraction, 16S rRNA Polymerase Chain Reaction (PCR) Amplification, and Sequencing
2.4. Bioinformatics Analysis and Statistical Analysis
2.5. Primers for Quantitative PCR (qPCR)
2.6. qPCR Analysis
2.7. Preparation of qPCR Standards
3. Results and Discussion
3.1. Temperature and Salinity Profiles
3.2. Dissolved Oxygen (DO) Profile
3.3. Nutrients and Chlorophyll a
3.4. Patterns in Prokaryotic Diversity
3.5. OTUs Change with Dissolved Oxygen
3.6. Community Composition and Structure
3.7. Vertical Distribution of Thaumarchaeota and Thaumarchaeota Ecotypes
3.8. Vertical Distribution of Nitrospinae and Nitrospinae Ecotypes
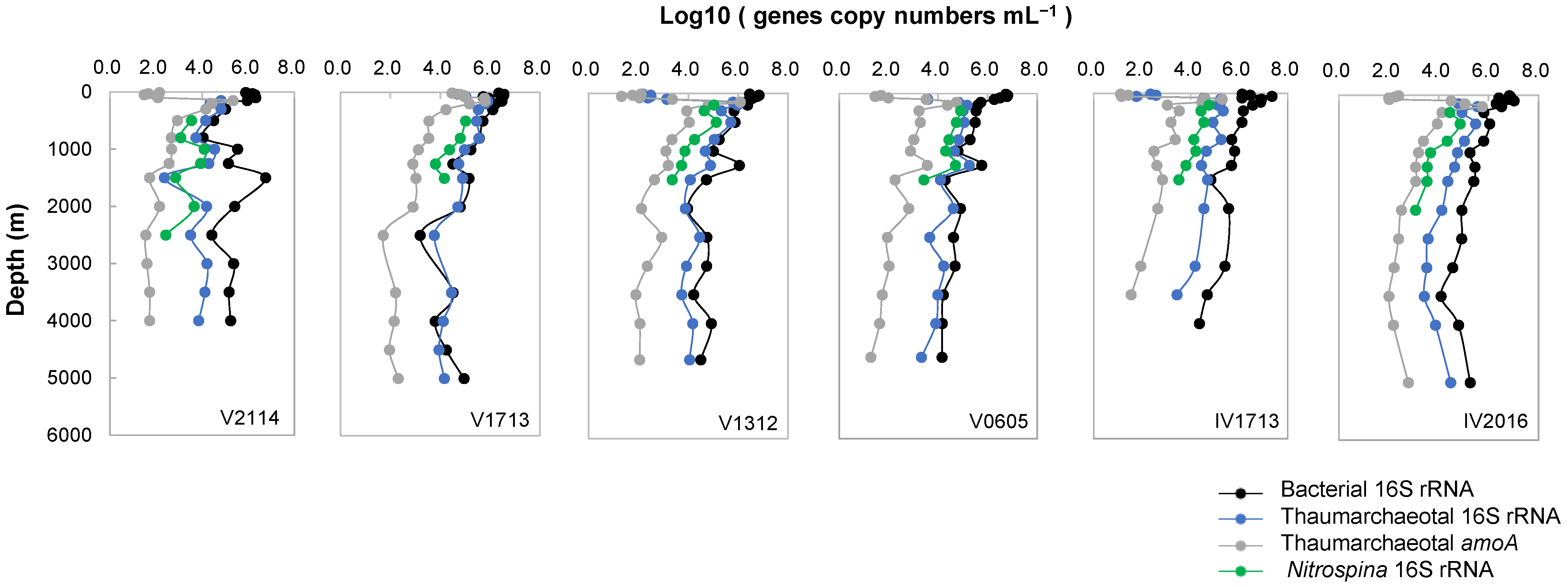
3.9. Quantitative PCR Analysis
3.10. Correlation Analysis and Potential Mechanisms of the Nitrifying Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breitburg, D.L.; Crump, B.C.; Dabiri, J.O.; Gallegos, C.L. Ecosystem engineers in the pelagic realm: Alteration of habitat by species ranging from microbes to jellyfish. Integr. Comp. Biol. 2010, 50, 188–200. [Google Scholar] [CrossRef]
- Wyrtki, K. The oxygen minima in relation to ocean circulation. Deep Sea Res. Oceanogr. Abstr. 1962, 9, 11–23. [Google Scholar] [CrossRef]
- Karstensen, J.; Stramma, L.; Visbeck, M. Oxygen minimum zones in the eastern tropical Atlantic and Pacific oceans. Prog. Oceanogr. 2008, 77, 331–350. [Google Scholar] [CrossRef]
- Beman, J.M.; Carolan, M.T. Deoxygenation alters bacterial diversity and community composition in the ocean’s largest oxygen minimum zone. Nat. Commun. 2013, 4, 2705. [Google Scholar] [CrossRef]
- Wright, J.J.; Konwar, K.M.; Hallam, S.J. Microbial ecology of expanding oxygen minimum zones. Nat. Rev. Microbiol. 2012, 10, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Kuypers, M.M. Microbial nitrogen cycling processes in oxygen minimum zones. Annu. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Lavik, G.; Stührmann, T.; Brüchert, V.; van der Plas, A.; Mohrholz, V.; Lam, P.; Mussmann, M.; Fuchs, B.M.; Amann, R.; Lass, U.; et al. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature 2009, 457, 581–584. [Google Scholar] [CrossRef]
- Karner, M.B.; DeLong, E.F.; Karl, D.M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 2001, 409, 507–510. [Google Scholar] [CrossRef]
- Tolar, B.B.; King, G.M.; Hollibaugh, J.T. An analysis of thaumarchaeota populations from the northern gulf of Mexico. Front. Microbiol. 2013, 4, 72. [Google Scholar] [CrossRef]
- Ulloa, O.; Canfield, D.E.; DeLong, E.F.; Letelier, R.M.; Stewart, F.J. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl. Acad. Sci. USA 2012, 109, 15996–16003. [Google Scholar] [CrossRef]
- Peng, X.; Fuchsman, C.A.; Jayakumar, A.; Warner, M.J.; Devol, A.H.; Ward, B.B. Revisiting nitrification in the Eastern Tropical South Pacific: A focus on controls. J. Geophys. Res. Oceans 2016, 121, 1667–1684. [Google Scholar] [CrossRef]
- DeLong, E.F.; Preston, C.M.; Mincer, T.; Rich, V.; Hallam, S.J.; Frigaard, N.U.; Martinez, A.; Sullivan, M.B.; Edwards, R.; Brito, B.R. Community genomics among stratified microbial assemblages in the ocean’s interior. Science 2006, 311, 496–503. [Google Scholar] [CrossRef]
- Levipan, H.A.; Molina, V.; Fernandez, C. Nitrospina-like bacteria are the main drivers of nitrite oxidation in the seasonal upwelling area of the Eastern South Pacific (Central Chile ∼36 °S). Environ. Microbiol. Rep. 2014, 6, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.B. Nitrification in marine systems. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 199–262. [Google Scholar]
- Zhang, Y.; Qin, W.; Hou, L.; Zakem, E.J.; Wan, X.; Zhao, Z.; Liu, L.; Hunt, K.A.; Jiao, N.; Kao, S.J.; et al. Nitrifier adaptation to low energy flux controls inventory of reduced nitrogen in the dark ocean. Proc. Natl. Acad. Sci. USA 2020, 117, 4823–4830. [Google Scholar] [CrossRef]
- Peng, X.; Fawcett, S.E.; van Oostende, N.; Wolf, M.J.; Marconi, D.; Sigman, D.M.; Ward, B.B. Nitrogen uptake and nitrification in the subarctic North Atlantic Ocean. Limnol. Oceanogr. 2018, 63, 1462–1487. [Google Scholar] [CrossRef]
- Clark, D.R.; Rees, A.P.; Joint, I. Ammonium regeneration and nitrification rates in the oligotrophic Atlantic Ocean: Implications for new production estimates. Limnol. Oceanogr. 2008, 53, 52–62. [Google Scholar] [CrossRef]
- Ward, B.B. Nitrogen transformations in the Southern California Bight. Deep Sea Res. Part I Oceanogr. Res. Pap. 1987, 34, 785–805. [Google Scholar] [CrossRef]
- Kalvelage, T.; Jensen, M.M.; Contreras, S.; Revsbech, N.P.; Lam, P.; Günter, M.; LaRoche, J.; Lavik, G.; Kuypers, M.M. Oxygen sensitivity of anammox and coupled N-cycle processes in oxygen minimum zones. PLoS ONE 2011, 6, e29299. [Google Scholar] [CrossRef]
- Jensen, M.M.; Kuypers, M.M.M.; Lavik, G.; Thamdrup, B. Rates and regulation of anaerobic ammonium oxidation and denitrification in the Black Sea. Limnol. Oceanogr. 2008, 53, 23–36. [Google Scholar] [CrossRef]
- Lipschultz, F.; Wofsy, S.C.; Ward, B.B.; Codispoti, L.A.; Friedrich, G.; Elkins, J.W. Bacterial transformations of inorganic nitrogen in the oxygen-deficient waters of the Eastern Tropical South Pacific Ocean. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1513–1541. [Google Scholar] [CrossRef]
- Bristow, L.A.; Dalsgaard, T.; Tiano, L.; Mills, D.B.; Thamdrup, B. Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proc. Natl. Acad. Sci. USA 2016, 113, 10601–10606. [Google Scholar] [CrossRef]
- Sun, X.; Frey, C.; Garcia-Robledo, E.; Jayakumar, A.; Ward, B.B. Microbial niche differentiation explains nitrite oxidation in marine oxygen minimum zones. ISME J. 2021, 15, 1317–1329. [Google Scholar] [CrossRef]
- Stramma, L.; Johnson, G.C.; Sprintall, J.; Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science 2008, 320, 655–658. [Google Scholar] [CrossRef]
- Carpenter, J.H. The accuracy of the winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- GB/T 12763.4-2007; Specifications for Oceanographic Survey—Part 4: Survey of Chemical Parameters in Sea Water. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Certification and Accreditation Administration: Beijing, China, 2007.
- Strickland, J.; Parsons, T.R. A Practical Handbook of Seawater Analysis, Ottawa, Canada. Fish. Res. Board Can. Bull. 1972, 167, 129. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Su-Cheng, P.; Yang, C.C.; Riley, J.P. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex. Anal. Chim. Acta 1990, 229, 115–120. [Google Scholar] [CrossRef]
- Fanning, K.A.; Pilson, M. On the spectrophotometric determination of dissolved silica in natural waters. Anal. Chim. Acta 1973, 45, 136–140. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; pp. 107–109. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 3 February 2021).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Moore, D.S.; Notz, W.I.; Flinger, M.A. The Basic Practice of Statistics, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2013. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Coskun, Ö.K.; Ortega-Arbulú, A.S.; Conci, N.; Wörheide, G.; Vargas, S.; Orsi, W.D. A 16S rRNA gene sequencing and analysis protocol for the Illumina MiniSeq platform. MicrobiologyOpen 2018, 7, e00611. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Zeng, Y.; Fan, Y.; Chen, H.; Yuan, C.; Li, Y.; Huang, M.; Shi, X.; He, P. Diversity and distribution of archaeal and bacterial nitrifiers in deep oceans. J. Sea Res. 2023, 193, 102389. [Google Scholar] [CrossRef]
- Mincer, T.J.; Church, M.J.; Taylor, L.T.; Preston, C.; Karl, D.M.; DeLong, E.F. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 2007, 9, 1162–1175. [Google Scholar] [CrossRef]
- Wuchter, C.; Abbas, B.; Coolen, M.J.; Herfort, L.; van Bleijswijk, J.; Timmers, P.; Strous, M.; Teira, E.; Herndl, G.J.; Middelburg, J.J.; et al. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. USA 2006, 103, 12317–12322. [Google Scholar] [CrossRef]
- Stubner, S. Enumeration of 16S rDNA of Desesulfotomaculum lineage 1 in rice field soil by real-time PCR with sybrgreen detection. J. Microbiol. Methods 2002, 50, 155–164. [Google Scholar] [CrossRef]
- Kamykowski, D.; Zentara, S.-J. Hypoxia in the world ocean as recorded in the historical data set. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1861–1874. [Google Scholar] [CrossRef]
- Bryant, J.A.; Stewart, F.J.; Eppley, J.M.; DeLong, E.F. Microbial community phylogenetic and trait diversity declines with depth in a marine oxygen minimum zone. Ecology 2012, 93, 1659–1673. [Google Scholar] [CrossRef]
- Mattes, T.E.; Ingalls, A.E.; Burke, S.; Morris, R.M. Metabolic flexibility of SUP05 under low DO growth conditions. Environ. Microbiol. 2021, 23, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Emery, W.J. Water Types and Water Masses. Encycl. Ocean Sci. 2001, 4, 3179–3187. [Google Scholar]
- Morris, R.M.; Rappé, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Dekaezemacker, J.; Turk-Kubo, K.A.; Moutin, T.; Hamersley, R.M.; Grosso, O.; Zehr, J.P.; Capone, D.G. Aphotic N2 fixation in the Eastern Tropical South Pacific Ocean. PLoS ONE 2013, 8, e81265. [Google Scholar] [CrossRef]
- Fernandez, C.; Farias, L.; Ulloa, O. Nitrogen fixation in denitrified marine waters. PLoS ONE 2011, 6, e20539. [Google Scholar] [CrossRef]
- Turk-Kubo, K.A.; Karamchandani, M.; Capone, D.G.; Zehr, J.P. The paradox of marine heterotrophic nitrogen fixation: Abundances of heterotrophic diazotrophs do not account for nitrogen fixation rates in the Eastern Tropical South Pacific. Environ. Microbiol. 2014, 16, 3095–3114. [Google Scholar] [CrossRef]
- Ivars-Martinez, E.; Martin-Cuadrado, A.B.; D’Auria, G.; Mira, A.; Ferriera, S.; Johnson, J.; Friedman, R.; Rodriguez-Valera, F. Comparative genomics of two ecotypes of the marine planktonic copiotroph Alteromonas macleodii suggests alternative lifestyles associated with different kinds of particulate organic matter. ISME J. 2008, 2, 1194–1212. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Landa, M.; Burns, A.S.; Durham, B.P.; Esson, K.; Nowinski, B.; Sharma, S.; Vorobev, A.; Nielsen, T.; Kiene, R.P.; Moran, M.A. Sulfur metabolites that facilitate oceanic phytoplankton-bacteria carbon flux. ISME J. 2019, 13, 2536–2550. [Google Scholar] [CrossRef] [PubMed]
- Hansman, R.L.; Griffin, S.; Watson, J.T.; Druffel, E.R.; Ingalls, A.E.; Pearson, A.; Aluwihare, L.I. The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc. Natl. Acad. Sci. USA 2009, 106, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Swan, B.K.; Martinez-Garcia, M.; Preston, C.M.; Sczyrba, A.; Woyke, T.; Lamy, D.; Reinthaler, T.; Poulton, N.J.; Masland, E.D.; Gomez, M.L.; et al. Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. Science 2011, 333, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; Vergin, K.L. Seasonality in ocean microbial communities. Science 2012, 335, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Bonin, P.; Vieira, C.; Grimaud, R.; Militon, C.; Cuny, P.; Lima, O.; Guasco, S.; Brussaard, C.P.; Michotey, V. Substrates specialization in lipid compounds and hydrocarbons of Marinobacter genus. Environ. Sci. Pollut. Res. Int. 2015, 22, 15347–15359. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Golyshin, P.N.; Crisafi, F.; Denaro, R.; Giuliano, L. Marine, aerobic hydrocarbon-degrading Gammaproteobacteria: The family Alcanivoracaceae. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Teramoto, M.; Suzuki, M.; Hatmanti, A.; Harayama, S. The potential of Cycloclasticus and Altererythrobacter strains for use in bioremediation of petroleum-aromatic-contaminated tropical marine environments. J. Biosci. Bioeng. 2010, 110, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, J.D.; Schäfer, H.; Cox, M.J.; Boden, R.; McDonald, I.R.; Murrell, J.C. Stable-isotope probing implicates Methylophaga spp. and novel Gammaproteobacteria in marine methanol and methylamine metabolism. ISME J. 2007, 1, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Ruff, S.E.; Arnds, J.; Knittel, K.; Amann, R.; Wegener, G.; Ramette, A.; Boetius, A. Microbial communities of deep-sea methane seeps at Hikurangi continental margin (New Zealand). PLoS ONE 2013, 8, e72627. [Google Scholar] [CrossRef]
- Wang, S.; Mu, D.; Du, Z.J. Persicimonas caeni gen. nov, sp. nov, the representative of a novel wide-ranging predatory taxon in Bradymonadales. Front. Microbiol. 2020, 11, 698. [Google Scholar] [CrossRef]
- van Vliet, D.M.; von Meijenfeldt, F.A.B.; Dutilh, B.E.; Villanueva, L.; Sinninghe Damsté, J.S.; Stams, A.J.M.; Sánchez-Andrea, I. The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environ. Microbiol. 2021, 3, 2834–2857. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, P.; Zhang, C.; Zhang, C.; Du, J.; Du, X.; Zhu, C.; Liu, J.; Xie, P.; Li, S. Comparative study of the gut microbiota among four different marine mammals in an aquarium. Front. Microbiol. 2021, 12, 769012. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.M.; Zhou, Y.L.; Almeida, A.; Finn, R.D.; Danchin, A.; He, L.S. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genom. 2020, 21, 408. [Google Scholar] [CrossRef]
- Fincker, M.; Huber, J.A.; Orphan, V.J.; Rappé, M.S.; Teske, A.; Spormann, A.M. Metabolic strategies of marine subseafloor Chloroflexi inferred from genome reconstructions. Environ. Microbiol. 2020, 22, 3188–3204. [Google Scholar] [CrossRef]
- Kalanetra, K.M.; Bano, N.; Hollibaugh, J.T. Ammonia-oxidizing Archaea in the Arctic Ocean and Antarctic coastal waters. Environ. Microbiol. 2009, 11, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Long, E.; Evans, F.; Demaere, M.Z.; Lauro, F.M.; Raftery, M.J.; Ducklow, H.; Grzymski, J.J.; Murray, A.E.; Cavicchioli, R.A. A metaproteomic assessment of winter and summer bacterioplankton from Antarctic Peninsula coastal surface waters. ISME J. 2012, 6, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Mincer, T.J.; Schleper, C.; Preston, C.M.; Roberts, K.; Richardson, P.M.; DeLong, E.F. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biol. 2006, 4, e95. [Google Scholar] [CrossRef] [PubMed]
- Beman, J.M.; Popp, B.N.; Francis, C.A. Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J. 2008, 2, 429–441. [Google Scholar] [CrossRef]
- Pachiadaki, M.G.; Sintes, E.; Bergauer, K.; Brown, J.M.; Record, N.R.; Swan, B.K.; Mathyer, M.E.; Hallam, S.J.; Lopez-Garcia, P.; Takaki, Y.; et al. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science 2017, 358, 1046–1051. [Google Scholar] [CrossRef]
- Newell, S.E.; Fawcett, S.E.; Ward, B.B. Depth distribution of ammonia oxidation rates and ammonia-oxidizer community composition in the Sargasso Sea. Limnol. Oceanogr. 2013, 58, 1491–1500. [Google Scholar] [CrossRef]
- Church, M.J.; Wai, B.; Karl, D.M.; DeLong, E.F. Abundances of crenarchaeal amoA genes and transcripts in the Pacific Ocean. Environ. Microbiol. 2010, 12, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.E.; Saito, A.M.A.; Goepfert, T.J.; Lamborg, C.H.; Dupont, C.L.; DiTullio, G.R. Thaumarchaeal ecotype distributions across the equatorial Pacific Ocean and their potential roles in nitrification and sinking flux attenuation. Limnol. Oceanogr. 2017, 62, 1984–2003. [Google Scholar] [CrossRef]
- Sintes, E.; De Corte, D.; Haberleitner, E.; Herndl, G.J. Geographic distribution of archaeal ammonia oxidizing ecotypes in the Atlantic Ocean. Front. Microbiol. 2016, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Teira, E.; Lebaron, P.; van Aken, H.; Herndl, G.J. Distribution and activity of bacteria and archaea in the deep water masses of the North Atlantic. Limnol. Oceanogr. 2006, 51, 2131–2144. [Google Scholar] [CrossRef]
- Tyrrell, T.; Holligan, P.M.; Mobley, C.D. Optical impacts of oceanic coccolithophore blooms. J. Geophys. Res. Oceans 1999, 104, 3223–3241. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Pike, S.; Maiti, K.; Lamborg, C.H.; Siegel, D.A.; Trull, T.W. Thorium-234 as a tracer of spatial, temporal and vertical variability in particle flux in the North Pacific. Deep Sea Res. 2009, 56, 1143–1167. [Google Scholar] [CrossRef]
- Grzymski, J.J.; Riesenfeld, C.S.; Williams, T.J.; Dussaq, A.M.; Ducklow, H.; Erickson, M.; Cavicchioli, R.; Murray, A.E. A metagenomic assessment of winter and summer bacterioplankton from Antarctica peninsula coastal surface waters. ISME J. 2012, 6, 1901–1915. [Google Scholar] [CrossRef]
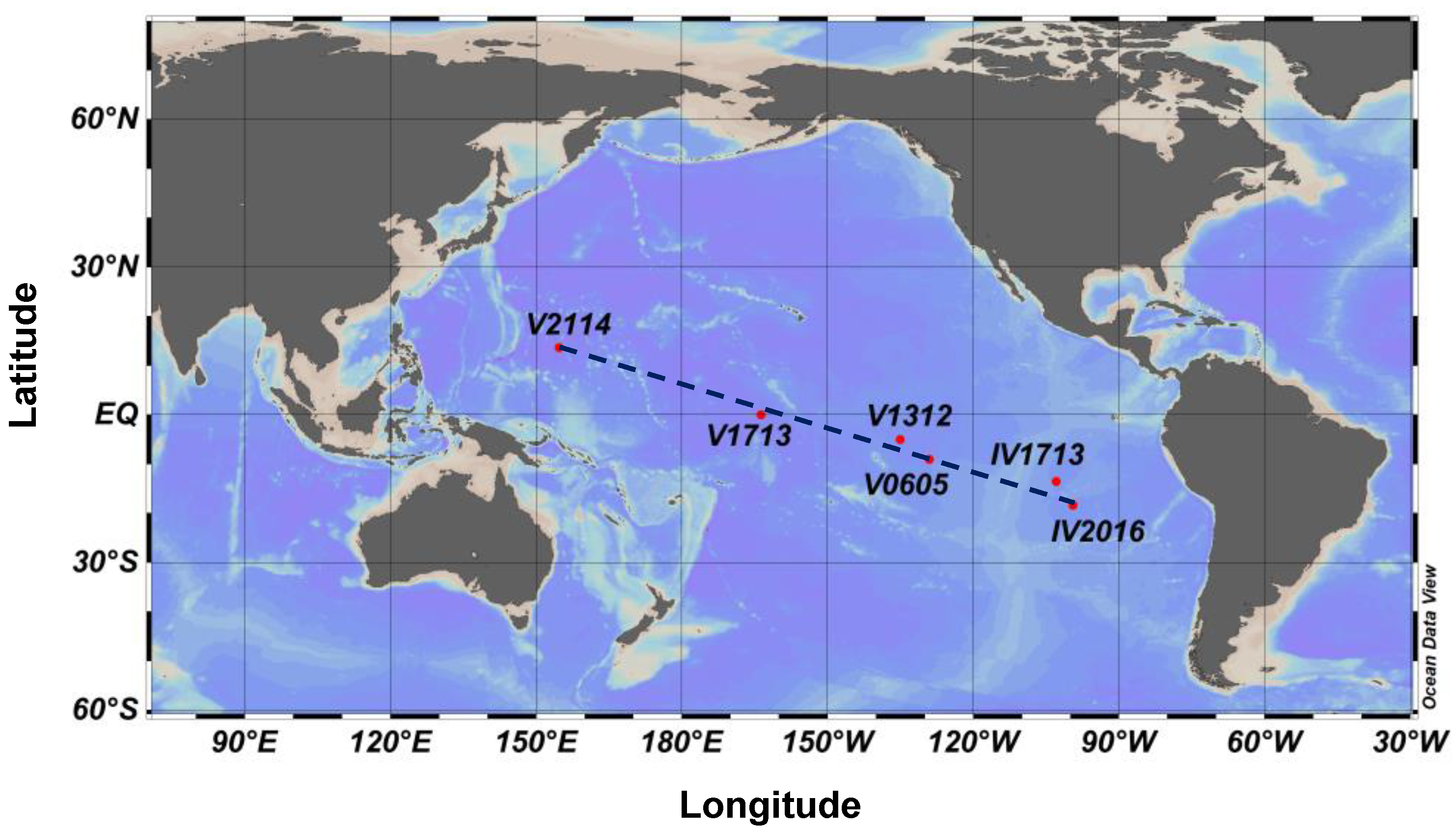

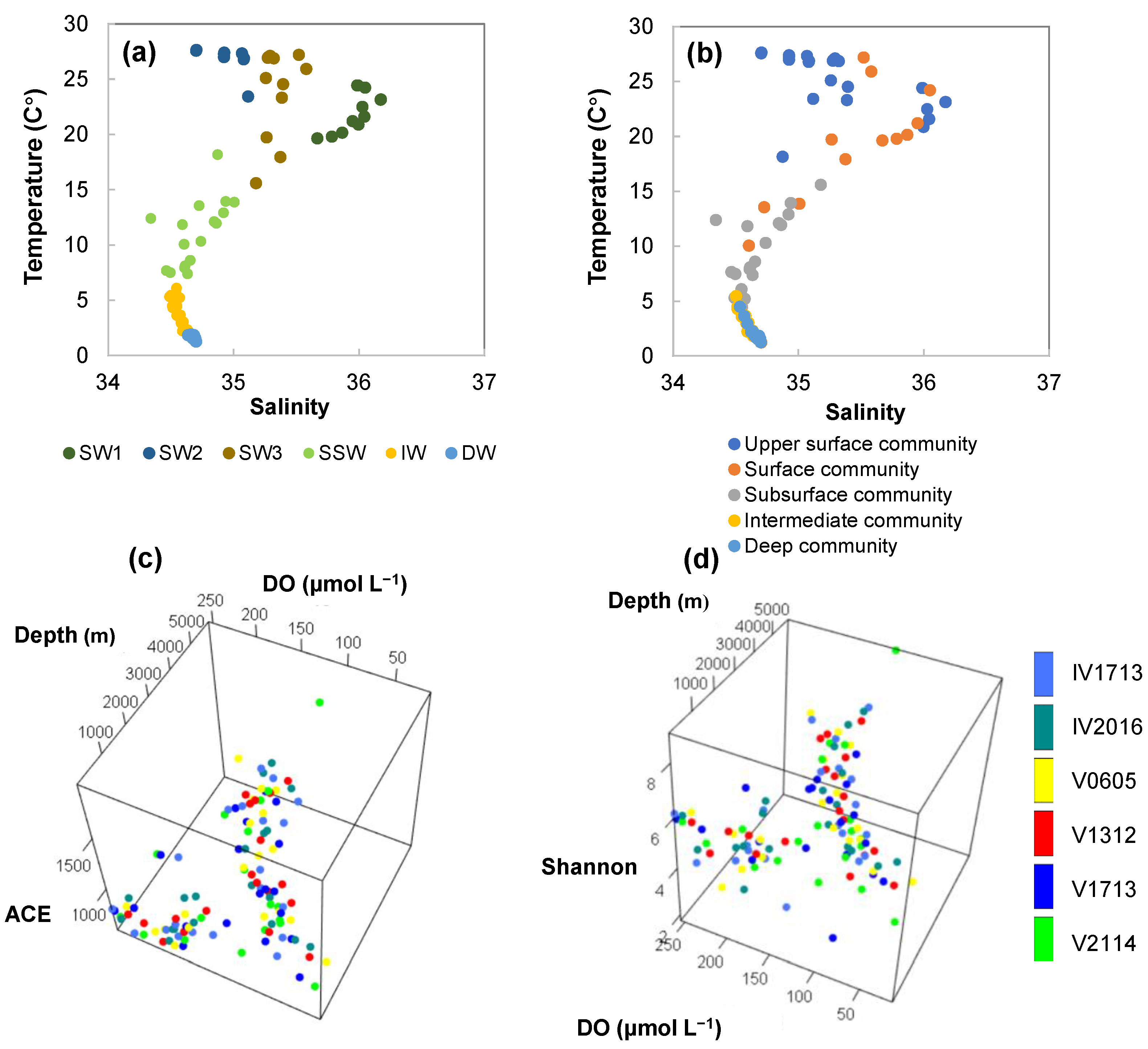


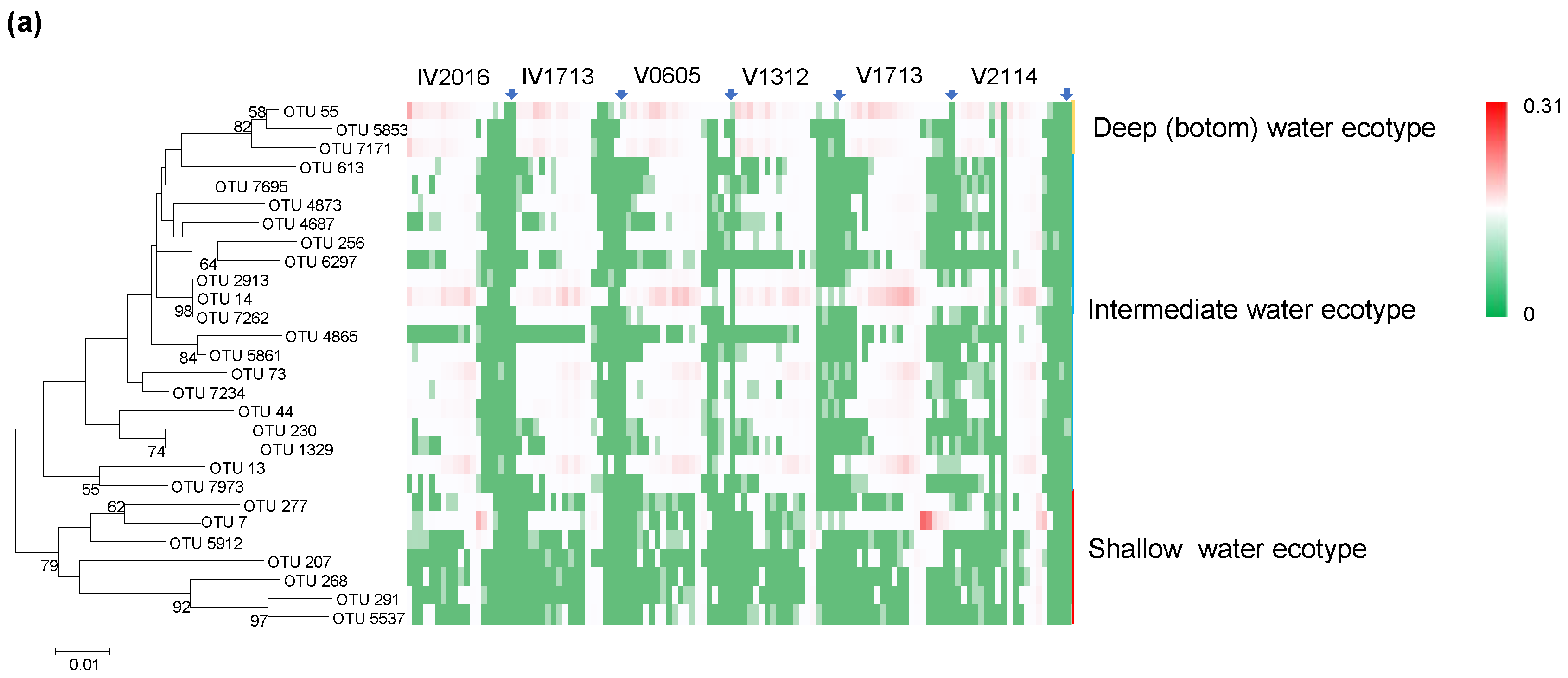
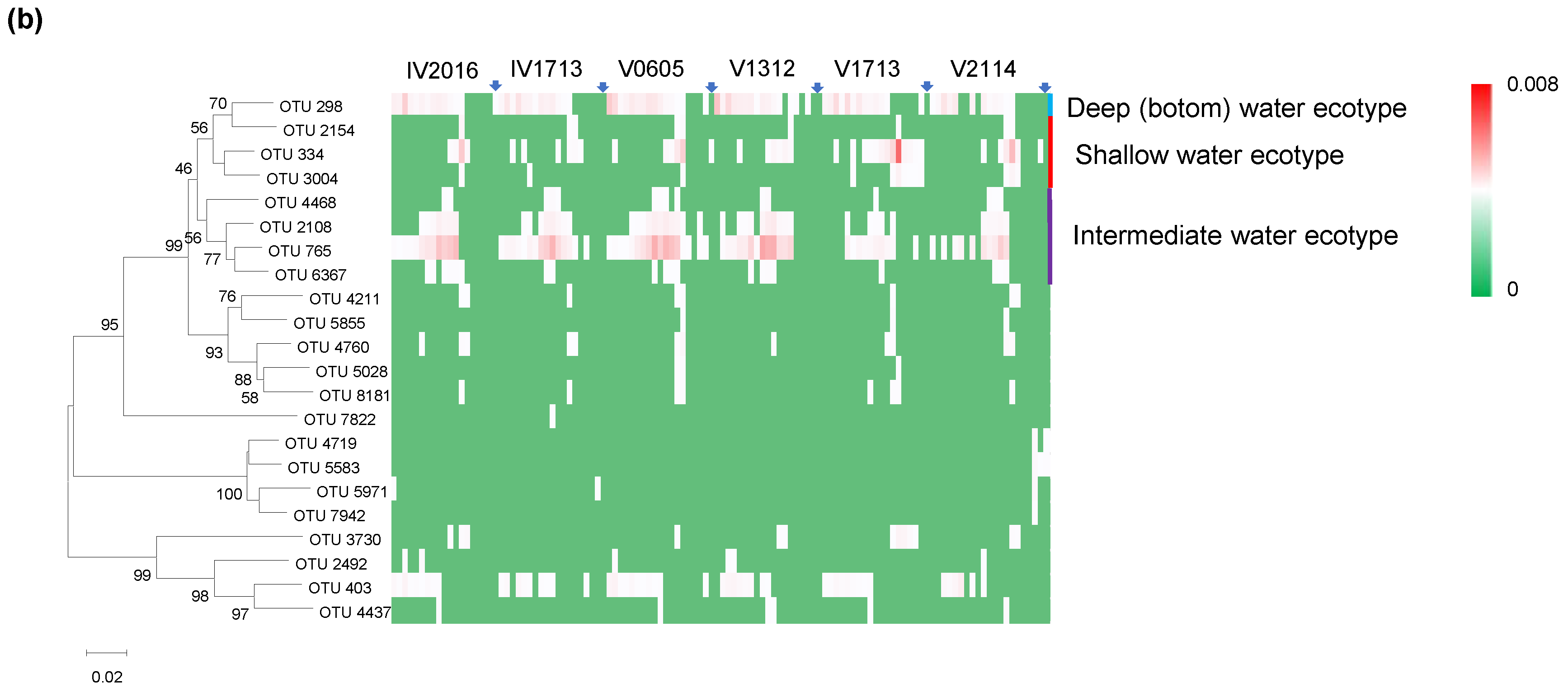

| Genes | Primers and Sequences (5′–3′) | Fragment Length (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| Bacterial 16S rRNA gene | 515f (GTGYCAGCMGCCGCGGTAA) 806r (GGACTACNVGGGTWTCTAAT) | 291 | 55 | [41] |
| Thaumarchaeotal 16S rRNA gene | TH95f (AATAAGGGGTGGGCA) TH279r (TACCGTCTACCTCTCCCACT) | 184 | 53 | [42] |
| Nitrospina 16S rRNA gene | NitSSU130f (GGGTGAGTAACACGTGAATAA) NitSSU282r (TCAGGCCGGCTAAMCA) | 152 | 49 | [43] |
| Thaumarchaeotal amoA gene | Arch-amoAf (CTGAYTGGGCYTGGACATC) Arch-amoAr (TTCTTCTTTGTTGCCCAGTA) | 256 | 54 | [44] |
| Water Masses | SW1 | SW2 | SW3 | SSW | IW | DW |
|---|---|---|---|---|---|---|
| Temperature (°C) | 19.65–24.43 | 23.43–27.65 | 15.59–27.20 | 7.38–18.16 | 2.22–6.09 | 1.25–1.88 |
| Salinity | 35.67–36.17 | 34.70–35.11 | 35.18–35.58 | 34.34–35.01 | 34.49–34.64 | 34.64–34.70 |
| Station | Depth (m) | |||||
| V2114 | – | 10–150 | - | 200–500 | 800–2000 | ≥2500 |
| V1713 | – | 10 | 30–200 | 300–500 | 800–2000 | ≥2500 |
| V1312 | – | 10–50 | 75 and 100 | 150–500 | 800–2000 | ≥2500 |
| V0605 | 100 and 150 | – | 10–75 | 200–500 | 800–2000 | ≥2500 |
| IV1713 | 10–100 | – | 150 | 200–500 | 800–2000 | ≥2500 |
| IV2016 | 30–200 | – | 10 | 300–500 | 800–2000 | ≥2500 |
| Domain | Phylum (Class) | OTUs | Number of OTUs | Positive + Negative − |
|---|---|---|---|---|
| Archaea | Euryarchaeota | Thermoplasmata | 4 | − |
| Marine Group III | 1 | − | ||
| Thaumarchaeota | Nitrosopumilaceae | 15 | − | |
| Bacteria | Marinimicrobia | Unclassified | 6 | − |
| Acidobacteria | Unclassified | 3 | − | |
| Verrucomicrobia | Unclassified | 4 | − | |
| Roseibacillus | 1 | − | ||
| Actinobacteria | Acidimicrobiia | 3 | − | |
| Solirubrobacterales | 1 | − | ||
| Corynebacterium lipophiloflavum | 1 | − | ||
| Bacteroidetes | Cytophagales | 1 | − | |
| Flavobacteriales | 1 | − | ||
| Flavobacteriaceae | 4 | + | ||
| Gemmatimonadetes | Unclassified | 3 | − | |
| Nitrospinae | Nitrospina | 2 | − | |
| Cyanobacteria | Unclassified | 2 | + | |
| Synechococcales | 2 | + | ||
| Phalacroma | 2 | + | ||
| Aureococcus anophagefferens | 1 | + | ||
| Planctomycetes | Unclassified | 2 | − | |
| Alphaproteobacteria | Unclassified | 6 | − | |
| Unclassified | 8 | + | ||
| Rhodobacteraceae | 2 | + | ||
| Rhodospirillales | 3 | − | ||
| Rickettsiales | 5 | + | ||
| Sneathiellaceae | 1 | − | ||
| Candidatus Pelagibacter ubique | 3 | + | ||
| Candidatus Puniceispirillum | 1 | + | ||
| Deltaproteobacteria | Unclassified | 2 | − | |
| SAR324 | 4 | − | ||
| Gammaproteobacteria | SAR86 | 2 | − | |
| Candidatus Thioglobus | 2 | − | ||
| Prochlorococcus | 1 | + | ||
| Unclassified | 2 | + | ||
| Unclassified | 8 | − | ||
| Arenicellaceae | 1 | − | ||
| Pseudohongiella | 1 | − | ||
| Thiotrichaceae | 1 | + | ||
| SUP05 | 3 | − | ||
| Woeseia | 1 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Wang, H.; Shi, J.; Xin, M.; Wang, W.; Xie, L.; Wei, Q.; Huang, M.; Shi, X.; Fan, Y.; et al. Prokaryote Distribution Patterns along a Dissolved Oxygen Gradient Section in the Tropical Pacific Ocean. Microorganisms 2023, 11, 2172. https://doi.org/10.3390/microorganisms11092172
He P, Wang H, Shi J, Xin M, Wang W, Xie L, Wei Q, Huang M, Shi X, Fan Y, et al. Prokaryote Distribution Patterns along a Dissolved Oxygen Gradient Section in the Tropical Pacific Ocean. Microorganisms. 2023; 11(9):2172. https://doi.org/10.3390/microorganisms11092172
Chicago/Turabian StyleHe, Peiqing, Huan Wang, Jie Shi, Ming Xin, Weimin Wang, Linping Xie, Qinsheng Wei, Mu Huang, Xuefa Shi, Yaqin Fan, and et al. 2023. "Prokaryote Distribution Patterns along a Dissolved Oxygen Gradient Section in the Tropical Pacific Ocean" Microorganisms 11, no. 9: 2172. https://doi.org/10.3390/microorganisms11092172




