Epinephrine Affects Ribosomes, Cell Division, and Catabolic Processes in Micrococcus luteus Skin Strain C01: Revelation of the Conditionally Extensive Hormone Effect Using Orbitrap Mass Spectrometry and Proteomic Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
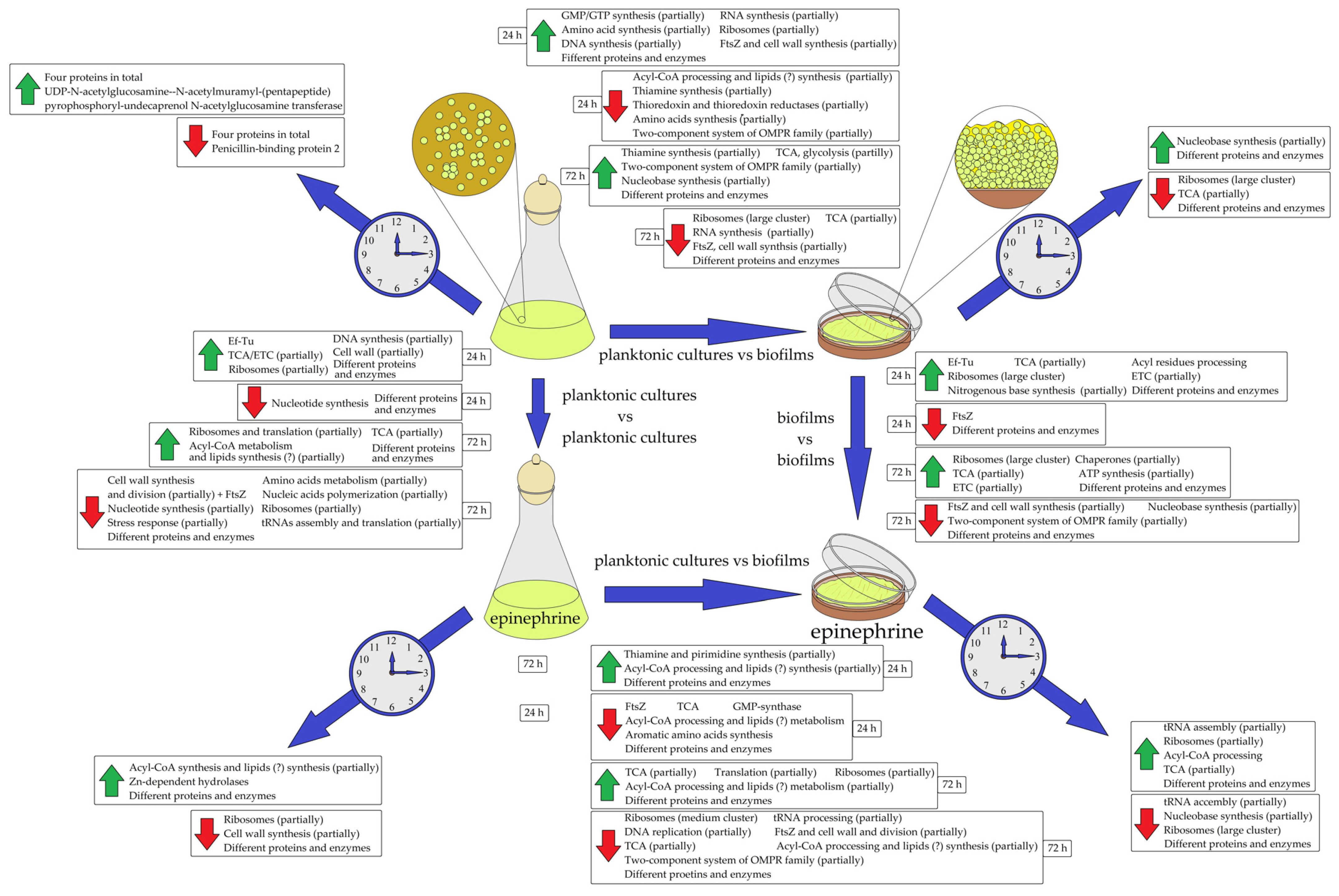
3.1. Proteome Composition of M. luteus C01: General Remarks
3.2. How the Proteome of Planktonic Cultures and Biofilms Changes between 24 h and 72 h Samples: Analysis of Differences within the Groups of Control and Epinephrine Samples
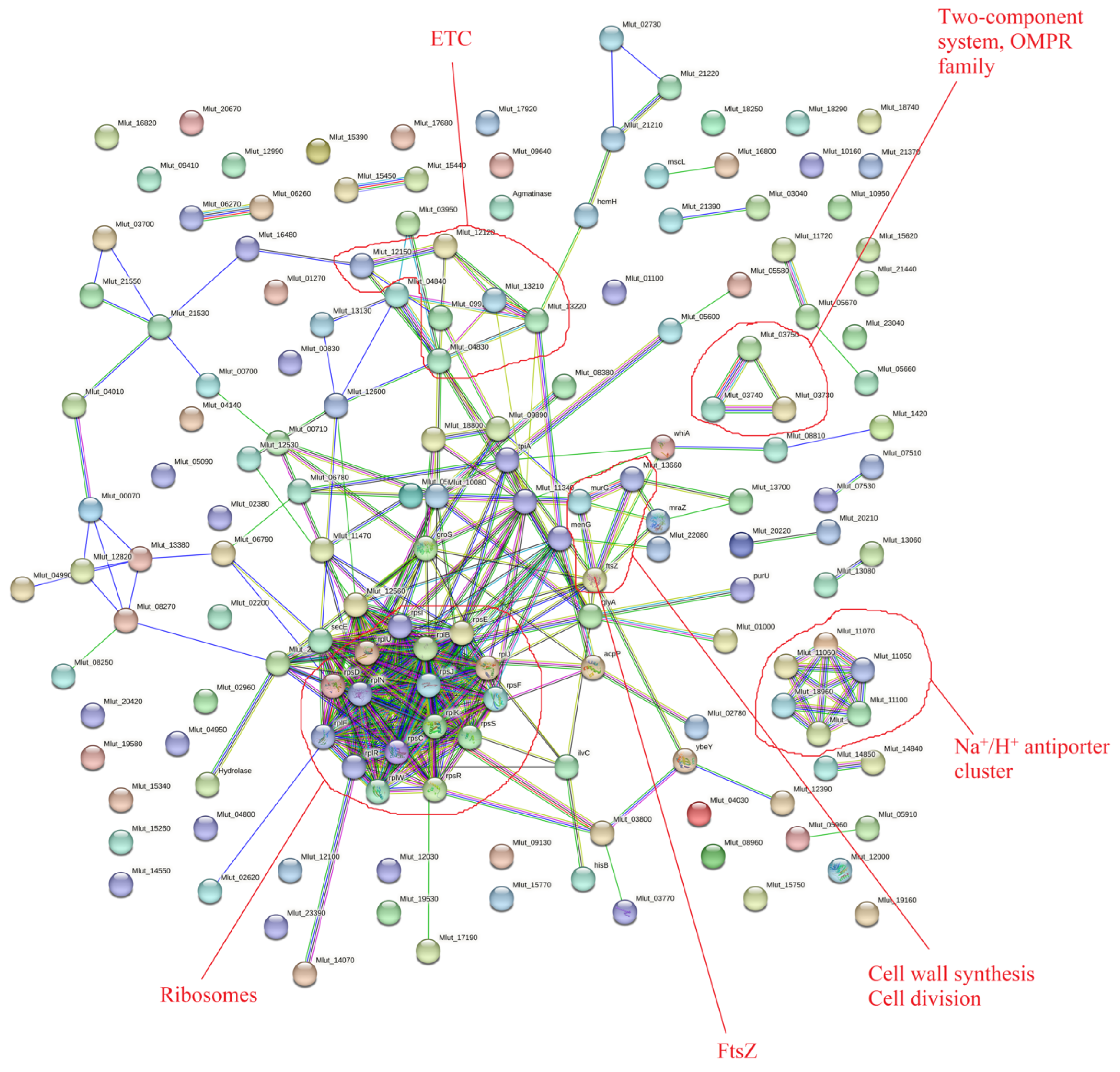
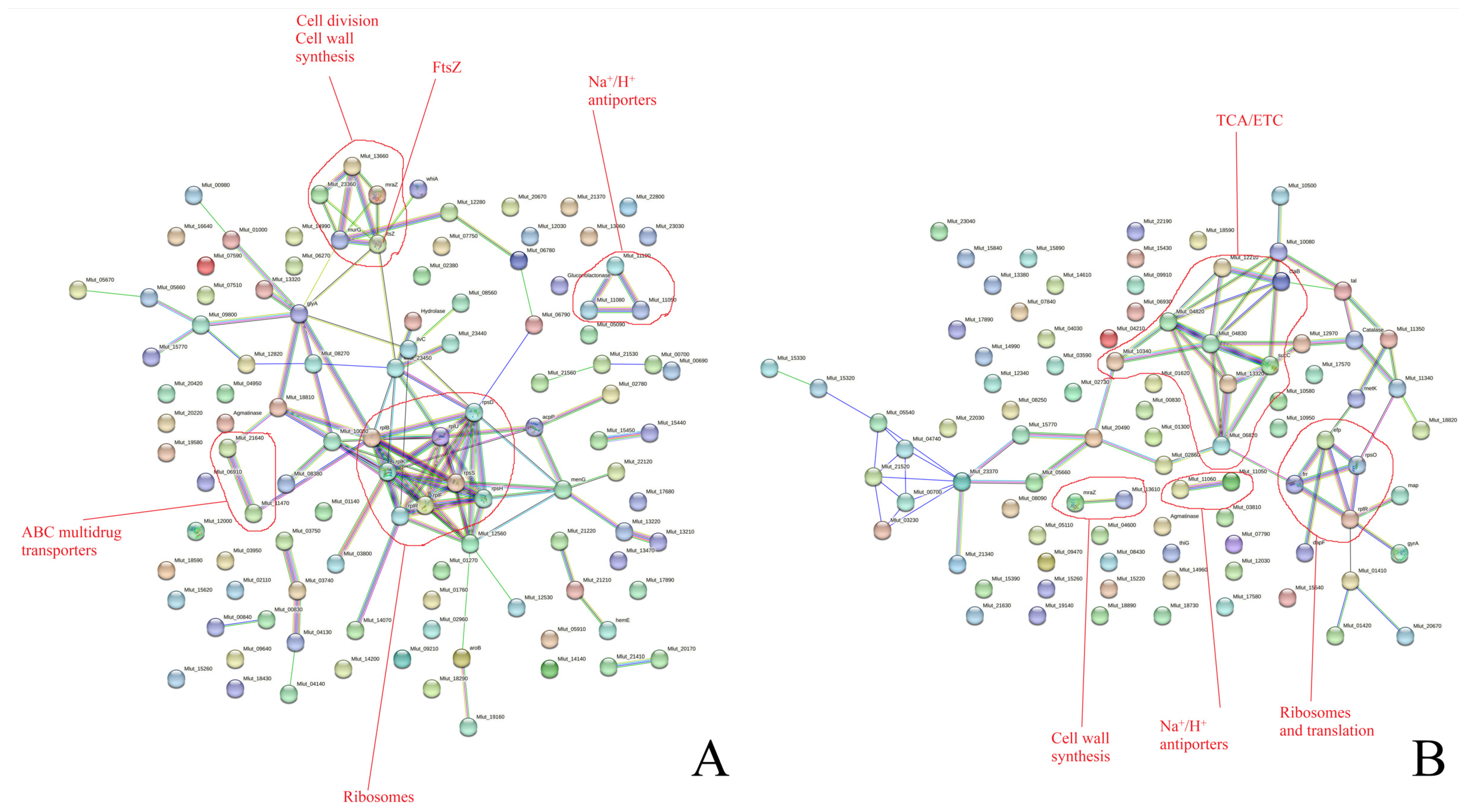
3.2.1. Proteomic Changes in Planktonic Cultures and Biofilms during Long-Term Incubation and in Control and Epinephrine Sample Groups
3.2.2. Proteomic Changes in Planktonic Cultures in Comparison with Biofilms in Control and Epinephrine Sample Groups
3.3. The Comparison of Control and Epinephrine Samples of Planktonic Cultures and Biofilms: Effect of the Hormone in Planktonic Culture and Biofilm Sample Groups
3.3.1. The Effect of Epinephrine on M. luteus C01 Planktonic Cultures
3.3.2. The Effect of Epinephrine on M. luteus C01 Biofilms
3.4. Comparison with the Biofilm Matrix Proteome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luqman, A. The orchestra of human bacteriome by hormones. Microb. Pathog. 2023, 180, 106125. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 3–24. [Google Scholar]
- Moreira, C.G.; Sperandio, V. The epinephrine/norepinephrine/autoinducer-3 interkingdom signaling system in Escherichia coli O157:H7. In Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health; Springer: New York, NY, USA, 2010; pp. 213–227. [Google Scholar]
- Borrel, V.; Thomas, P.; Catovic, C.; Racine, P.-J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Duclairoir-Poc, C.; Zouboulis, C.C.; Feuilloley, M.G.J. Acne and stress: Impact of catecholamines on Cutibacterium acnes. Front. Med. 2019, 6, 155–167. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Schelkunov, M.I.; Geras’kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Danilova, N.D.; Ovcharova, M.A.; Mart’yanov, S.V.; Pankratov, T.A.; Muzychenko, D.S.; et al. Epinephrine affects gene expression levels and has a complex effect on biofilm formation in Micrococcus luteus strain C01 isolated from human skin. Biofilm 2021, 3, 100058. [Google Scholar] [CrossRef] [PubMed]
- Mart’yanov, S.V.; Botchkova, E.A.; Plakunov, V.K.; Gannesen, A.V. The impact of norepinephrine on mono-species and dual-species staphylococcal biofilms. Microorganisms 2021, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Reading, N.C.; Rasko, D.A.; Torres, A.G.; Sperandio, V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 5889–5894. [Google Scholar] [CrossRef] [PubMed]
- Casida, L.E., Jr. Death of Micrococcus luteus in soil. Appl. Environ. Microbiol. 1980, 39, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, K.; Krishnaveni, M. Antibacterial activity of pigment produced from Micrococcus luteus KF532949. Int. J. Chem. Anal. Sci. 2013, 4, 149–152. [Google Scholar] [CrossRef]
- Laba, W.; Choinska, A.; Rodziewicz, A.; Piegza, M. Keratinolytic abilities of Micrococcus luteus from poultry waste. Braz. J. Microbiol. 2015, 46, 691–700. [Google Scholar] [CrossRef]
- Sher, S.; Hussain, S.Z.; Rehman, A. Phenotypic and genomic analysis of multiple heavy metal–resistant Micrococcus luteus strain AS2 isolated from industrial waste water and its potential use in arsenic bioremediation. Appl. Microbiol. Biotechnol. 2020, 104, 2243–2254. [Google Scholar] [CrossRef]
- Kloos, W.E.; Musselwhite, M.S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 1975, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Lange-Asschenfeld, B.; Marenbach, D.; Lang, C.; Patzelt, A.; Ulrich, M.; Maltusch, A.; Terhorst, D.; Stockfleth, E.; Sterry, W.; Lademann, J. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Ski. Pharmacol. Physiol. 2011, 24, 305–311. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Ianniello, N.M.; Andrade, D.C.; Ivancic, S.; Eckardt, P.A.; Ramirez, J.C.L. Native valve infective endocarditis due to Micrococcus luteus in a non-Hodgkin’s lymphoma patient. IDCases 2019, 18, e00657. [Google Scholar] [CrossRef]
- Rodriguez-Nava, G.; Mohamed, A.; Yanez-Bello, M.A.; Trelles-Garcia, D.P. Advances in medicine and positive natural selection: Prosthetic valve endocarditis due to biofilm producer Micrococcus luteus. IDCases 2020, 20, e00743. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical characteristics of patients with Micrococcus luteus bloodstream infection in a Chinese Tertiary-Care Hospital. Polish J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aung, T.T.; Chaudhuri, D. The first case of native mitral valve endocarditis due to Micrococcus luteus and review of the literature. Case Rep. Cardiol. 2019, 2019, 5907319. [Google Scholar] [CrossRef]
- Kiseleva, A.A.; Solovyeva, T.V.; Ovcharova, M.A.; Geras’kina, O.V.; Mart’yanov, S.V.; Cherdyntseva, T.A.; Danilova, N.D.; Zhurina, M.V.; Botchkova, E.A.; Feofanov, A.V.; et al. Effect of β-estradiol on mono-and mixed-species biofilms of human commensal bacteria Lactobacillus paracasei AK508 and Micrococcus luteus C01 on different model surfaces. Coatings 2022, 12, 436. [Google Scholar] [CrossRef]
- Matsuura, K.; Asano, Y.; Yamada, A.; Naruse, K. Detection of Micrococcus luteus biofilm formation in microfluidic environments by pH measurement using an ion-sensitive field-effect transistor. Sensors 2013, 13, 2484–2493. [Google Scholar] [CrossRef]
- Blakeman, J.T.; Morales-García, A.L.; Mukherjee, J.; Gori, K.; Hayward, A.S.; Lant, N.J.; Geoghegan, M. Extracellular DNA provides structural integrity to a Micrococcus luteus biofilm. Langmuir 2019, 35, 6468–6475. [Google Scholar] [CrossRef]
- Danilova, N.D.; Solovyeva, T.V.; Mart’Yanov, S.V.; Zhurina, M.V.; Gannesen, A.V. Stimulatory effect of epinephrine on biofilms of Micrococcus luteus C01. Microbiology 2020, 89, 493–497. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Ziganshin, R.H.; Zdorovenko, E.L.; Klimko, A.I.; Ianutsevich, E.A.; Danilova, O.A.; Tereshina, V.M.; Gorbachevskii, M.V.; Ovcharova, M.A.; Nevolina, E.D.; et al. Epinephrine extensively changes the biofilm matrix composition in Micrococcus luteus C01 isolated from human skin. Front. Microbiol. 2022, 13, 1003942. [Google Scholar] [CrossRef] [PubMed]
- Zhurina, M.V.; Gannesen, A.V.; Mart’Yanov, S.V.; Teteneva, N.A.; Shtratnikova, V.Y.; Plakunov, V.K. Niclosamide as a promising antibiofilm agent. Microbiology 2017, 86, 455–462. [Google Scholar] [CrossRef]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and Simple Preparation of Ultrahigh-performance Capillary Columns for LC-MS*[S]. Mol. Cell. Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Perry, R.P. Balanced production of ribosomal proteins. Gene 2007, 401, 1–3. [Google Scholar] [CrossRef]
- Kadowaki, T.; Nishiyama, Y.; Hisabori, T.; Hihara, Y. Identification of OmpR-family response regulators interacting with thioredoxin in the cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2015, 10, e0119107. [Google Scholar]
- Racine, P.J.; Janvier, X.; Clabaut, M.; Catovic, C.; Souak, D.; Boukerb, A.M.; Groboillot, A.; Konto-Ghiorghi, Y.; Duclairoir-Poc, C.; Lesouhaitier, O.; et al. Dialog between skin and its microbiota: Emergence of “Cutaneous Bacterial Endocrinology”. Exp. Dermatol. 2020, 29, 790–800. [Google Scholar] [CrossRef]
- Carmina, E.; Stanczyk, F.Z.; Lobo, R.A. Evaluation of Hormonal Status. In Yen & Jaffe’s Reproductive Endocrinology E-Book: Physiology, Pathophysiology, and Clinical Management, 8th ed.; Strauss, J.F., III, Barbieri, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 887–915. [Google Scholar]
- Louis, M.; Clamens, T.; Tahrioui, A.; Desriac, F.; Rodrigues, S.; Rosay, T.; Harmer, N.; Diaz, S.; Barreau, M.; Racine, P.J.; et al. Pseudomonas aeruginosa biofilm dispersion by the human atrial natriuretic peptide. Adv. Sci. 2022, 9, 2103262. [Google Scholar] [CrossRef] [PubMed]
- Kalaycı-Yüksek, F.; Gümüş, D.; Anğ-Küçüker, M. Hormones Can Influence Antibiotic Susceptibilities Even in Mono-and Co-Culture Conditions. Acta Biol. Marisiensis 2021, 4, 39–49. [Google Scholar] [CrossRef]
- Centeleghe, I.; Norville, P.; Hughes, L.; Maillard, J.Y. Dual species dry surface biofilms; Bacillus species impact on Staphylococcus aureus survival and surface disinfection. J. Appl. Microbiol. 2022, 133, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, N.; Sadiq, F.A.; He, G. Interspecies Interactions in Dual-Species Biofilms Formed by Psychrotrophic Bacteria and the Tolerance of Sessile Communities to Disinfectants. J. Food Protection. 2020, 83, 951–958. [Google Scholar] [CrossRef]
- Ovcharova, M.A.; Geraskina, O.V.; Danilova, N.D.; Botchkova, E.A.; Martyanov, S.V.; Feofanov, A.V.; Plakunov, V.K.; Gannesen, A.V. Atrial natriuretic peptide affects skin commensal Staphylococcus epidermidis and Cutibacterium acnes dual-species biofilms. Microorganisms 2021, 9, 552. [Google Scholar] [CrossRef]
- Ovcharova, M.A.; Schelkunov, M.I.; Geras’kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Martyanov, S.V.; Nevolina, E.D.; Zhurina, M.V.; Feofanov, A.V.; Botchkova, E.A.; et al. C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms. Biology 2023, 12, 436. [Google Scholar] [CrossRef]
- Diuvenji, E.V.; Nevolina, E.D.; Mart’yanov, S.V.; Zhurina, M.A.; Kalmantaeva, O.V.; Makarova, M.A.; Botchkova, E.A.; Firstova, V.V.; Plakunov, V.K.; Gannesen, A.V. Binary biofilms of Staphylococcus aureus 209P and Kytococcus schroeteri H01: Dualistic role of kytococci and cell adhesion alterations in the presence of the A-type natriuretic peptide. Microbiology 2022, 91, 563–576. [Google Scholar] [CrossRef]
- Lephart, E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin—Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Wang, M.; Zhao, H.; Zhao, F. Advances on Hormones in Cosmetics: Illegal Addition Status, Sample Preparation, and Detection Technology. Molecules 2023, 28, 1980. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Tian, Q.; Stepaniants, S.B.; Mao, M.; Collins, S.J.; Hanlon, W.A.; Hood, L.E. Integrated genomic and proteomic analyses of gene expression in mammalian cells. Mol. Cell. Proteom. 2004, 3, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Hwa, T. Shaping bacterial gene expression by physiological and proteome allocation constraints. Nat. Rev. Microbiol. 2023, 21, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Chen, Y.; Kuo, J.T.; Lai, Y.C.; Wu, C.C.; Huang, C.F.; Lin, C.T. Phosphorylated OmpR is required for type 3 fimbriae expression in Klebsiella pneumoniae under hypertonic conditions. Front. Microbiol. 2018, 9, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Hewapathirana, S.; García-Seisdedos, D.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Freriks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A Hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, 543–552. [Google Scholar] [CrossRef]

| Fold Change | Log Student’s t-Test p-Value | Student’s t-Test Significant | Student’s t-Test q-Value | Andromeda Score | Peptides | Unique Peptides | Majority Protein IDs | Protein Name |
|---|---|---|---|---|---|---|---|---|
| 0.046 | 0.0000575 | + | 0.000 | 167.950 | 11 | 11 | A0A4Y8PNI0 | Acetohydroxy acid synthase small subunit |
| 0.367 | 0.0000009 | + | 0.000 | 35.724 | 6 | 5 | A0A5F0I5D1 | UDP-N-acetylglucosamine–N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase |
| 0.611 | 0.0000143 | + | 0.000 | 70.148 | 12 | 8 | C5CA30 | DASS family sodium-coupled anion symporter |
| 0.677 | 0.0001048 | + | 0.040 | 202.960 | 9 | 9 | D3LL93 | PKD domain-containing protein luteus |
| 1.765 | 0.0000462 | + | 0.000 | 190.430 | 8 | 6 | A0A562G5G6 | Enoyl-CoA hydratase/carnithine racemase luteus |
| 2.069 | 0.0000536 | + | 0.000 | 138.000 | 7 | 1 | A0A5F0IA97 | tRNA(Ile)-lysidine synthase |
| 12.426 | 0.0000283 | + | 0.000 | 100.360 | 8 | 2 | A0A562FUE9 | Penicillin-binding protein 2 |
| 31.097 | 0.0000167 | + | 0.000 | 3.151 | 17 | 0 | A0A6N4C4E7 | Adenylosuccinate lyase |
| Fold Change | Student’s t-Test p-Value | Student’s t-Test Significant | Student’s t-Test q-Value | Andromeda Score | Peptides | Unique Peptides | Majority Protein IDs | Protein Name |
|---|---|---|---|---|---|---|---|---|
| 0.069341 | 0.000904343 | + | 0.055778 | 4.3162 | 38 | 1 | A0A5F0IA49 | AMP-dependent synthetase |
| 0.078911 | 0.000186892 | + | 0.031636 | 53.67 | 30 | 0 | D3LKU1 | Gamma-glutamyl phosphate reductase |
| 0.080156 | 6.10057 × 10−5 | + | 0.044 | 10.718 | 12 | 0 | A0A378NGS6 | NUDIX domain |
| 0.106835 | 5.3882 × 10−5 | + | 0.055 | 323.31 | 34 | 1 | A0A6N4C449 | Methylcrotonoyl-CoA carboxylase |
| 0.130453 | 0.000716674 | + | 0.051625 | 11.453 | 19 | 0 | A0A6N4F6V6 | Sulfurtransferase |
| 0.142135 | 0.000366145 | + | 0.032952 | 323.31 | 16 | 8 | A0A5F0IA94 | 3-hydroxyisobutyrate dehydrogenase |
| 0.145986 | 0.000147486 | + | 0.0348 | 301.7 | 28 | 1 | A0A5F0I7U0 | Acetyl/propionyl-CoA carboxylase subunit alpha |
| 0.154537 | 0.000474668 | + | 0.054 | 102.96 | 17 | 5 | A0A509Y5D9 | Long-chain fatty acid–CoA ligase |
| 0.161394 | 3.96082 × 10−5 | + | 0.073333 | 323.31 | 39 | 0 | C5C8P4 | Acyl-CoA synthetase/AMP-acid ligase |
| 0.180196 | 0.000301722 | + | 0.033111 | 128.37 | 13 | 13 | A0A1M7AJD2 | Glutaryl-CoA dehydrogenase |
| 0.191962 | 0.000600747 | + | 0.054571 | 14.963 | 18 | 1 | A0A6N4FA22 | Lysine–tRNA ligase |
| 0.195861 | 2.78807 × 10−6 | + | 0.096 | 323.31 | 17 | 7 | A0A653J4T6 | Acyl-CoA dehydrogenase |
| 0.207599 | 0.000241677 | + | 0.03725 | 323.31 | 13 | 7 | A0A031GG35 | GroES-like protein |
| 0.220707 | 0.00020476 | + | 0.029 | 205.13 | 18 | 0 | A0A2N6RHX4 | Acyl-CoA synthetase |
| 0.23262 | 0.000575452 | + | 0.056593 | 323.31 | 20 | 1 | A0A4Y8PJX4 | Acetyl-CoA C-acetyltransferase |
| 0.245841 | 0.000497764 | + | 0.0568 | 323.31 | 27 | 2 | A0A5E8QG57 | Enoyl-CoA hydratase/isomerase family protein |
| 0.250082 | 0.000268107 | + | 0.035059 | 323.31 | 23 | 23 | A0A1M7AJR6 | KR domain-containing protein |
| 0.273894 | 0.0009515 | + | 0.0502 | 237.02 | 21 | 19 | A0A509Y4M5 | Enoyl-CoA hydratase |
| 0.282804 | 6.30992 × 10−5 | + | 0.031429 | 115.88 | 22 | 0 | A0A5F0IB54 | 3-hydroxyacyl-CoA dehydrogenase |
| 0.310135 | 0.000949188 | + | 0.051487 | 51.029 | 6 | 6 | A0A5F0IBJ1 | N-acetyltransferase |
| 0.347456 | 0.001145003 | + | 0.05234 | 323.31 | 30 | 3 | A0A4Y8ZJ73 | Aldehyde dehydrogenase family protein |
| 0.371518 | 0.00068292 | + | 0.055067 | 64.194 | 12 | 3 | A0A5F0IAP2 | Acyl-CoA dehydrogenase OX = 1391911 |
| 0.417764 | 0.000218222 | + | 0.036308 | 296.24 | 20 | 20 | A0A031IKQ5 | Alpha-ketoacid dehydrogenase subunit beta |
| 0.422328 | 0.000110642 | + | 0.024444 | 9.4998 | 50 | 0 | A0A4U1LK82 | Aldehyde dehydrogenase family protein |
| 0.451917 | 0.000898155 | + | 0.057371 | 264.55 | 13 | 0 | D3LRU4 | GroES-like protein SK58 |
| 0.483193 | 0.000621288 | + | 0.05269 | 98.964 | 10 | 10 | A0A031G8N2 | ATP-grasp superfamily enzyme |
| 0.50694 | 0.00098185 | + | 0.048976 | 161.74 | 8 | 1 | C5C833 | Predicted Zn-dependent hydrolase of beta-lactamase |
| 0.525612 | 0.001371883 | + | 0.0492 | 323.31 | 17 | 1 | C5CBR0 | Iron-regulated ABC transporter ATPase subunit SufC |
| 0.543036 | 0.001042716 | + | 0.048696 | 277.87 | 20 | 20 | A0A2N6RPP3 | Pyruvate dehydrogenase (Acetyl-transferring) E1 component subunit alpha |
| 0.593636 | 0.000842068 | + | 0.055882 | 323.31 | 51 | 37 | A0A031GU24 | Acyl-CoA synthetase (NDP forming) |
| 0.730556 | 0.001022347 | + | 0.048091 | 187.01 | 18 | 16 | A0A378NRK2 | 4-hydroxy-3-methylbut-2-enyl diphosphate reductase |
| 0.787446 | 7.26814 × 10−5 | + | 0.0275 | 323.31 | 34 | 1 | A0A562FU00 | Gamma-glutamyl phosphate reductase |
| 0.863303 | 0.000219033 | + | 0.033714 | 78.573 | 11 | 1 | A0A378NK55 | Farnesyl diphosphate synthase |
| 1.354815 | 0.000808918 | + | 0.057576 | 259.17 | 21 | 21 | A0A5F0I7M5 | 4-aminobutyrate–2-oxoglutarate transaminase |
| 1.395987 | 0.00102733 | + | 0.047022 | 27.183 | 4 | 4 | A0A5E8QGG7 | Vitamin K epoxide reductase |
| 1.463339 | 0.000229784 | + | 0.031467 | 21.431 | 6 | 6 | A0A378NL43 | HTH-type transcriptional regulator gltC |
| 1.510947 | 0.000996293 | + | 0.046698 | 125.83 | 12 | 12 | A0A031IW78 | Glutamate racemase |
| 1.581977 | 0.000349861 | + | 0.036421 | 278.5 | 25 | 25 | C5CC59 | 50S ribosomal protein L2 |
| 1.609 | 0.001319842 | + | 0.05125 | 81.518 | 8 | 8 | D3LR26 | 30S ribosomal protein S19 |
| 1.671859 | 0.000985313 | + | 0.04781 | 149.72 | 13 | 13 | A0A562FW33 | 5-oxoprolinase subunit A |
| 1.777925 | 0.000927898 | + | 0.05427 | 59.002 | 10 | 10 | A0A6N4FDF3 | Pantothenate kinase |
| 1.842939 | 0.00052194 | + | 0.054615 | 323.31 | 93 | 0 | A0A5F0I860 | DNA-directed RNA polymerase subunit beta |
| 1.895257 | 0.000947341 | + | 0.052842 | 196.1 | 19 | 19 | A0A562FSS5 | 50S ribosomal protein L3 |
| 2.401196 | 1.73241 × 10−5 | + | 0.11 | 5.7013 | 2 | 2 | A0A2N6RLH0 | Methyl viologen resistance protein SmvA |
| 2.782218 | 0.000355313 | + | 0.0346 | 81.878 | 6 | 6 | A0A2N6RLI0 | TetR/AcrR family transcriptional regulator |
| 2.947873 | 0.000401354 | + | 0.04087 | 75.154 | 23 | 19 | A0A378NLV1 | Linear gramicidin synthase subunit D |
| 3.011034 | 0.001340022 | + | 0.050204 | 23.859 | 2 | 1 | D3LMW7 | Sortase family protein |
| 3.126505 | 0.00039416 | + | 0.037091 | 106.79 | 10 | 9 | A0A132HXJ2 | Siderophore-interacting protein |
| 5.335081 | 0.000709395 | + | 0.05329 | 81.796 | 8 | 2 | A0A562FUE9 | PKD domain-containing protein |
| 14.36734 | 6.13161 × 10−5 | + | 0.036667 | 10.524 | 19 | 0 | A0A653IYK6 | UDP-N-acetylmuramoylalanine–D-glutamate ligase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gannesen, A.V.; Ziganshin, R.H.; Ovcharova, M.A.; Nevolina, E.D.; Klimko, A.I.; Martyanov, S.V.; Plakunov, V.K. Epinephrine Affects Ribosomes, Cell Division, and Catabolic Processes in Micrococcus luteus Skin Strain C01: Revelation of the Conditionally Extensive Hormone Effect Using Orbitrap Mass Spectrometry and Proteomic Analysis. Microorganisms 2023, 11, 2181. https://doi.org/10.3390/microorganisms11092181
Gannesen AV, Ziganshin RH, Ovcharova MA, Nevolina ED, Klimko AI, Martyanov SV, Plakunov VK. Epinephrine Affects Ribosomes, Cell Division, and Catabolic Processes in Micrococcus luteus Skin Strain C01: Revelation of the Conditionally Extensive Hormone Effect Using Orbitrap Mass Spectrometry and Proteomic Analysis. Microorganisms. 2023; 11(9):2181. https://doi.org/10.3390/microorganisms11092181
Chicago/Turabian StyleGannesen, Andrei V., Rustam H. Ziganshin, Maria A. Ovcharova, Ekaterina D. Nevolina, Alena I. Klimko, Sergey V. Martyanov, and Vladimir K. Plakunov. 2023. "Epinephrine Affects Ribosomes, Cell Division, and Catabolic Processes in Micrococcus luteus Skin Strain C01: Revelation of the Conditionally Extensive Hormone Effect Using Orbitrap Mass Spectrometry and Proteomic Analysis" Microorganisms 11, no. 9: 2181. https://doi.org/10.3390/microorganisms11092181





