Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020
Abstract
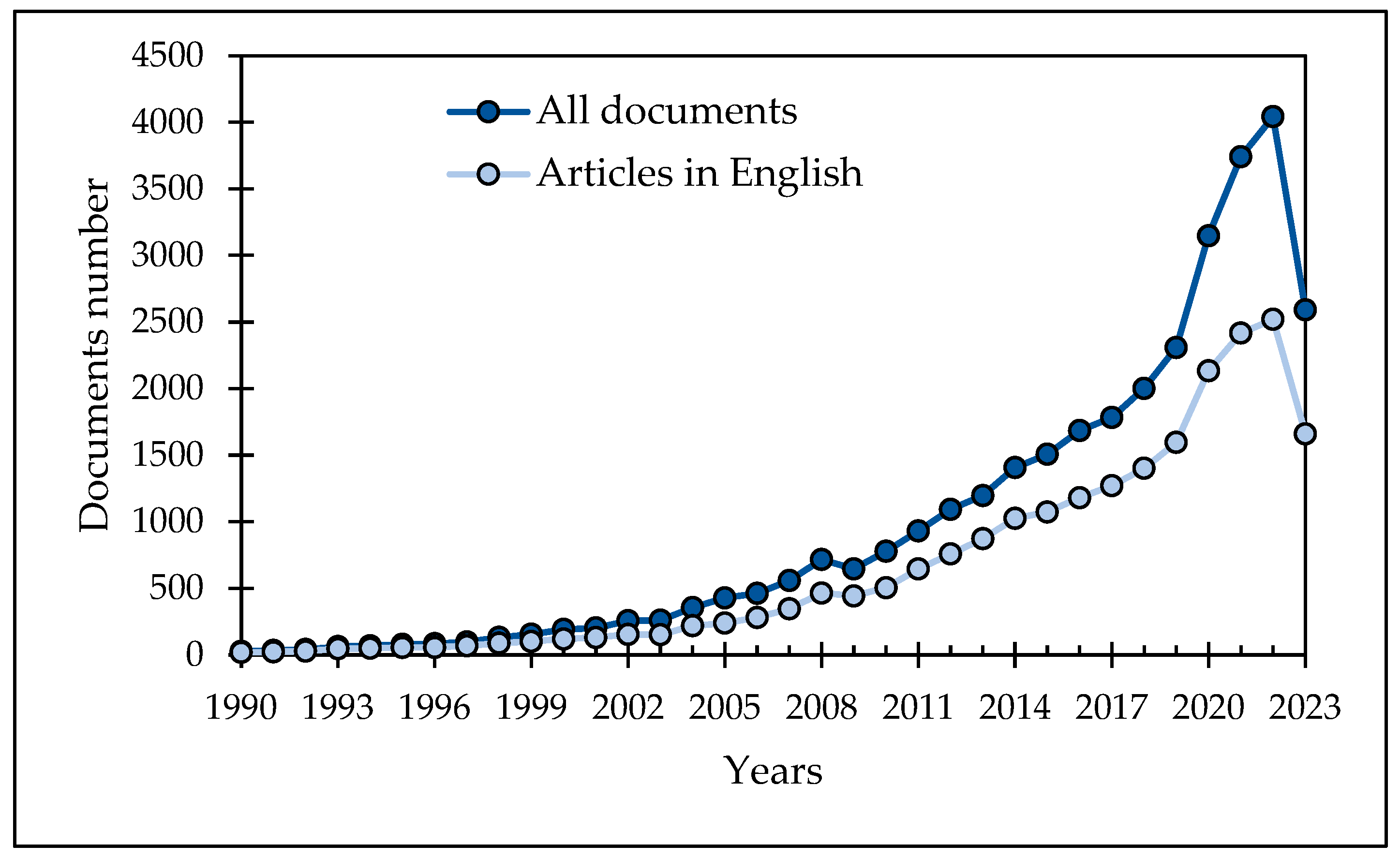
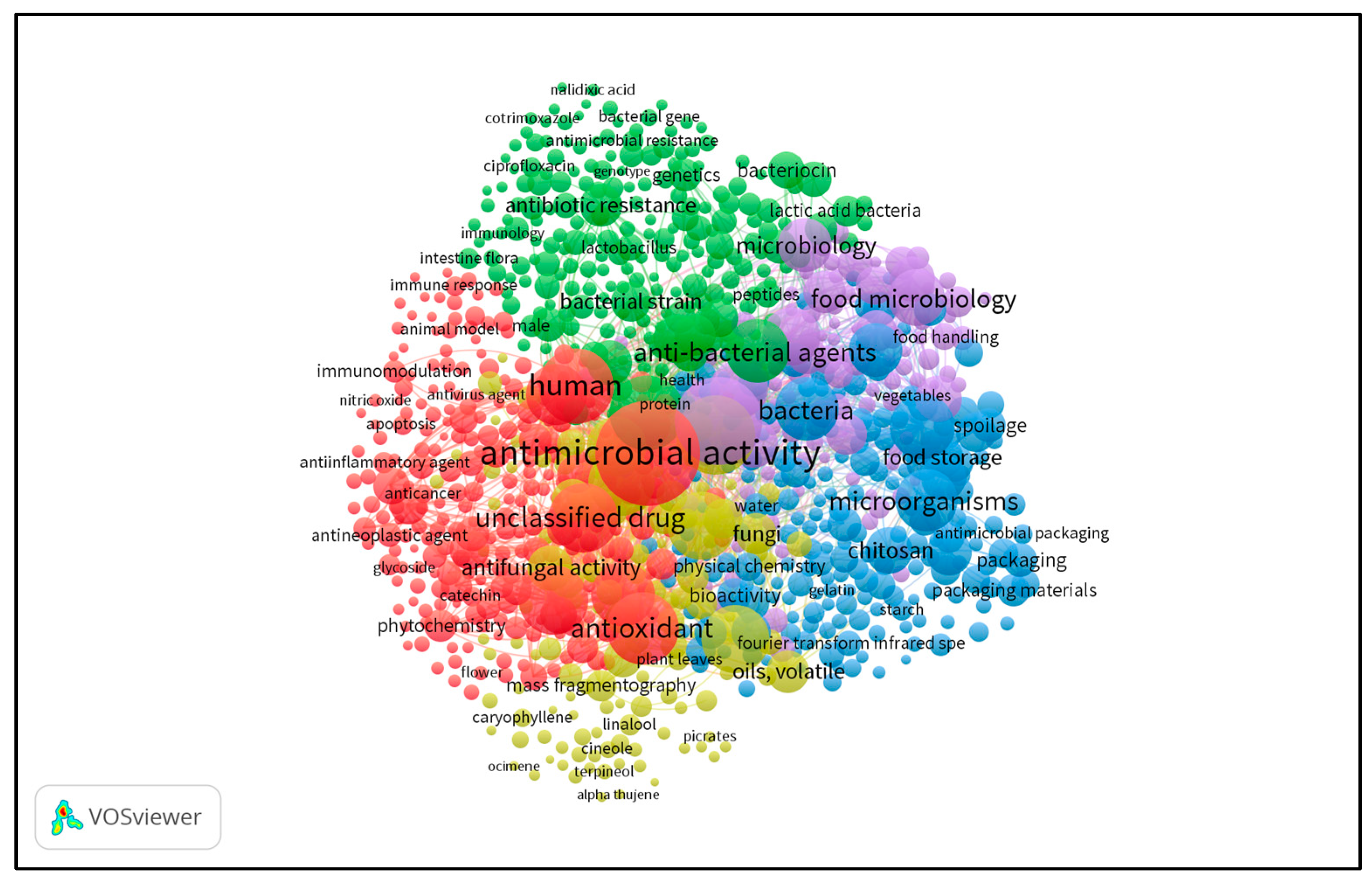
:1. Introduction
2. Food Waste Composition
3. Application of Natural Antimicrobials
3.1. Application of Natural Antimicrobials in Food Products
- Bakery products
- Meat products
- Dairy products
3.2. Application of Natural Antimicrobials in Agriculture and Livestock
3.3. Application of Natural Antimicrobials in Medicine and Pharmacology
| Natural Antimicrobials | Applications | References |
|---|---|---|
| Chitosan, aloe vera, and olive leaf extract | Surgical site infections | [87] |
| Lactobacillus | Cardiovascular-related diseases | [88] |
| Plant-based nanoparticles | Nanotherapeutic drugs with antimicrobial properties | [89] |
| Andrographis paniculate | Infectious disease | [90] |
| Nanomaterials & bacteriocins | Therapeutic strategies | [91] |
| Essential Oils | Antimicrobial, Antiviral | [92] |
| Different antimicrobials from nature | Peroxidase-catalyzed systems | [93] |
| Nigella sativa L. | Therapeutic use | [94] |
| Aloe vera, Angelica gigas, Astragalus membranaceus, Ganoderma lucidum, Panax ginseng, Scutellaria baicalensis | Coronavirus disease | [95] |
| Natural volatiles | Food-based natural antimicrobials | [96] |
| Phyllanthus emblica | Vitamin C, minerals, amino acids source | [97] |
| European barberry, garlic, sweet potato, biter gourd | Effect on hypoglycemic | [98] |
| Garlic & turmeric | Antimicrobial application | [81] |
| Vernonia amygdalina | Hair shampoo | [99] |
| Bryophyllum pinnatum | Hair shampoo | [100] |
3.4. Application of Natural Antimicrobials in Polymers
3.5. Application of Natural Antimicrobials in Textile
3.6. Application of Natural Antimicrobials in Nanotechnology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elshama, S.S. Synthetic and natural food additives: Toxicological hazards and health benefits. J. Toxicol. 2020, 4, 555643. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Chapter Five—Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 157–225. [Google Scholar]
- Ding, Z.; Ge, Y.; Sar, T.; Kumar, V.; Harirchi, S.; Binod, P.; Sirohi, R.; Sindhu, R.; Wu, P.; Lin, F.; et al. Valorization of tropical fruits waste for production of commercial biorefinery products—A review. Bioresour. Technol. 2023, 374, 128793. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Harirchi, S.; Sar, T.; Vs, V.; Rajendran, K.; Gómez-García, R.; Hellwig, C.; Binod, P.; Sindhu, R.; Madhavan, A.; et al. Myco-biorefinery approaches for food waste valorization: Present status and future prospects. Bioresour. Technol. 2022, 360, 127592. [Google Scholar] [CrossRef]
- Jiang, Y.; Subbiah, V.; Wu, H.; Bk, A.; Sharifi-Rad, J.; Suleria, H.A.R. Phenolic profiling of berries waste and determination of their antioxidant potential. J. Food Qual. 2022, 2022, 5605739. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Tundis, R.; Loizzo, M.R. Reuse of food waste: The chemical composition and health properties of pomelo (Citrus maxima) cultivar essential oils. Molecules 2022, 27, 3273. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Yan, X.; Chao, X.; Zhang, W.; Yuan, C.; Zeng, Q. Anti-diabetic effect of banana peel dietary fibers on type 2 diabetic mellitus mice induced by streptozotocin and high-sugar and high-fat diet. J. Food Biochem. 2022, 46, e14275. [Google Scholar] [CrossRef]
- Dhua, S.; Kumar, K.; Sharanagat, V.S.; Nema, P.K. Bioactive compounds and its optimization from food waste: Review on novel extraction techniques. Nutr. Food Sci. 2022, 52, 1270–1288. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Md Norodin, N.S.; Sarkawi, N.S.; Hamzah, M.H.S.; Mohd Nasir, H.; Abang Zaidel, D.N.; Che Yunus, M.A.; Md Salleh, L. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of ultrasound-assisted extraction and encapsulation of antioxidants from orange peels in alginate-chitosan microparticles. Antioxidants 2022, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Alvi, T.; Asif, Z.; Iqbal Khan, M.K. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique—A review. Food Biosci. 2022, 46, 101580. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of bioactive compounds from Pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative technologies for extraction and microencapsulation of bioactives from plant-based food waste and their applications in functional food development. Foods 2021, 10, 279. [Google Scholar] [CrossRef]
- Budiati, T.; Suryaningsih, W.; Bethiana, T.N. Antimicrobial of tropical fruit and vegetable waste extract for food-borne pathogenic bacteria. Ital. J. Food Saf. 2022, 11, 10510. [Google Scholar] [CrossRef]
- Gonçalves, L.A.; Lorenzo, J.M.; Trindade, M.A. Fruit and agro-industrial waste extracts as potential antimicrobials in meat products: A brief review. Foods 2021, 10, 1469. [Google Scholar] [CrossRef]
- Naksing, T.; Teeka, J.; Rattanavichai, W.; Pongthai, P.; Kaewpa, D.; Areesirisuk, A. Determination of bioactive compounds, antimicrobial activity, and the phytochemistry of the organic banana peel in Thailand. J. Biosci 2021, 37, 1981–3163. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of food waste to produce value-added products based on its bioactive compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A critical review on food waste management for the production of materials and biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Pandey, K.; Yadav, A.K.; Goel, C. Utilization of Food Waste for Biofuel Production. In Food Waste to Green Fuel: Trend & Development; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–23. [Google Scholar]
- Chong, C.H.; Wahi, R.; Choo, C.M.; Chew, S.J.; Dassanayake, M.K. Drying and Valorisation of Food Processing Waste; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2017, 59, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef] [PubMed]
- Sherazi, S.T.H.; Mahesar, S.A. Vegetable oil deodorizer distillate: A rich source of the natural bioactive components. J. Oleo Sci. 2016, 65, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and poultry wastes: Management practices, feedstocks for renewable energy production, and recovery of value added products. Biomass Convers. Biorefin. 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Ullah, N.; Nadhman, A.; Siddiq, S.; Mehwish, S.; Islam, A.; Jafri, L.; Hamayun, M. Plants as antileishmanial agents: Current scenario. Phytother. Res. 2016, 30, 1905–1925. [Google Scholar] [CrossRef]
- El-Sawi, S.A.; Ibrahim, M.E.; Bassuiny, R.I.; Merghany, R.M. Antioxidant, cytotoxic and antimicrobial efficacy of potato peels, taro peels, and husk and silk of corn. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 619–626. [Google Scholar] [CrossRef]
- Wang, L.; Teplitski, M. Microbiological food safety considerations in shelf-life extension of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2023, 80, 102895. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Balaban, M.; Koc, C.; Sar, T.; Akbas, M.Y. Antibiofilm effects of pomegranate peel extracts against B. cereus, B. subtilis, and E. faecalis. Int. J. Food Sci. Technol. 2021, 56, 4915–4924. [Google Scholar] [CrossRef]
- Balaban, M.; Koc, C.; Sar, T.; Yesilcimen Akbas, M. Screening for bioactive compound rich pomegranate peel extracts and their antimicrobial activities. Johns. Matthey Technol. Rev. 2021, 66, 81–89. [Google Scholar] [CrossRef]
- Sar, T.; Akbas, M.Y. Antimicrobial activities of olive oil mill wastewater extracts against selected microorganisms. Sustainability 2023, 15, 8179. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical characteristics, mineral content, and antioxidant and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef]
- Abu El-Hassan, A.; Roby, M.; Rabiee, L.A.; Abd-Eltawab, S.A. Chemical, microbiological and antioxidant effects of banana peels on butter cake. Egypt. J. Food Sci. 2022, 50, 241–250. [Google Scholar] [CrossRef]
- Abdel-Hameed, S.M.; Abd Allah, N.A.R.; Hamed, M.M.; Soltan, O.I.A. Papaya fruit by-products as novel food ingredients in cupcakes. Ann. Agric. Sci. 2023, 68, 60–74. [Google Scholar] [CrossRef]
- Zaki, H.S.; Abd-Elhak, N.L.; Abd El-Rahman, N.A. The utilization of yellow and red onion peels and their extracts as antioxidant and antimicrobial in preservation of beef burger during storage. Am. J. Food Sci. Technol. 2022, 10, 1–9. [Google Scholar]
- Wang, C.; An, X.; Gao, Z.; Li, Z.; Tian, S.; Lu, Y. Effects of ethanolic extract from onion skin on the quality characteristics of beef patties during refrigerated storage. Food Sci. Technol. 2022, 42, e118121. [Google Scholar] [CrossRef]
- Eldahrawy, M.; Salem, A.M.; Nabil, M. The efficiency of citrus peel powders in improvement of meat quality during chilled storage. Benha Vet. Med. J. 2022, 42, 208–213. [Google Scholar] [CrossRef]
- Ghimire, A.; Paudel, N.; Poudel, R. Effect of pomegranate peel extract on the storage stability of ground buffalo (Bubalus bubalis) meat. LWT 2022, 154, 112690. [Google Scholar] [CrossRef]
- Hadab, N.S.; Dakheel, M.M. Application of pomegranate pomace as a natural antibacterial and antioxidant preservative in beef. Iraqi J. Vet. Sci. 2022, 36, 211–216. [Google Scholar] [CrossRef]
- Thanoun, E.A.; Al-Jammaas, O.H.A. Storage stability of chicken patties after treatment with pomegranate, potato and apple peel extracts. Agraarteadus 2022, 33. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pașcalău, R.; Sallam, K.I. Antioxidant and antibacterial effect of fruit peel powders in chicken patties. Foods 2022, 11, 301. [Google Scholar] [CrossRef]
- El-Kholany, E.A.; El-Deeb, A.M.; Elsheikh, D.M. Impact of lemon peel extract utilization on the biological values of Labneh during storage. Egypt. J. Agric. Res. 2022, 100, 555–569. [Google Scholar] [CrossRef]
- Alsubhi, N.H.; Al-Quwaie, D.A.; Alrefaei, G.I.; Alharbi, M.; Binothman, N.; Aljadani, M.; Qahl, S.H.; Jaber, F.A.; Huwaikem, M.; Sheikh, H.M.; et al. Pomegranate pomace extract with antioxidant, anticancer, antimicrobial, and antiviral activity enhances the quality of strawberry-yogurt smoothie. Bioengineering 2022, 9, 735. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. From by-product to functional ingredient: Incorporation of avocado peel extract as an antioxidant and antibacterial agent. Innov. Food Sci. Emerg. Technol. 2022, 80, 103116. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Mehdizadeh, T.; Aliakbarlu, J. Chemical and microbiological stability and sensorial properties of traditional Iranian butter incorporated with pomegranate peel extract. Int. J. Dairy Technol. 2023, 76, 178–186. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Barreira, J.C.M.; Soković, M.; Calhelha, R.C.; Bento, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Castanea sativa male flower extracts as an alternative additive in the Portuguese pastry delicacy “pastel de nata”. Food Funct. 2020, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- da Silva Veloso, F.; Caleja, C.; Calhelha, R.C.; Pires, T.C.S.; Alves, M.J.; Barros, L.; Genena, A.K.; Barreira, J.C.M.; Ferreira, I.C.F.R. Characterization and application of pomegranate epicarp extracts as functional ingredients in a typical Brazilian pastry product. Molecules 2020, 25, 1481. [Google Scholar] [CrossRef]
- Pires, E.d.O.; Pereira, E.; Carocho, M.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Garcia, C.C.; Ferreira, I.C.F.R.; et al. Study on the potential application of impatiens balsamina L. flowers extract as a natural colouring ingredient in a pastry product. Int. J. Environ. Res. Public Health 2021, 18, 9062. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Chin, Y.-W.; Paik, H.-D. Application of natural preservatives for meat and meat products against food-borne pathogens and spoilage bacteria: A review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Patil, P.; Siddibhavi, M.; Ankola, A.V.; Sankeshwari, R.; Kumar, V. Antimicrobial and antibiofilm effect of cranberry extract on Streptococcus mutans and Lactobacillus acidophilus: An in vitro study. Int. J. Clin. Pediatr. Dent. 2020, 13, 11. [Google Scholar] [PubMed]
- Jain, P.; Rajasekar, A. Evaluation of antimicrobial property of herbal mouthwash using cranberry extract: A pilot study. Plant Cell Biotechnol. Mol. Biol. 2020, 31, 135–144. [Google Scholar]
- Daoutidou, M.; Plessas, S.; Alexopoulos, A.; Mantzourani, I. Assessment of antimicrobial activity of pomegranate, cranberry, and black chokeberry extracts against foodborne pathogens. Foods 2021, 10, 486. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The role of essential oils against pathogenic Escherichia coli in food products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef]
- Salami, S.O.; Afolayan, A.J. Suitability of roselle Hibiscus sabdariffa as raw material for soft drink production. J. Food Qual. 2020, 2020, 8864142. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of milk permeate with selected lactic acid bacteria strains and apple by-products into beverages with antimicrobial properties and enriched with Galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L. Effects of natural bioactive compounds on microbial safety and quality of dairy products. J. Eng. Sci. 2021, 28, 149–160. [Google Scholar] [CrossRef]
- Yuningtyas, S.; Roswiem, A.P.; Azahra, D.; Alfarabi, M. Antioxidant activity and characterization of arrowroot (Maranta arundinacea) tuber yogurt. Biodivers. J. Biol. Divers. 2023, 24. [Google Scholar] [CrossRef]
- Shirani, K.; Falah, F.; Vasiee, A.; Yazdi, F.T.; Behbahani, B.A.; Zanganeh, H. Effects of incorporation of Echinops setifer extract on quality, functionality, and viability of strains in probiotic yogurt. J. Food Meas. Charact. 2022, 16, 2899–2907. [Google Scholar] [CrossRef]
- Maiza, A.; Kamgang Nzekoue, F.; Ghazouani, T.; Afif, M.; Caprioli, G.; Fiorini, D.; Vittori, S.; Maggi, F.; Buccioni, M.; Martì Navia, A.; et al. Butter oil (ghee) enrichment with aromatic plants: Chemical characterization and effects on fibroblast migration in anin-vitro wound healing model. Arab. J. Chem. 2020, 13, 8909–8919. [Google Scholar] [CrossRef]
- Afiyah, D.N.; Sarbini, R.N.; Arief, I.I.; Suryati, T. Effect of storage on microbiological properties of probiotic yogurt with podang urang mango (Mangifera indica L.) extracts. IOP Conf. Ser. Earth Environ. Sci. 2023, 1168, 012024. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Mirsaeedghazi, H.; Azarikia, F.; Abedinia, A. Investigation of antimicrobial activity of orange and pomegranate peels extracts and their use as a natural preservative in a functional beverage. J. Food Meas. Charact. 2021, 15, 5683–5694. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Salamiah, S.; Orbani, R.H. Moler disease control in shallots using botanical pesticides Jengkol peel powder and its impact on microbial biodiversity in Peatlands. Pak. J. Phytopathol. 2022, 34, 117–133. [Google Scholar] [CrossRef]
- Suyanto, A.; Agnes Tutik, P.I.; Akbar, T. Jengkol peel extract (Pithecellobium jiringa (Jack) Prain) as a biofungicide against fungus Curvularia sp., the cause of leaf spot disease on oil palm (Elaeis guineensis Jacq) seedlings. J. Smart Sci. Technol. JSST 2022, 2, 1–6. [Google Scholar] [CrossRef]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Martín-Ramos, P.; Oliveira, R. Antifungal activity of plant waste extracts against phytopathogenic fungi: Allium sativum peels extract as a promising product targeting the fungal plasma membrane and cell wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, N.; Rastegari, A.A.; Yadav, A.N. Chapter 7—Biodiversity, phylogenetic profiling, and mechanisms of colonization of seed microbiomes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–125. [Google Scholar]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Chapter 2—Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar]
- Das, I.; Sonowal, A.; Gogoi, B.; Sharma, B.; Patowary, K.; Borah, D. Citrus maxima (Burm.) Merr. fruit juice and peel extract mediated synthesis of silver nanoparticles (AgNPs) and their applications as antimicrobial agents and plant growth enhancers. Kuwait J. Sci. 2023, 50. [Google Scholar] [CrossRef]
- El Houda, A.K.N.; Ali, B.; Ahmed, A.; Salah, O.; Yazid, F.-C. Chemical composition, antimicrobial and insecticidal activities of Citrus paradisi peel essential oil from Algeria. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1093–1098. [Google Scholar] [CrossRef]
- Redhead, A.K.; Azman, N.F.I.N.; Nasaruddin, A.I.; Vu, T.; Santos, F.; Malheiros, R.; Hussin, A.S.M.; Toomer, O.T. Peanut skins as a natural antimicrobial feed additive to reduce the transmission of Salmonella in poultry meat produced for human consumption. J. Food Prot. 2022, 85, 1479–1487. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Mishra, G.; Malaiya, A.; Jain, A.; Mody, N.; Raichur, A.M. Antimicrobial application potential of phytoconstituents from turmeric and garlic. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 409–435. [Google Scholar]
- Pleeging, C.C.; Coenye, T.; Mossialos, D.; De Rooster, H.; Chrysostomou, D.; Wagener, F.A.; Cremers, N.A. Synergistic antimicrobial activity of supplemented medical-grade honey against Pseudomonas aeruginosa biofilm formation and eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Hegazi, N.M.; Abd Elghani, G.E.; Farag, M.A. Manuka honey, a unique mono-floral honey. A comprehensive review of its bioactives, metabolism, action mechanisms, and therapeutic merits. Food Biosci. 2021, 42, 101038. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Si, J.-J.; Li, S.-S.; Zhang, G.-Z.; Wang, S.; Zheng, H.-Q.; Hu, F.-L. Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to Manuka honey (12+ and 20+). Molecules 2021, 26, 2778. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Godhi, B.S.; Beeraka, N.M.; Buddi, J.S.H.P.; Mahadevaiah, S.; Madhunapantula, S.V. Updates in the analytical isolation of indian propolis chemical constituents and their role in dental pharmacology-A review. Nat. Prod. J. 2022, 12, 77–88. [Google Scholar] [CrossRef]
- Seydibeyoğlu, E.A.; Ayşe, I. The effect of natural antimicrobial agents on the characteristics of surgical sutures. Dokuz Eylül Univ. Muhendis. Fak. Muhendis. Derg. 2020, 22, 11–20. [Google Scholar]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and improving function of lactobacilli in the prevention and treatment of cardiovascular-related diseases: A novel perspective from gut microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Proćków, J.; Simal-Gandara, J. Safer plant-based nanoparticles for combating antibiotic resistance in bacteria: A comprehensive review on its potential applications, recent advances, and future perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (burm. F.) wall. Ex nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.-S. Potential novel food-related and biomedical applications of nanomaterials combined with bacteriocins. Pharmaceutics 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Tonoyan, L.; Montagner, D.; Friel, R.; O’Flaherty, V. Antimicrobials offered from nature: Peroxidase-catalyzed systems and their mimics. Biochem. Pharmacol. 2020, 182, 114281. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed. Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Leuner, O.; Melnikovova, I.; Fernandez-Cusimamani, E. Pharmacology of natural volatiles and essential oils in food, therapy, and disease prophylaxis. Front. Pharmacol. 2021, 12, 740302. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Mishra, V.; Hemani, V.; Verma, A.; Jain, A.; Jain, S.; Charyulu, R.N. Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends Food Sci. Technol. 2021, 110, 594–610. [Google Scholar] [CrossRef]
- Tinrat, S.; Singhapol, C. Evaluation of antioxidant and antibacterial activities of Vernonia amygdalina leaf extracts as an auxiliary in natural hair shampoo. Evaluation 2020, 13, 50–57. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yachawad, A.V.; Zambare, K.K.; Landge, S. Formulation and evaluation of herbal shampoo: Bryophyllum pinnatum. Asian J. Pharm. Res. 2020, 10, 86–88. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Gaba, S.; Saini, A.; Singh, G.; Monga, V. An insight into the medicinal attributes of berberine derivatives: A review. Bioorg. Med. Chem. 2021, 38, 116143. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Jafarpour, D.; Hashemi, S.M.B.; Ghaedi, A. Study the antibacterial properties of different parts of saffron extract and their application in cream. J. Food Sci. Technol. 2021, 18, 339–349. [Google Scholar] [CrossRef]
- Hadizadeh-Talasaz, F.; Mardani, F.; Bahri, N.; Rakhshandeh, H.; Khajavian, N.; Taghieh, M. Effect of Rosemary cream on episiotomy wound healing in primiparous women: A randomized clinical trial. BMC Complement. Med. Ther. 2022, 22, 226. [Google Scholar] [CrossRef]
- Lam, N.S.K.; Long, X.X.; Li, X.; Yang, L.; Griffin, R.C.; Doery, J.C. Comparison of the efficacy of tea tree (Melaleuca alternifolia) oil with other current pharmacological management in human demodicosis: A Systematic Review. Parasitology 2020, 147, 1587–1613. [Google Scholar] [CrossRef]
- Kairey, L.; Agnew, T.; Bowles, E.J.; Barkla, B.J.; Wardle, J.; Lauche, R. Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front. Pharmacol. 2023, 14, 1116077. [Google Scholar] [CrossRef] [PubMed]
- Fauziyah, R.N.; Widyasanti, A. The valorization of neem leaves infused coconut oil at various concentrations for the production of natural liquid shampoo. Adv. Food Sci. Sustain. Agric. Agroind. Eng. AFSSAAE 2021, 4, 131–137. [Google Scholar]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antimicrobial polymer-based materials for food packaging applications. Polymers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural antimicrobials as additives for edible food packaging applications: A review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, J.; Rasane, P.; Gupta, P.; Kaur, S.; Sharma, N.; Sowdhanya, D. Natural additives as active components in edible films and coatings. Food Biosci. 2023, 53, 102689. [Google Scholar] [CrossRef]
- Jamali, S.N.; Assadpour, E.; Feng, J.; Jafari, S.M. Natural antimicrobial-loaded nanoemulsions for the control of food spoilage/pathogenic microorganisms. Adv. Colloid Interface Sci. 2021, 295, 102504. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Kurek, M. Coacervation as a novel method of microencapsulation of essential oils—A review. Molecules 2022, 27, 5142. [Google Scholar] [CrossRef]
- Can, F.Ö. Encapsulation of lemongrass oil for antimicrobial and biodegradable food packaging applications. Sci. Adv. Mater. 2021, 13, 803–811. [Google Scholar]
- Santiesteban-López, N.A.; Gómez-Salazar, J.A.; Santos, E.M.; Campagnol, P.C.B.; Teixeira, A.; Lorenzo, J.M.; Sosa-Morales, M.E.; Domínguez, R. Natural antimicrobials: A clean label strategy to improve the shelf life and safety of reformulated meat products. Foods 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sanches Silva, A.; Chávez-González, M.L.; Dubey, N. Novel tools to improve food quality and shelf life: Advances and future perspectives. Front. Sustain. Food Syst. 2023, 7, 1148977. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Lima, R.C.; Carvalho, A.P.; Vieira, C.P.; Moreira, R.V.; Conte-Junior, C.A. Green and healthier alternatives to chemical additives as cheese preservative: Natural antimicrobials in active nanopackaging/coatings. Polymers 2021, 13, 2675. [Google Scholar] [CrossRef]
- Saleh, I.; Abu-Dieyeh, M.H. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 2021, 11, 7871. [Google Scholar] [CrossRef]
- Tao, M.; Chen, J.; Huang, K. Bio-based antimicrobial delivery systems for improving microbial safety and quality of raw or minimally processed foods. Curr. Opin. Food Sci. 2021, 41, 189–200. [Google Scholar] [CrossRef]
- Deepti, P.; Shahnaz, J.; Manisha, G. Functional properties of natural dyed textiles. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; Ashis Kumar, S., Nasser, S.A., Hamed Majdooa, A., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–19. [Google Scholar]
- Werede, E.; Jabasingh, S.A.; Demsash, H.D.; Jaya, N.; Gebrehiwot, G. Eco-friendly cotton fabric dyeing using a green, sustainable natural dye from Gunda Gundo (Citrus sinensis) orange peels. Biomass Convers. Biorefin. 2023, 13, 5219–5234. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Gharanjig, K.; Imani, H.; Razani, N. Green dyeing of wool yarns with yellow and black myrobalan extract as bio-mordant with natural dyes. J. Nat. Fibers 2022, 19, 3893–3915. [Google Scholar] [CrossRef]
- Adeel, S.; Rehman, F.-U.; Amin, A.; Amin, N.; Batool, F.; Hassan, A.; Ozomay, M. Sustainable exploration of coffee extracts for dyeing of microwave-treated bio-mordanted cotton fabric. Pigment Resin Technol. 2023, 52, 331–340. [Google Scholar] [CrossRef]
- Samant, L.; Jose, S.; Rose, N.M.; Shakyawar, D.B. Antimicrobial and UV protection properties of cotton fabric using enzymatic pretreatment and dyeing with Acacia Catechu. J. Nat. Fibers 2022, 19, 2243–2253. [Google Scholar] [CrossRef]
- Bisht, G.; Srivastava, S.; Kulshreshtha, R.; Sourirajan, A.; Baumler, D.J.; Dev, K. Applications of red pigments from psychrophilic Rhodonellum psychrophilum GL8 in health, food and antimicrobial finishes on textiles. Process Biochem. 2020, 94, 15–29. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential use of olive oil mill wastewater for bacterial cellulose production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef]
- Yilmaz, F. Application of Glycyrrhiza glabra L. root as a natural antibacterial agent in finishing of textile. Ind. Crops Prod. 2020, 157, 112899. [Google Scholar] [CrossRef]
- Muruganandham, M.; Sivasubramanian, K.; Velmurugan, P.; Suresh Kumar, S.; Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Manickam, S.; Pang, C.H.; Sivakumar, S. An eco-friendly ultrasound approach to extracting yellow dye from Cassia alata flower petals: Characterization, dyeing, and antibacterial properties. Ultrason. Sonochem. 2023, 98, 106519. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.; Kumar, L.; Gaikwad, K.K. Screen printing of catechu (Senegalia catechu)/guar gum based edible ink for food printing and packaging applications. Prog. Org. Coat. 2023, 182, 107629. [Google Scholar] [CrossRef]
- Puttipan, R.; Khankaew, S. Novel bio-based, time-temperature dependent colorimetric ink and film containing colorants from the red pitaya (Hylocereus costaricensis). Prog. Org. Coat. 2023, 178, 107470. [Google Scholar] [CrossRef]
- Cabañas-Romero, L.V.; Valls, C.; Valenzuela, S.V.; Roncero, M.B.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Bacterial cellulose–chitosan paper with antimicrobial and antioxidant activities. Biomacromolecules 2020, 21, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, M.W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex situ development and characterization of green antibacterial bacterial cellulose-based composites for potential biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Sun, Y.; Zheng, Y.-D.; He, W.; Yang, Y.-Y.; Xie, Y.-J.; Feng, Z.-X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Wang, H.; Wu, K.; Gao, J.; Li, J. Characterization and antimicrobial properties of ferulic acid grafted self-assembled bacterial cellulose-chitosan membranes. J. Appl. Polym. Sci. 2021, 138, 50824. [Google Scholar] [CrossRef]
- Ayres Cacciatore, F.; Dalmás, M.; Maders, C.; Ataíde Isaía, H.; Brandelli, A.; da Silva Malheiros, P. Carvacrol encapsulation into nanostructures: Characterization and antimicrobial activity against foodborne pathogens adhered to stainless steel. Food Res. Int. 2020, 133, 109143. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.A.; Brandelli, A.; Malheiros, P.d.S. Combining natural antimicrobials and nanotechnology for disinfecting food surfaces and control microbial biofilm formation. Crit. Rev. Food Sci. Nutr. 2021, 61, 3771–3782. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef]
- Heckler, C.; Marques Maders Silva, C.; Ayres Cacciatore, F.; Daroit, D.J.; da Silva Malheiros, P. Thymol and carvacrol in nanoliposomes: Characterization and a comparison with free counterparts against planktonic and glass-adhered Salmonella. LWT 2020, 127, 109382. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Ma, K.; Zhe, T.; Li, F.; Zhang, Y.; Yu, M.; Li, R.; Wang, L. Sustainable films containing AIE-active berberine-based nanoparticles: A promising antibacterial food packaging. Food Hydrocoll. 2022, 123, 107147. [Google Scholar] [CrossRef]
- Arumugam, S.; Kandasamy, J.; Thiyaku, T.; Saxena, P. Effect of low concentration of SiO2 nanoparticles on grape seed essential Oil/PBAT composite films for sustainable food packaging application. Sustainability 2022, 14, 8073. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.-s.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, antioxidant and anticancer activities of gold nanoparticles green synthesized using Mangifera indica seed aqueous extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef]
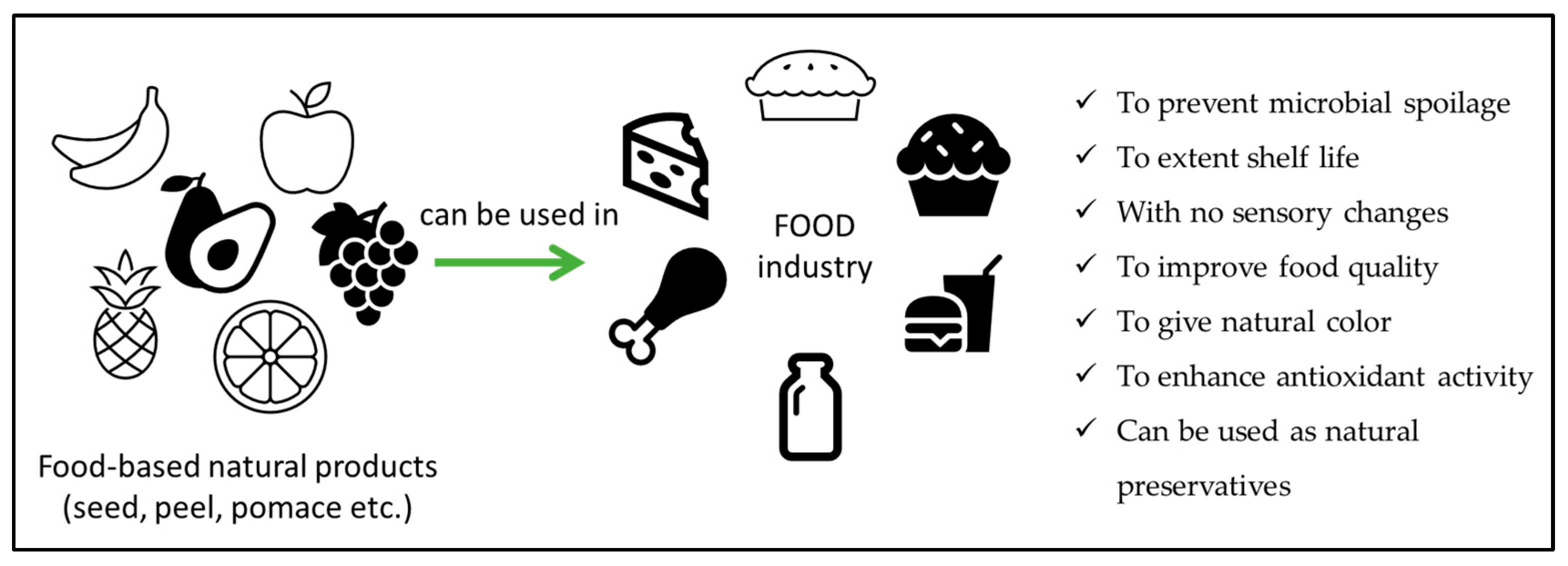
| Food | Products | Material | Outcomes | References |
|---|---|---|---|---|
| Bakery products | Extract | Orange peel Pomegranate peel | Antimicrobial affects against P. aeruginosa, S. marcescens, E. coli, S. aureus, B. subtilis, B. cereus, and K. pneumonia. Extend the shelf life of cakes and delay microbial spoilage. | [41] |
| Powder | Banana peel | Improvement in sensory characteristics, increased mineral content, and antimicrobial and antioxidant activity when added to cake. | [42] | |
| Powder or seed oil | Papaya seed | Antimicrobial activity against B. subtilis, S. aureus, E. faecalis, P. aeruginosa, B. cereus, Salmonella spp., C. albicans, and Aspergillus spp. Enhance the flavor and nutritional value when added to chocolate cakes. | [43] | |
| Meat products | Peels or extracts | Onion peels | Increased the total phenolic and flavonoid content and delayed microbial spoilage when added to meat. | [44] |
| Extract | Onion peels | Reduced microbial spoilage, antioxidant, and antimicrobial activity, and extended shelf life of beef meatballs. | [45] | |
| Powder | Lemon peels Orange peels | Improved microbial and chemical values, enhance sensory characteristics when added to meat. | [46] | |
| Extract | Pomegranate peel | Reduced total colony count when added to buffalo meat. | [47] | |
| Pomace | Pomegranate pomace | Positive effects on total bacterial count, lipid oxidation, pH values, and extended shelf life of beef. | [48] | |
| Extract | Pomegranate peel Potato peel Apple peel | Reduced total bacterial growth when added to chicken meatballs. In addition, showed antioxidant properties | [49] | |
| Powder | Lemon peel Orange peel Grapefruit peel Banana peel | Lower microbial loads when added to chicken meatballs. | [50] | |
| Dairy products | Extract | Lemon peel | Antimicrobial activity against B. cereus, S. aureus, L. monocytogenes, B. subtilis, E. coli, S. typhimurium, P. aeruginosa, C. albicans, and A. fumigatus. Extended shelf life and reduced microbial contamination | [51] |
| Extract | Pomegranate pomace | Antimicrobial activity against L. monocytogenes, P. aeruginosa, K. pneumoniae, A. niger, and C. glabrata. Improvement in color, texture, quality, and lower antimicrobial load when added to smoothies. | [52] | |
| Extract | Avocado peel | Antimicrobial activity against S. aureus, S. epidermidis, and E. coli. When added, chemical and physical properties did not change the mayonnaise. | [53] | |
| Extract | Pomegranate peel | Lower microbial load when added to butter. | [54] |
| Use | Natural Antimicrobials | References |
|---|---|---|
| Antimicrobial Packaging | Essential oils, bacteriocins, lysozyme, grapefruit seed extracts, chitosan. | [110] |
| Starch, sugar beets, corn. | [111] | |
| Different natural antimicrobials. | [36] | |
| Biomass, microorganisms, Bio-based monomers. | [112] | |
| Plant-Derived, Animal-Derived, Microorganism-Derived. | [113] | |
| Pineapple, green tea, coconut, pecans, fenugreek seeds, mangoes, olive leaf, yellow onions, and soybean seeds. | [109] | |
| Carotenoids, tannins, alkaloids, anthocyanins, flavonoids, terpenoids, caffeic acid, and other organic acids. | [114] | |
| Minimization of Synthetic Additives | Oils of eugenol, carvacrol, thymol, and basil. | [115] |
| Peppermint, oregano, coriander | [116] | |
| Lemongrass oil | [117] | |
| Extended Shelf Life | Rosemary, oregano, basil, and cedar essence. | [118] |
| Bacteriocins, enzymes, essential oils, grapefruit seed extract, green tea extract, and cranberry extract | [119] | |
| Antimicrobial properties of carbon nanomaterials | [120] | |
| Bacteriocins | [121] | |
| Preservation of Nutritional Quality | Lemongrass, garlic, cumin, green propolis, black cumin cedar, fennel, pennyroyal, and ginger | [122] |
| Prosopis juliflora leaf | [123] | |
| Microparticles, microgels, nanoliposomes, nano micelles, and nanostructured lipid carriers generated from yeast. | [124] |
| Natural Antimicrobials | Application | Tested Microorganisms | Finding Results | References |
|---|---|---|---|---|
| Carvacrol | Encapsulation into liposomes or nanocapsules | S. aureus Salmonella E. coli L. monocytogenes | This can be used as a surface sanitizer | [142] |
| Thymol and/or carvacrol | Salmonella | Antimicrobial-loaded nanoliposomes displayed decreased anti-Salmonella activities. | [145] | |
| Mandarin essential oil | Essential-oil-loaded chitosan nanoparticles | S. aureus and E. coli | Biofilm formation was inhibited, and mature biofilms were removed It can be used for pork preservation | [146] |
| Curcumin | Bacterial cellulose hydrogel containing silver nanoparticles using curcumin | S. aureus, P. aeruginosa and C. auris | It has potential wound-dressing application, and has broad-spectrum antimicrobial activity | [147] |
| Berberine-cinnamic acid | Nanocomposite films | S. aureus and E. coli | Microbial growth was inhibited, and food shelf life was extended | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. https://doi.org/10.3390/microorganisms11092234
Sar T, Kiraz P, Braho V, Harirchi S, Akbas MY. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms. 2023; 11(9):2234. https://doi.org/10.3390/microorganisms11092234
Chicago/Turabian StyleSar, Taner, Pelin Kiraz, Vjola Braho, Sharareh Harirchi, and Meltem Yesilcimen Akbas. 2023. "Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020" Microorganisms 11, no. 9: 2234. https://doi.org/10.3390/microorganisms11092234
APA StyleSar, T., Kiraz, P., Braho, V., Harirchi, S., & Akbas, M. Y. (2023). Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms, 11(9), 2234. https://doi.org/10.3390/microorganisms11092234








