Genetic Variability of the Internal Transcribed Spacer and Pyruvate:Ferredoxin Oxidoreductase Partial Gene of Trichomonas vaginalis from Female Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Sampling
2.2. PCR Sequencing
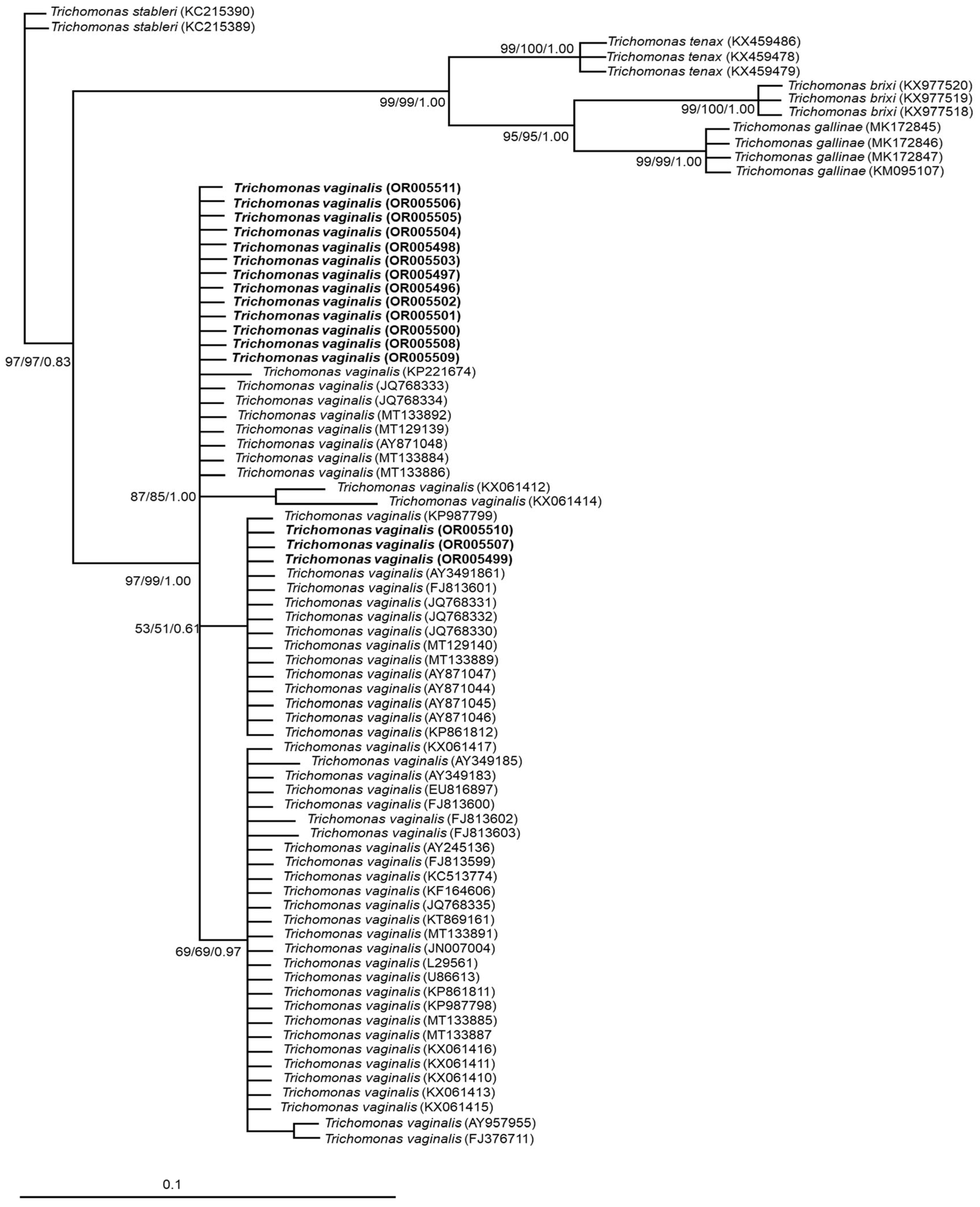
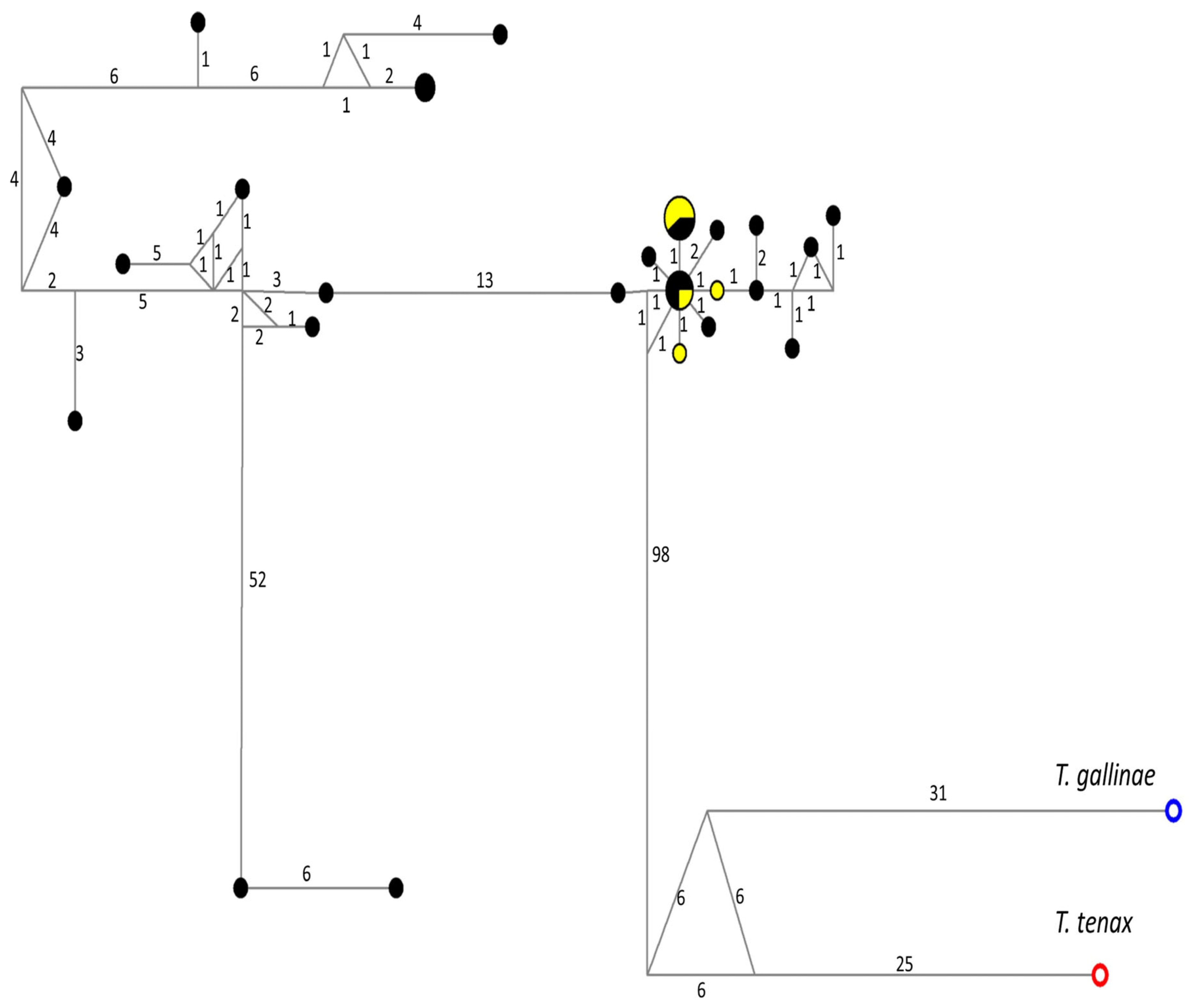
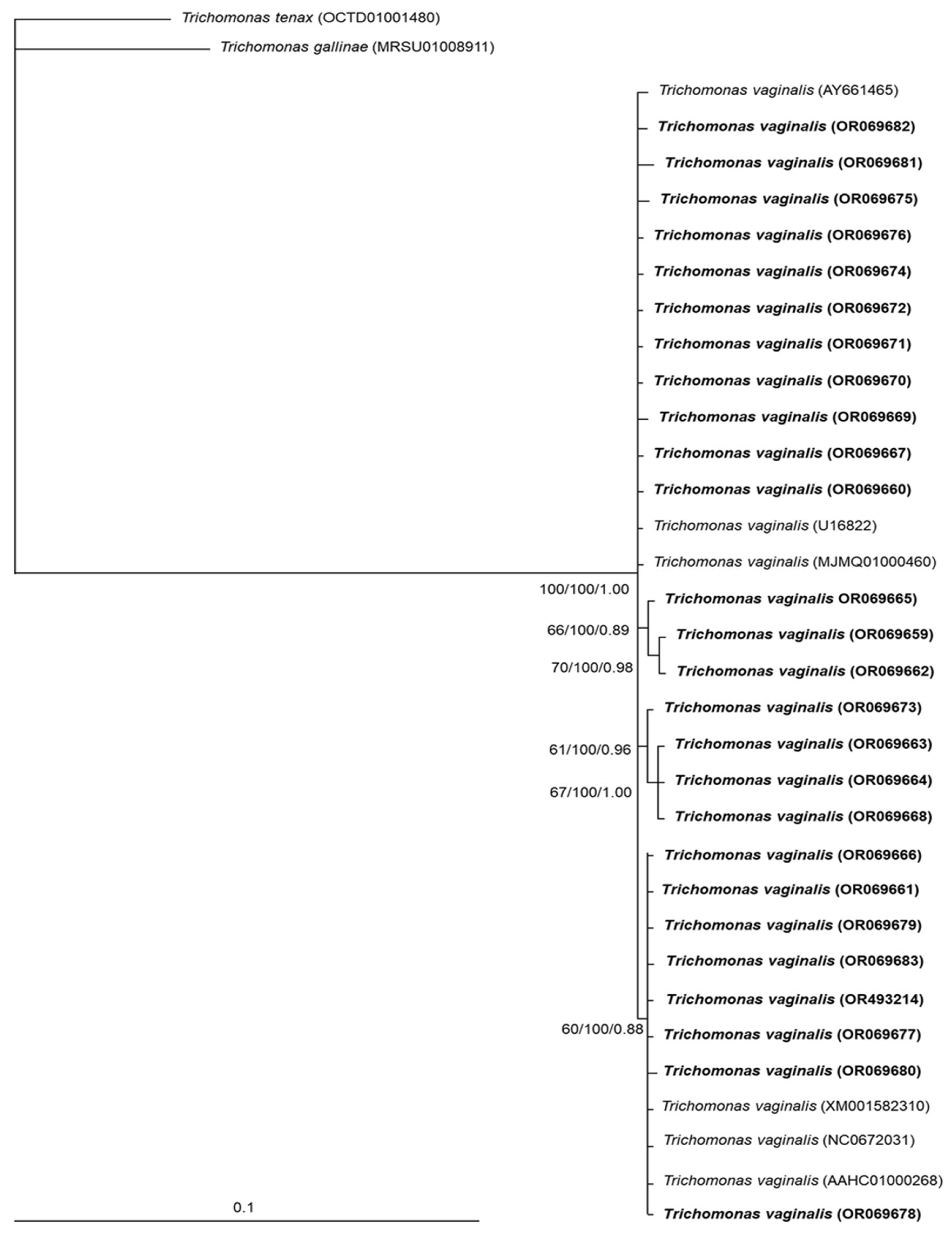
2.3. Phylogenetic and Genetic Variation Analysis
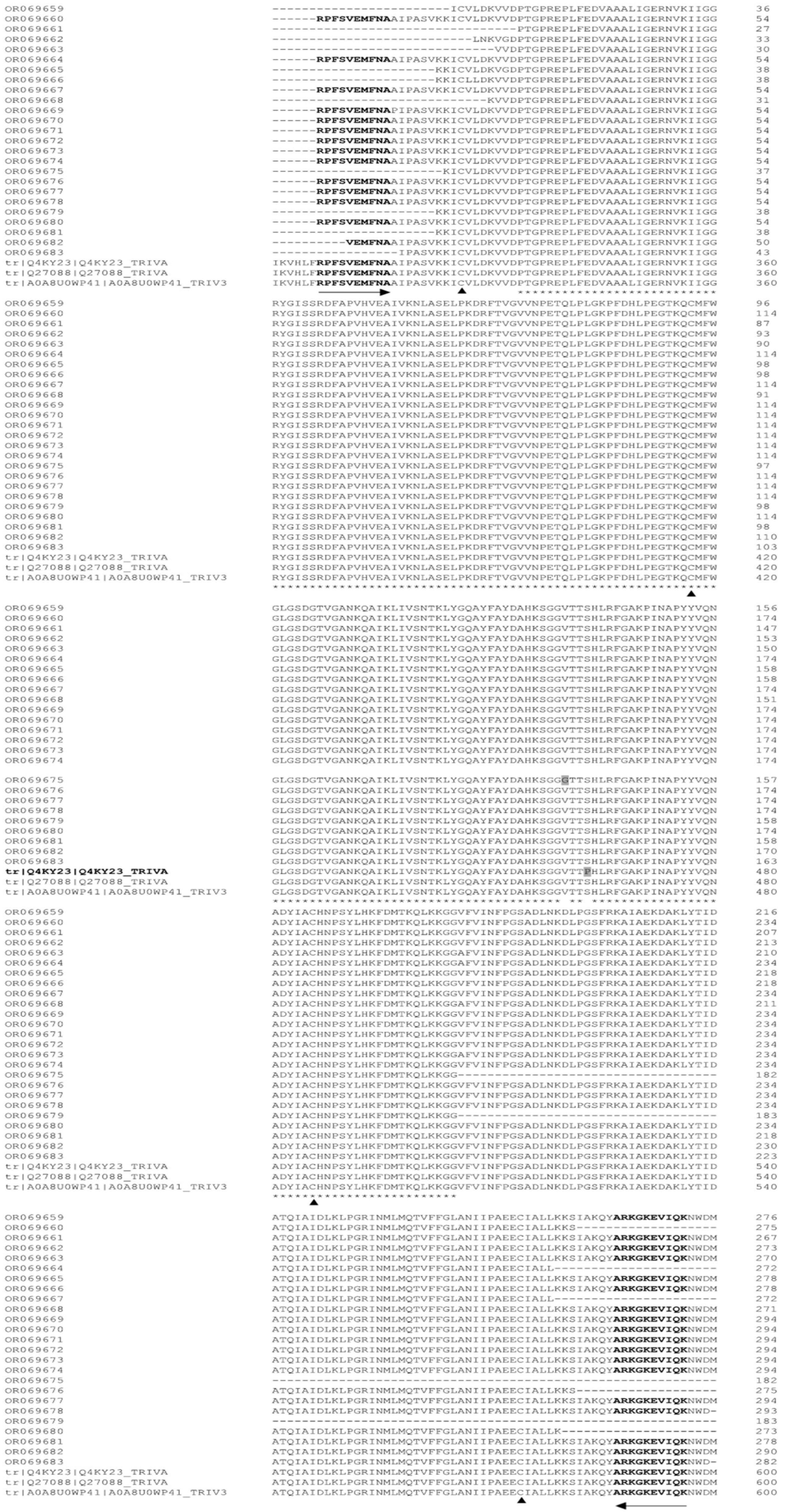
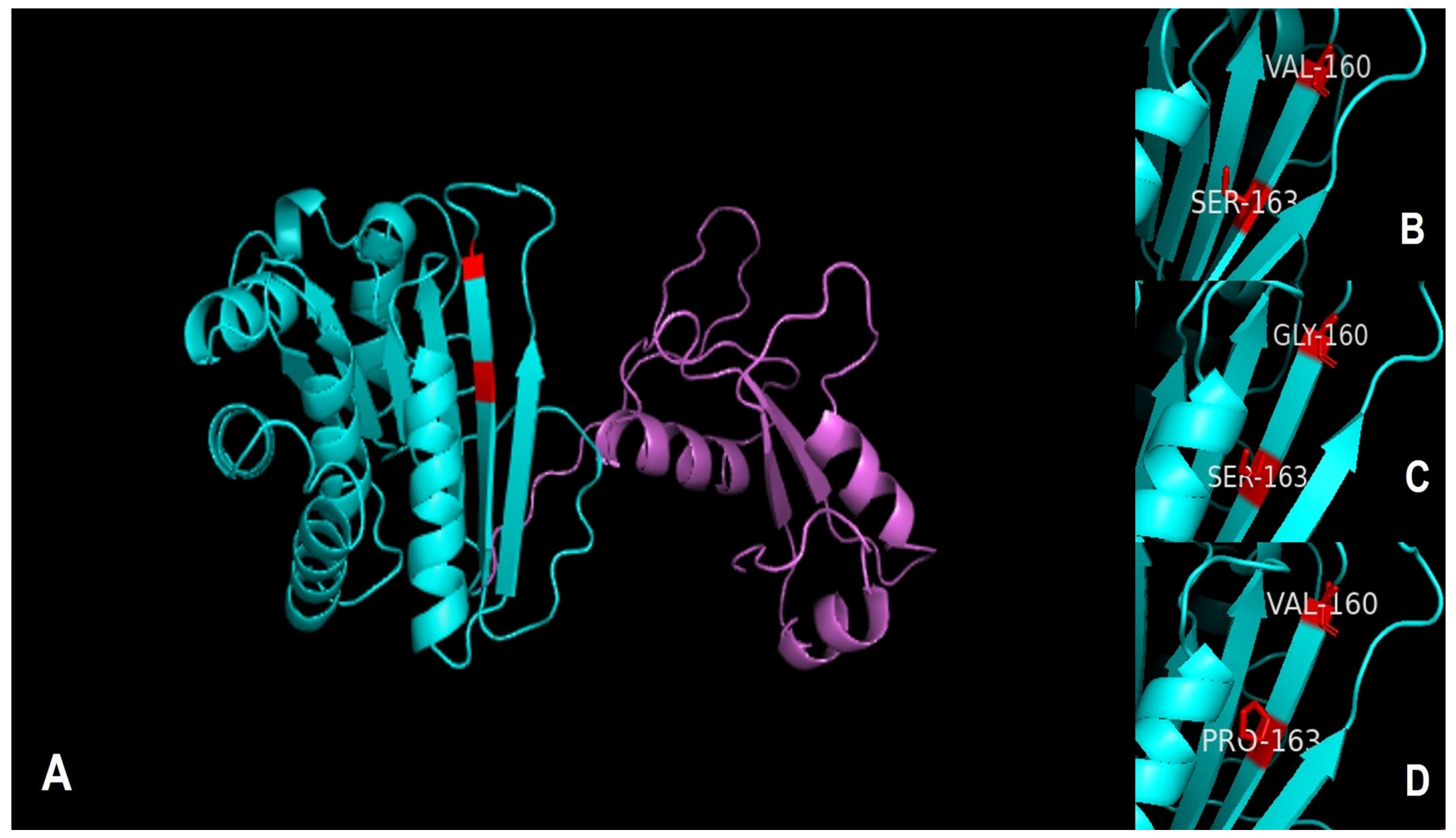
2.4. Alignment of Protein Sequences and Prediction of Three-Dimensional Structure
3. Results
3.1. Characteristics of the Study Population
3.2. Phylogenetic and Genetic Variation Findings
3.3. Alignment of pfor A and Prediction of Its Three-Dimensional Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Symptoms | Number of Patients | GenBank Accession Numbers | pfor A Subclustering (Clusters according to Figure 4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A n = 11 | p Value * A vs. B+C+D | B n = 3 | p Value * B vs. A+C+D | C n = 4 | p Value * C vs. A+B+D | D n = 8 | p Value * D vs. A+B+C | |||
| Genital burning/itching | 9 | OR069659 OR069661 OR069663 OR069665 OR069667 OR069670 OR069673 OR069679 OR069664 | 2 | 0.216 | 2 | 0.267 | 3 | 0.103 | 2 | 0.675 |
| Cervicovaginitis | 6 | OR069661 OR069663 OR069673 OR069664 OR069682 OR069670 | 2 | 1.000 | 0 | 1.000 | 3 | 0.027 ** | 1 | 0.627 |
| Vaginal discharge with unpleasant smell | 5 | OR069659 OR069662 OR069671 OR069681 OR069683 | 2 | 1.000 | 2 | 0.084 | 0 | 0.555 | 1 | 1.000 |
| Myomatosis | 1 | OR069667 | 1 | 0.423 | 0 | 1.000 | 0 | 1.000 | 0 | 1.000 |
| Dysuria | 1 | OR069668 | 0 | 1.000 | 0 | 1.000 | 1 | 0.153 | 0 | 1.000 |
| Dyspareunia | 1 | OR069673 | 0 | 1.000 | 0 | 1.000 | 1 | 0.153 | 0 | 1.000 |
| Gestational diabetes | 3 | OR069663 OR069673 OR069678 | 0 | 0.230 | 0 | 1.000 | 2 | 0.565 | 1 | 1.000 |
| Overweight/obesity | 15 | OR069659 OR069662 OR069667 OR069668 OR069669 OR069670 OR069673 OR069674 OR069676 OR069680 OR069677 OR069678 OR069679 OR069681 OR069664 | 6 | 1. 000 | 3 | 0.238 | 2 | 1.000 | 3 | 0.2183 |
| Pregnancy | 15 | OR069661 OR069660 OR069663 OR069665 OR069666 OR069671 OR069673 OR069674 OR069676 OR069675 OR069677 OR069678 OR069664 OR069682 OR069683 | 6 | 1.000 | 3 | 0.238 | 2 | 1.000 | 5 | 1.000 |
Appendix B
References
- Rowley, J.; Vander, H.S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562P. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Burguess, D. Trichomoniasis. Clin. Microbiol. Rev. 2004, 17, 794–803. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307. [Google Scholar] [CrossRef]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Van der Giezen, M.; Tovar, J.; Clark, C.G. Mitochondrion-derived organelles in protest and fungi. Int. Rev. Cytol. 2005, 244, 175–225. [Google Scholar]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef]
- Jeffery, C.J. Mass spectrometry and the search for moonlighting proteins. Mass Spectrom. Rev. 2005, 24, 772–782. [Google Scholar] [CrossRef]
- Hug, L.A.; Stechmann, A.; Roger, A. Phylogenetic distribution and histories of proteins involved in anaerobic pyruvate metabolism in eukaryotes. Mol. Biol. Evol. 2009, 27, 311–324. [Google Scholar] [CrossRef]
- Meza-Cervantes, P.; Gonzalez-Robles, A.; Cardenas-Guerra, R.E.; Ortega-Lopez, J.; Saavedra, E.; Pineda, E.; Arroyo, R. Pyruvate: Ferredoxin oxidoreductase (PFO) is a surface-associated cell-binding protein in Trichomonas vaginalis and is involved in trichomonal adherence to host cell. Microbiology 2011, 157, 3469–3482. [Google Scholar] [CrossRef]
- Hrdy, I.; Müller, M. Primary structure and eubacterial relationship of the pyruvate: Ferredoxin oxidoreductase of the amitochondriate eukaryote Trichomonas vaginalis. J. Mol. Evol. 1995, 41, 388–396. [Google Scholar] [CrossRef]
- Alderete, J.F.; Garza, G.E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect. Immun. 1985, 50, 701–707. [Google Scholar] [CrossRef]
- Song, H.O. Influence of 120 kDa Pyruvate: Ferredoxin Oxidoreductase on Pathogenicity of Trichomonas vaginalis. Korean J. Parasitol. 2016, 54, 71–74. [Google Scholar] [CrossRef]
- Moreno-Brito, V.; Yañez-Gomez, C.; Meza-Cervantes, P.; Avila-Gonzalez, L.; Rodriguez, M.A.; Ortega-Lopez, J.; Gonzalez-Robles, A.; Arroyo, R. A Trichomonas vaginalis 120 KDa protein with identity to hydrogenosome pyruvate: Ferrodoxin oxidoreductase is a surface adhesin induce by iron. Cell Microbiol. 2005, 7, 245–258. [Google Scholar] [CrossRef]
- Upcroft, J.A.; Delgadillo-Correa, M.G.; Dunne, R.L.; Sturm, A.W.; Johnson, P.J.; Upcroft, P. Genotyping Trichomonas vaginalis. Int. J. Parasitol. 2006, 36, 821–828. [Google Scholar] [CrossRef]
- Villalobos, G.; Orozco-Mosqueda, G.E.; Lopez-Perez, M.; Lopez-Escamilla, E.; Córdoba-Aguilar, A.; Rangel-Gamboa, L.; Olivo-Diaz, A.; Romero-Valdovinos, M.; Maravilla, P.; Martinez-Hernandez, F. Suitability of internal transcribed spacers (ITS) as markers for the population genetic structure of Blastocystis spp. Parasit. Vectors 2014, 7, 461. [Google Scholar] [CrossRef]
- Snipes, L.J.; Gamard, P.M.; Narcisi, E.M.; Beard, C.B.; Lehmann, T.; Secor, W.E. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J. Clin. Microbiol. 2000, 38, 3004–3009. [Google Scholar] [CrossRef]
- Rivera, W.L.; Ong, V.A.; Masalunga, M.C. Molecular characterization of Trichomonas vaginalis isolates from the Philippines. Parasitol. Res. 2009, 106, 105–110. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Nogal-Ruiz, J.J.; Arán, V.J.; Escario, J.A.; Gómez-Barrio, A.; Alderete, J.F. Determination of internal transcribed spacer regions (ITS) in Trichomonas vaginalis isolates and differentiation among Trichomonas species. Parasitol. Int. 2014, 63, 427–431. [Google Scholar] [CrossRef]
- Ertabaklar, H.; Ertuğ, S.; Çalışkan, S.Ö.; Malatyalı, E.; Bozdoğan, B. Use of internal transcribed spacer sequence polymorphisms as a method for Trichomonas vaginalis genotyping. Turkiye Parazitol. Derg. 2018, 42, 6–10. [Google Scholar] [CrossRef]
- Villalobos, G.; Sanchez-Aguillon, F.; Carmona-Maldonado, M.V.; Gonzalez-Arenas, N.R.; Lopez-Escamilla, E.; Hernandez-Castro, R.; Romero-Valdovinos, M.; Martinez-Flores, W.A.; Ramirez-Hinojosa, J.P.; Maravilla, P.; et al. Unexpected presence of Blastocystis subtype 1-3 DNA in human vaginal and sperm samples coinfected with Trichomonas vaginalis. Korean J. Parasitol. 2022, 60, 195–200. [Google Scholar] [CrossRef]
- Lopez-Escamilla, E.; Sanchez-Aguillon, F.; Alatorre-Fernandez, C.P.; Aguilar-Zapata, D.; Arroyo-Escalante, S.; Arellano, T.; Moncada-Barron, D.; Romero-Valdovinos, M.; Martinez-Hernandez, F.; Rodriguez-Zulueta, P.; et al. New Tetratrichomonas species in two patients with pleural empyema. J. Clin. Microbiol. 2013, 51, 3143–3146. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. (Version 3.81). Available online: http://www.mesquiteproject.org/ (accessed on 30 July 2023).
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Ronquis, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rozas, J.; Sanchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Hedrick, P.W. Measures of Genetics Variation. In Genetics of Populations, 2nd ed.; Jones and Bartlett Publishers: Sudbury, MA, USA, 1999; pp. 47–87. [Google Scholar]
- Fu, Y.-X.; Li, W.-H. Statistical test of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Schrodinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.8; Schrodinger, LLC: New York, NY, USA, 2015. [Google Scholar]
- Hirt, R.P. Trichomonas vaginalis virulence factors: An integrative overview. Sex. Transm. Infect. 2013, 89, 439–443. [Google Scholar] [CrossRef]
- Conrad, M.D.; Gorman, A.W.; Schillinger, J.A.; Fiori, P.R.; Arroyo, R.; Malla, N.; Dubey, M.L.; Gonzalez, J.; Blank, S.; Secor, W.E.; et al. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Neglected Trop. Dis. 2012, 6, e1573. [Google Scholar] [CrossRef]
- Dessì, D.; Rappelli, P.; Diaz, N.; Cappuccinelli, P.; Fiori, P.L. Mycoplasma hominis and Trichomonas vaginalis: A unique case of symbiotic relationship between two obligate human parasites. Front. Biosci. 2006, 11, 2028–2034. [Google Scholar]
- Brotman, R.M.; Bradford, L.L.; Conrad, M.; Gajer, P.; Ault, K.; Peralta, L.; Forney, L.J.; Carlton, J.N.; Abdo, Z.; Ravel, J. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex. Transm. Dis. 2012, 39, 807–812. [Google Scholar] [CrossRef]
- Pineda, E.; Encalada, R.; Rodríguez-Zavala, J.S.; Olivos-García, A.; Moreno-Sánchez, R.; Saavedra, E. Pyruvate: Ferredoxin oxidoreductase and bifunctional aldehyde-alcohol dehydrogenase are essential for energy metabolism under oxidative stress in Entamoeba histolytica. FEBS J. 2010, 277, 3382–3395. [Google Scholar] [CrossRef]
- Townson, S.M.; Upcroft, J.A.; Upcroft, P. Characterization and purification of pyruvate: Ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 1996, 79, 183–193. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Müller, M. Anaerobic pyruvate metabolism of Tritrichomonas foetus and Trichomonas vaginalis hydrogenosomes. Mol. Biochem. Parasitol. 1986, 20, 57–65. [Google Scholar] [CrossRef]
- Alderete, J.F.; Millsap, K.W.; Lehker, M.W.; Benchimol, M. Enzymes of microbial pathogens and Trichomonas vaginalis: Molecular mimicry and functional diversity. Cell. Microbiol. 2001, 3, 359–370. [Google Scholar] [CrossRef]
- Baum, D.A.; Smith, S.D. Tree Thinking: An Introduction to Phylogenetic Biology; Roberts and Company Publishers: Greenwood Village, CO, USA, 2013; pp. 56–78. [Google Scholar]
- Alarcon-Valdes, P.; Villalobos, G.; Martinez-Flores, W.A.; Lopez-Escamilla, E.; Gonzalez-Arenas, N.R.; Romero-Valdovinos, M.; Martinez-Hernandez, F.; Santillan-Benitez, J.G.; Maravilla, P. Can the pyruvate: Ferredoxin oxidoreductase (PFOR) gene be used as an additional marker to discriminate among Blastocystis strains or subtypes? Parasit. Vectors 2018, 11, 564. [Google Scholar] [CrossRef]
- Alrefaei, A.F.; Albeshr, M.F.; Alharbi, S.N.; Alrefaei, A.F.; Almutairi, M.H.; Almutairi, B.O.; Nader, J.L.; Manoharadas, S. Molecular characterization of the Fe-hydrogenase gene marker in Trichomonas gallinae isolated from birds in Riyadh, Saudi Arabia. Parasitol. Int. 2021, 8, 102263. [Google Scholar] [CrossRef]
- Mao, M.; Liu, H.L. Genetic diversity of Trichomonas vaginalis clinical isolates from Henan province in central China. Pathog. Glob. Health 2015, 109, 242–246. [Google Scholar] [CrossRef]
- Paulish-Miller, T.E.; Augostini, P.; Schuyler, J.A.; Smith, W.L.; Mordechai, E.; Adelson, M.E.; Gygax, S.E.; Secor, W.E.; Hilbert, D.W. Trichomonas vaginalis metronidazole resistance is associated with single nucleotide polymorphisms in the nitroreductase genes ntr4Tv and ntr6Tv. Antimicrob. Agents Chemother. 2014, 58, 2938–2943. [Google Scholar] [CrossRef]

| Population | n | Expected Heterozygosity (Hd) | Nucleotide Diversity (π) | Haplotype Polymorphism (θ) | Tajima’s D (p) | Fu and Li’s D (p) |
|---|---|---|---|---|---|---|
| Whole pfor A * | 32 | 0.790 | 0.0019 | 0.0033 | −1.267 (p > 0.10) | −1.540 (p > 0.10) |
| Mexican samples pfor A | 26 | 0.815 | 0.0021 | 0.0031 | −1.041 (p > 0.10) | −1.069 (p > 0.10) |
| Whole ITS ‡ | 68 | 0.493 | 0.0027 | 0.0087 | −1.942 (p < 0.05) | −3.253 (p < 0.05) |
| Mexican samples ITS | 16 | 0.325 | 0.0011 | 0.0010 | 0.155 (p > 0.10) | 0.688 (p > 0.10) |
| ITS type 1 | 28 | 0.648 | 0.0042 | 0.0087 | −1.723 (p > 0.10) | −1.997 (p > 0.10) |
| ITS type 2 + | 42 | 0.487 | 0.0058 | 0.0022 | −2.443 (p < 0.05) | −2.708 (p < 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Hernandez, F.; Sanchez-Aguillon, F.; Martinez-Ocaña, J.; Gonzalez-Arenas, N.R.; Romero-Valdovinos, M.; Lopez-Escamilla, E.; Maravilla, P.; Villalobos, G. Genetic Variability of the Internal Transcribed Spacer and Pyruvate:Ferredoxin Oxidoreductase Partial Gene of Trichomonas vaginalis from Female Patients. Microorganisms 2023, 11, 2240. https://doi.org/10.3390/microorganisms11092240
Martinez-Hernandez F, Sanchez-Aguillon F, Martinez-Ocaña J, Gonzalez-Arenas NR, Romero-Valdovinos M, Lopez-Escamilla E, Maravilla P, Villalobos G. Genetic Variability of the Internal Transcribed Spacer and Pyruvate:Ferredoxin Oxidoreductase Partial Gene of Trichomonas vaginalis from Female Patients. Microorganisms. 2023; 11(9):2240. https://doi.org/10.3390/microorganisms11092240
Chicago/Turabian StyleMartinez-Hernandez, Fernando, Fabiola Sanchez-Aguillon, Joel Martinez-Ocaña, Nelly Raquel Gonzalez-Arenas, Mirza Romero-Valdovinos, Eduardo Lopez-Escamilla, Pablo Maravilla, and Guiehdani Villalobos. 2023. "Genetic Variability of the Internal Transcribed Spacer and Pyruvate:Ferredoxin Oxidoreductase Partial Gene of Trichomonas vaginalis from Female Patients" Microorganisms 11, no. 9: 2240. https://doi.org/10.3390/microorganisms11092240






