Impact of Protein Aggregates on Sporulation and Germination of Bacillus subtilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Sporulation Induction for TLFM
2.3. Spore Harvesting
2.4. Germination Induction for TLFM
2.5. Semi-Lethal Treatment of Spore Suspensions
2.6. Time-Lapse Fluorescence Microscopy (TLFM)
2.7. Statistical Analysis
3. Results
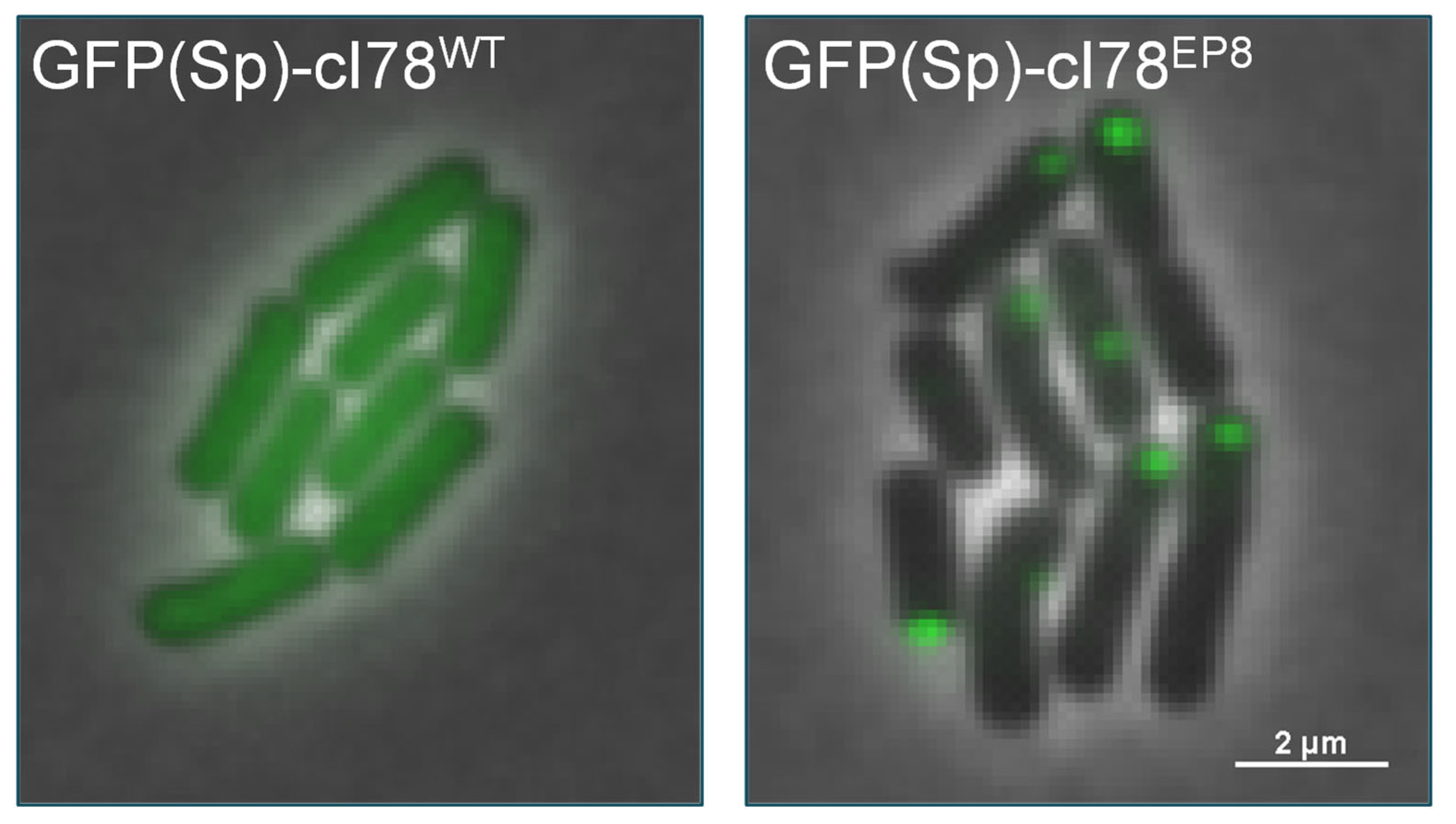
3.1. Implementing a Synthetic PA Model System in B. subtilis
3.2. PAs Readily Persist throughout the Sporulation and Germination Processes
3.3. PA-Harboring Endospores Are Not Affected in Heat or UV Survival
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein aggregation in bacteria. FEMS Microbiol. Rev. 2020, 44, 54–72. [Google Scholar] [CrossRef]
- Govers, S.K.; Mortier, J.; Adam, A.; Aertsen, A. Protein aggregates encode epigenetic memory of stressful encounters in individual Escherichia coli cells. PLoS Biol. 2018, 16, e2003853. [Google Scholar] [CrossRef] [PubMed]
- Govers, S.K.; Dutré, P.; Aertsen, A. In Vivo Disassembly and Reassembly of Protein Aggregates in Escherichia coli. J. Bacteriol. 2014, 196, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Mortier, J.; Tadesse, W.; Govers, S.K.; Aertsen, A. Stress-induced protein aggregates shape population heterogeneity in bacteria. Curr. Genet. 2019, 65, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.B.; Madden, R.; Demarez, A.; Stewart, E.J.; Taddei, F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. USA 2008, 105, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Coquel, A.-S.; Jacob, J.-P.; Primet, M.; Demarez, A.; Dimiccoli, M.; Julou, T.; Moisan, L.; Lindner, A.B.; Berry, H. Localization of Protein Aggregation in Escherichia coli Is Governed by Diffusion and Nucleoid Macromolecular Crowding Effect. PLoS Comput. Biol. 2013, 9, e1003038. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Seybert, A.; König, L.; Pruggnaller, S.; Haselmann, U.; Sourjik, V.; Weiss, M.; Frangakis, A.S.; Mogk, A.; Bukau, B. Quantitative and spatio-temporal features of protein aggregation in Escherichia coli and consequences on protein quality control and cellular ageing. EMBO J. 2010, 29, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Rokney, A.; Shagan, M.; Kessel, M.; Smith, Y.; Rosenshine, I.; Oppenheim, A.B.E. coli Transports Aggregated Proteins to the Poles by a Specific and Energy-Dependent Process. J. Mol. Biol. 2009, 392, 589–601. [Google Scholar] [CrossRef]
- Gray, W.T.; Govers, S.K.; Xiang, Y.; Parry, B.R.; Campos, M.; Kim, S.; Jacobs-Wagner, C. Nucleoid Size Scaling and Intracellular Organization of Translation across Bacteria. Cell 2019, 177, 1632–1648.e20. [Google Scholar] [CrossRef]
- Schramm, F.D.; Schroeder, K.; Alvelid, J.; Testa, I.; Jonas, K. Growth-driven displacement of protein aggregates along the cell length ensures partitioning to both daughter cells inCaulobacter crescentus. Mol. Microbiol. 2019, 111, 1430–1448. [Google Scholar] [CrossRef]
- Dewachter, L.; Bollen, C.; Wilmaerts, D.; Louwagie, E.; Herpels, P.; Matthay, P.; Khodaparast, L.; Khodaparast, L.; Rousseau, F.; Schymkowitz, J.; et al. The Dynamic Transition of Persistence toward the Viable but Nonculturable State during Stationary Phase Is Driven by Protein Aggregation. mBio 2021, 12, e0070321. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.-Y.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Article ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth. Mol. Cell 2018, 73, 143–156.e4. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.B.; Demarez, A. Protein aggregation as a paradigm of aging. Biochim. Biophys. Acta BBA Gen. Subj. 2009, 1790, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Proenca, A.M.; Rang, C.U.; Qiu, A.; Shi, C.; Chao, L. Cell aging preserves cellular immortality in the presence of lethal levels of damage. PLoS Biol. 2019, 17, e3000266. [Google Scholar] [CrossRef] [PubMed]
- Proenca, A.M.; Rang, C.U.; Buetz, C.; Shi, C.; Chao, L. Age structure landscapes emerge from the equilibrium between aging and rejuvenation in bacterial populations. Nat. Commun. 2018, 9, 3722. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Chao, L.; Proenca, A.M.; Qiu, A.; Chao, J.; Rang, C.U. Allocation of gene products to daughter cells is determined by the age of the mother in single Escherichia coli cells. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200569. [Google Scholar] [CrossRef] [PubMed]
- Mortier, J.; Gayán, E.; Van Eyken, R.; Montaguth, O.E.T.; Khodaparast, L.; Khodaparast, L.; Houben, B.; Carpentier, S.; Rousseau, F.; Schymkowitz, J.; et al. Gene Erosion Can Lead to Gain-of-Function Alleles That Contribute to Bacterial Fitness. mBio 2021, 12, e0112921. [Google Scholar] [CrossRef]
- Saarikangas, J.; Barral, Y. Protein aggregates are associated with replicative aging without compromising protein quality control. eLife 2015, 4, e06197. [Google Scholar] [CrossRef]
- Riley, E.P.; Schwarz, C.; Derman, A.I.; Lopez-Garrido, J. Milestones in Bacillus subtilis sporulation research. Microb. Cell 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Khanna, K.; Lopez-Garrido, J.; Pogliano, K. Shaping an Endospore: Architectural Transformations During Bacillus subtilis Sporulation. Annu. Rev. Microbiol. 2020, 74, 361–386. [Google Scholar] [CrossRef]
- Bejerano-Sagie, M.; Oppenheimer-Shaanan, Y.; Berlatzky, I.; Rouvinski, A.; Meyerovich, M.; Ben-Yehuda, S. A Checkpoint Protein That Scans the Chromosome for Damage at the Start of Sporulation in Bacillus subtilis. Cell 2006, 125, 679–690. [Google Scholar] [CrossRef]
- De Jong, I.G.; Beilharz, K.; Kuipers, O.P.; Veening, J.W. Live Cell Imaging of Bacillus subtilis and Streptococcus pneumoniae using Automated Time-lapse Microscopy. J. Vis. Exp. 2011, 53, e3145. [Google Scholar]
- Veening, J.-W.; Stewart, E.J.; Berngruber, T.W.; Taddei, F.; Kuipers, O.P.; Hamoen, L.W. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc. Natl. Acad. Sci. USA 2008, 105, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Overkamp, W.; Beilharz, K.; Weme, R.D.O.; Solopova, A.; Karsens, H.; Kovács, T.; Kok, J.; Kuipers, O.P.; Veening, J.-W. Benchmarking Various Green Fluorescent Protein Variants in Bacillus subtilis, Streptococcus pneumoniae, and Lactococcus lactis for Live Cell Imaging. Appl. Environ. Microbiol. 2013, 79, 6481–6490. [Google Scholar] [CrossRef] [PubMed]
- Matavacas, J.; Anand, D.; von Wachenfeldt, C. New insights into the disulfide stress response by the Bacillus subtilis Spx system at a single-cell level. Mol. Microbiol. 2023, 120, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Errington, J. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Genet. 2003, 1, 117–126. [Google Scholar] [CrossRef]
- Kain, J.; He, G.G.; Losick, R. Polar Localization and Compartmentalization of ClpP Proteases during Growth and Sporulation in Bacillus subtilis. J. Bacteriol. 2008, 190, 6749–6757. [Google Scholar] [CrossRef]
- Simmons, L.A.; Grossman, A.D.; Walker, G.C. Clp and Lon Proteases Occupy Distinct Subcellular Positions in Bacillus subtilis. J. Bacteriol. 2008, 190, 6758–6768. [Google Scholar] [CrossRef]
- Kirstein, J.; Strahl, H.; Molière, N.; Hamoen, L.W.; Turgay, K. Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol. Microbiol. 2008, 70, 682–694. [Google Scholar] [CrossRef]
- Matavacas, J.; von Wachenfeldt, C. Update on the Protein Homeostasis Network in Bacillus subtilis. Front. Microbiol. 2022, 13, 865141. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Okumus, B.; Chien, P.; Baker, T.A.; Paulsson, J. Segregation of molecules at cell division reveals native protein localization. Nat. Methods 2012, 9, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Garsin, D.A.; Losick, R. Self-Reinforcing Activation of a Cell-Specific Transcription Factor by Proteolysis of an Anti-σ Factor in B. subtilis. Mol. Cell 2001, 8, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Prepiak, P.; Defrancesco, M.; Spadavecchia, S.; Mirouze, N.; Albano, M.; Persuh, M.; Fujita, M.; Dubnau, D. MecA dampens transitions to spore, biofilm exopolysaccharide and competence expression by two different mechanisms. Mol. Microbiol. 2011, 80, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Gerth, U.; Krüger, E.; Derré, I.; Msadek, T.; Hecker, M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 2002, 28, 787–802. [Google Scholar] [CrossRef]
- Liu, J.; Cosby, W.M.; Zuber, P. Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol. Microbiol. 1999, 33, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Msadek, T.; Dartois, V.; Kunst, F.; Herbaud, M.; Denizot, F.; Rapoport, G. ClpP of Bacillus subtilisis required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 1998, 27, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Nanamiyaa, H.; Takahashia, K.; Fujitab, M.; Kawamura, F. Deficiency of the Initiation Events of Sporulation in Bacillus subtilis clpP Mutant Can Be Suppressed by a Lack of the Spo0E Protein Phosphatase. Biochem. Biophys. Res. Commun. 2000, 279, 229–233. [Google Scholar] [CrossRef]
- Turgay, K.; Hahn, J.; Burghoorn, J.; Dubnau, D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998, 17, 6730–6738. [Google Scholar]
- Runde, S.; Molière, N.; Heinz, A.; Maisonneuve, E.; Janczikowski, A.; Elsholz, A.K.W.; Gerth, U.; Hecker, M.; Turgay, K. The role of thiol oxidative stress response in heat-induced protein aggregate formation during thermotolerance in Bacillus subtilis. Mol. Microbiol. 2014, 91, 1036–1052. [Google Scholar] [CrossRef]
- Hantke, I.; Schäfer, H.; Janczikowski, A.; Turgay, K. YocM a small heat shock protein can protect Bacillus subtilis cells during salt stress. Mol. Microbiol. 2018, 111, 423–440. [Google Scholar] [CrossRef]






| Strain | Description | Source or Reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | Used for transformation of plasmids | Laboratory collection |
| Bacillus subtilis | ||
| PS832 | B. subtilis PS832 wild type; prototrophic derivative of B. subtilis 168 | Received from Peter Setlow (University of Connecticut) |
| PS832 amyE::PHyperspank gfp(Sp)-cI78EP8 | PS832 with PHyperspank gfp(Sp)-cI78EP8 cassette inserted in its amyE locus | This study |
| PS832 amyE::PHyperspank gfp(Sp)-cI78WT | PS832 with PHyperspank gfp(Sp)-cI78WT cassette inserted in its amyE locus | This study |
| PS832 amyE::PHyperspank gfp(Sp) | PS832 with PHyperspank gfp(Sp) cassette inserted in its amyE locus | This study |
| Plasmid | Description | Source or Reference |
|---|---|---|
| pTrc99A-Ptrc-mCer-cI78EP8 | Contains cI78EP8 which encodes an aggregate-prone truncated version of the lambda prophage repressor protein cI | [1] |
| pTrc99A-Ptrc-mCer-cI78WT | Contains cI78WT which encodes a soluble truncated version of the lambda prophage repressor protein cI | [1] |
| pDR111-gfp(Sp) | Contains gfp(Sp) under IPTG-inducible PHyperspank to integrate into the amyE locus | [20] |
| pDR111-gfp(Sp)-cI78EP8 | Contains gfp(Sp)-cI78EP8 under IPTG-inducible PHyperspank to integrate into the amyE locus | This study |
| pDR111-gfp(Sp)-cI78WT | Contains gfp(Sp)-cI78WT under IPTG-inducible PHyperspank to integrate into the amyE locus | This study |
| Name | Sequence | Purpose |
|---|---|---|
| P1 | ACACATGGTATGGATGAATTGTATAAAGGCTCTGGCTCTGGCTCTAGCCCTTCAATCGCCAGAGAA | Creates an amplicon of cI78EP8 and cI78WT to insert into pDR111-gfp(Sp) |
| P2 | CAGAATTGCCGACCTTGACTAGTGCTCATTATTAGCCAAACGTCTCTTCAGGCC | Creates an amplicon of cI78EP8 and cI78WT to insert into pDR111-gfp(Sp) |
| P3 | AGAGCCAGAGCCAGAGCCTTTATACAATTCATCCATACCATGTGT | Linearizes the pDR111-gfp(Sp) vector to insert the cI78EP8 and cI78WT amplicon |
| P4 | TAATAATGAGCACTAGTCAAGGTCG | Linearizes the pDR111-gfp(Sp) vector to insert the cI78EP8 and cI78WT amplicon |
| P5 | CGTTTCGGTGATGAAGATCTTC | Control and sequencing of insertions in pDR111-gfp(Sp) |
| P6 | CCTCGTTTCCACCGAATTAGC | Control and sequencing of insertions in pDR111-gfp(Sp) |
| P7 | GTTCTGTTTCTGCTTCGGTATG | Control and sequencing of insertions in the amyE locus |
| P8 | GCAAATGCATAACTGCTTCCAAC | Control and sequencing of insertions in the amyE locus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortier, J.; Cambré, A.; Schack, S.; Christie, G.; Aertsen, A. Impact of Protein Aggregates on Sporulation and Germination of Bacillus subtilis. Microorganisms 2023, 11, 2365. https://doi.org/10.3390/microorganisms11092365
Mortier J, Cambré A, Schack S, Christie G, Aertsen A. Impact of Protein Aggregates on Sporulation and Germination of Bacillus subtilis. Microorganisms. 2023; 11(9):2365. https://doi.org/10.3390/microorganisms11092365
Chicago/Turabian StyleMortier, Julien, Alexander Cambré, Sina Schack, Graham Christie, and Abram Aertsen. 2023. "Impact of Protein Aggregates on Sporulation and Germination of Bacillus subtilis" Microorganisms 11, no. 9: 2365. https://doi.org/10.3390/microorganisms11092365





