Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Ethical Approval
2.2. Diagnostic Tools
2.2.1. Combination of Vaginal Discharge and Vaginal pH
2.2.2. Amsel-Like Criteria
2.2.3. Nugent Score
2.2.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
DNA Extraction
Quantitative PCR Assay
2.2.5. MALDI-TOF Mass Spectrometry (“VAGI-TOF”)
2.3. Statistical Methods
3. Results
3.1. Main Patient Characteristics
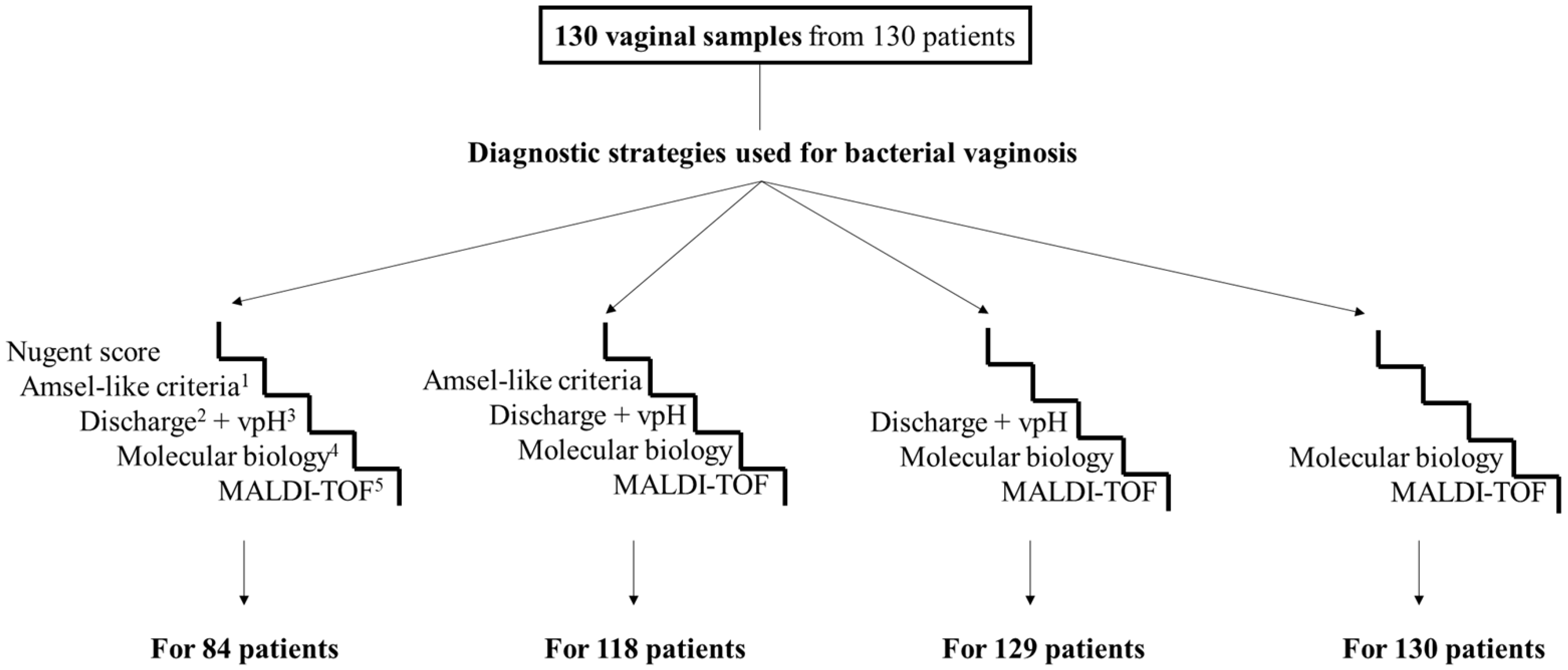
3.2. Diagnosis of Bacterial Vaginosis According to the Different Strategies
3.3. Performance of Diagnostic Strategies for Bacterial Vaginosis
3.4. Development of a New Diagnostic Tool Using Mass Spectrometry, “VAGI-TOF”
Reproducibility and Global Aspect Ratio of the Spectra
3.5. Analysis of the Spectra According to the Diagnostic Tools Already Used
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial vaginosis: What do we currently know? Front. Cell Infect. Microbiol. 2022, 11, 672429. [Google Scholar] [CrossRef] [PubMed]
- Bitew, A.; Abebaw, Y.; Bekele, D.; Mihret, A. Prevalence of bacterial vaginosis and associated risk factors among women complaining of genital tract infection. Int. J. Microbiol. 2017, 2017, 4919404. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Thomas, K.K.; Fiedler, T.L.; Ringwood, K.; Fredricks, D.N. Risks for acquisition of bacterial vaginosis among women who report sex with women: A cohort study. PLoS ONE 2010, 5, e11139. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Blanchard, E.; Taylor, C.M.; Aaron, K.J.; Talluri, R.; Griswold, M.E.; Redden, D.T.; Luo, M.; Welsh, D.A.; Van Der Pol, W.J.; et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: A prospective study. J. Infect. Dis. 2018, 218, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Panici, P.B. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016, 293, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Achilles, S.L.; Austin, M.N.; Meyn, L.A.; Mhlanga, F.; Chirenje, Z.M.; Hillier, S.L. Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet. Gynecol. 2018, 218, 622.e1. [Google Scholar] [CrossRef] [PubMed]
- Fethers, K.A.; Fairley, C.K.; Morton, A.; Hocking, J.S.; Hopkins, C.; Kennedy, L.J.; Fehler, G.; Bradshaw, C.S. Early sexual experiences and risk factors for bacterial vaginosis. J. Infect. Dis. 2009, 200, 1662–1670. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Nansel, T.R.; Brotman, R.M.; Zhang, J.; Yu, K.-F.; Schwebke, J.R.; Andrews, W.W. Personal hygienic behaviors and bacterial vaginosis. Sex. Transm. Dis. 2010, 37, 94. [Google Scholar] [CrossRef]
- Joyisa, N.; Moodley, D.; Nkosi, T.; Talakgale, R.; Karim, Q.A. Asymptomatic bacterial vaginosis in pregnancy and missed opportunities for treatment: A cross-sectional observational study. Infect. Dis. Obstet. Gynecol. 2019, 2019, 7808179. [Google Scholar] [CrossRef]
- Hay, P. Bacterial vaginosis. Medicine 2014, 42, 359–363. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Schwebke, J.R.; Zhang, J.; Nansel, T.R.; Yu, K.-F.; Andrews, W.W. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet. Gynecol. 2004, 104, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Koumans, E.H.; Sternberg, M.; Bruce, C.B.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Bretelle, F.; Rozenberg, P.; Pascal, A.; Favre, R.; Bohec, C.; Loundou, A.; Senat, M.-V.; Aissi, G.; Lesavre, N.; Brunet, J.; et al. High Atopobium vaginae and Gardnerella vaginalis vaginal loads are associated with preterm birth. Clin. Infect. Dis. 2015, 60, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Redelinghuys, M.J.; Ehlers, M.M.; Dreyer, A.W.; Kock, M.M. Normal flora and bacterial vaginosis in pregnancy: An overview. Crit. Rev. Microbiol. 2016, 42, 352–363. [Google Scholar] [CrossRef]
- Tamarelle, J.; Thiébaut, A.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef]
- Coleman, J.S.; Gaydos, C.A. Molecular diagnosis of bacterial vaginosis: An update. J. Clin. Microbiol. 2018, 56, e00342.e18. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Smith, W.L.; Chadwick, S.G.; Toner, G.; Mordechai, E.; Adelson, M.E.; Aguin, T.J.; Sobel, J.D.; Gygax, S.E. Development and validation of a highly accurate quantitative real-time PCR assay for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 2016, 54, 1017–1024. [Google Scholar] [CrossRef]
- Cartwright, C.P.; Pherson, A.J.; Harris, A.B.; Clancey, M.S.; Nye, M.B. Multicenter study establishing the clinical validity of a nucleic-acid amplification–based assay for the diagnosis of bacterial vaginosis. Diagn. Microbiol. Infect. Dis. 2018, 92, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.P.; Lembke, B.D.; Ramachandran, K.; Body, B.A.; Nye, M.B.; Rivers, C.A.; Schwebke, J.R. Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 2012, 50, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Breding, K.; Selbing, A.; Farnebäck, M. Diagnosis of bacterial vaginosis using a novel molecular real-time PCR test. J. Women’s Health Gyn. 2020, 7, 1–7. [Google Scholar]
- Gaydos, C.A.; Beqaj, S.; Schwebke, J.R.; Lebed, J.; Smith, B.; Davis, T.E.; Fife, K.H.; Nyirjesy, P.; Spurrell, T.; Furgerson, D.; et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet. Gynecol. 2017, 130, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Munckhof, E.H.A.v.D.; van Sitter, R.L.; Boers, K.E.; Lamont, R.F.; Witt, R.T.; le Cessie, S.; Knetsch, C.W.; van Doorn, L.-J.; Quint, W.G.V.; Molijn, A.; et al. Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. Eur. J. Clin. Microbiol. 2019, 38, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Yourshaw, C.; Chintalapudi, M.R.; Turner, C.; Murphy, E. Diagnostic evaluation of a multiplex quantitative real-time PCR assay for bacterial vaginosis. J. Women’s Health Care 2016, 5, 2167–2420. [Google Scholar] [CrossRef]
- Menard, J.P.; Fenollar, F.; Henry, M.; Bretelle, F.; Raoult, D. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin. Infect. Dis. 2008, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.-P.; Mazouni, C.; Fenollar, F.; Raoult, D.; Boubli, L.; Bretelle, F. Diagnostic accuracy of quantitative real-time PCR assay versus clinical and Gram stain identification of bacterial vaginosis. Eur. J. Clin. Microbiol. 2010, 29, 1547–1552. [Google Scholar] [CrossRef]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-assisted laser desorption ionization–time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- La Scola, B.; Raoult, D. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS ONE 2009, 4, e8041. [Google Scholar] [CrossRef]
- Pinault, L.; Chabrière, E.; Raoult, D.; Fenollar, F. Direct identification of pathogens in urine by use of a specific matrix-assisted laser desorption ionization–time of flight spectrum database. J. Clin. Microbiol. 2019, 57, e01678.e18. [Google Scholar] [CrossRef] [PubMed]
- Forsum, U.; Hallén, A.; Larsson, P.-G. Bacterial vaginosis–a laboratory and clinical diagnostics enigma: Review article II. APMIS 2005, 113, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chavoustie, S.E.; Eder, S.E.; Koltun, W.D.; Lemon, T.R.; Mitchell, C.; Nyirjesy, P.; Sobel, J.D.; Sobel, R.; Villanueva, R. Experts explore the state of bacterial vaginosis and the unmet needs facing women and providers. Int. J. Gynaecol. Obstet. 2017, 137, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Antien, D. Prise en charge des leucorrhées par le médecin généraliste. Étude d’une population de 399 médecins généralistes. Sci. du Vivant [q-bio]. 2019, 52. [Google Scholar]
- Wilson, J. 2004, Managing recurrent bacterial vaginosis. Sex Transm Infect 2004, 80, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F.; Zeeshan, F.; Younus, R.; Yasmin, H.; Bugti, S.; Hassan, A. Prevalence of bacterial vaginosis in females of child-bearing age and utility of pH and Whiff Test in Diagnosis. J. Rawalpindi Med. Coll. 2020, 24, 51–56. [Google Scholar] [CrossRef]
- Majigo, M.V.; Kashindye, P.; Mtulo, Z. Bacterial vaginosis, the leading cause of genital discharge among women presenting with vaginal infection in Dar es Salaam, Tanzania. Afr. Health Sci. 2021, 21, 531–537. [Google Scholar] [CrossRef]
- Thomason, J.L.; Gelbart, S.M.; Scaglione, N.J. Bacterial vaginosis: Current review with indications for asymptomatic therapy. Am. J. Obstet. Gynecol. 1991, 165, 1210–1217. [Google Scholar] [CrossRef]
- Ugwumadu, A.; Reid, F.; Hay, P.; Manyonda, I. Natural history of bacterial vaginosis and intermediate flora in pregnancy and effect of oral clindamycin. Obstet. Gynecol. 2004, 104, 114–119. [Google Scholar] [CrossRef]
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of bacterial vaginosis and vaginal flora changes in peri-and postmenopausal women. J. Clin. Microbiol. 2002, 40, 2147–2152. [Google Scholar] [CrossRef]
- Leitich, H.; Kiss, H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best. Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef]
- Hillier, S.L.; Krohn, M.A.; Nugent, R.P.; Gibbs, R.S.; Vaginal Infections and Prematurity Study Group. Characteristics of three vaginal flora patterns assessed Gram stain among pregnant women. Am. J. Obstet. Gynecol. 1992, 166, 938–944. [Google Scholar] [CrossRef]
- Bretelle, F.; Loubière, S.; Desbriere, R.; Loundou, A.; Blanc, J.; Heckenroth, H.; Schmitz, T.; Benachi, A.; Haddad, B.; Mauviel, F.; et al. Effectiveness and costs of molecular screening and treatment for bacterial vaginosis to prevent preterm birth: The AuTop Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 894–902. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Unemo, M. Composition of the vaginal microbiota in women of reproductive age–sensitive and specific molecular diagnosis of bacterial vaginosis is possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef]
- Madhivanan, P.; Krupp, K.; Li, T.; Ravi, K.; Selezneva, J.; Srinivas, V.; Arun, A.; Klausner, J.D. Performance of BVBlue Rapid Test in detecting bacterial vaginosis among women in Mysore, India. Infect. Dis. Obstet. Gynecol. 2014, 2014, 908313. [Google Scholar] [CrossRef]
- Blankenstein, T.; Lytton, S.D.; Leidl, B.; Atweh, E.; Friese, K.; Mylonas, I. Point-of-care (POC) diagnosis of bacterial vaginosis using VGTestTM ion mobility spectrometry (IMS) in a routine ambulatory care gynecology clinic. Arch. Gynecol. Obstet. 2015, 292, 355–362. [Google Scholar] [CrossRef]
- Banks, M.; Amirghasemi, F.; Mitchell, E.; Mousavi, M.P.S. Home-Based Electrochemical Rapid Sensor (HERS): A diagnostic tool for bacterial vaginosis. Sensors 2023, 23, 1891. [Google Scholar] [CrossRef]
- Tanaka, K. The origin of macromolecule ionization by laser irradiation (Nobel lecture). Angew. Chem. Int. Ed. 2003, 42, 3860–3870. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhang, G.; Fan, Y.-Y.; Yang, X.; Sui, W.-J.; Lu, X.-X. Direct identification of bacteria causing urinary tract infections by combining matrix-assisted laser desorption ionization-time of flight mass spectrometry with UF-1000i urine flow cytometry. J. Microbiol. Methods 2013, 92, 231–235. [Google Scholar] [CrossRef]
- March Rosselló, G.A.; Gutiérrez Rodríguez, M.P.; Leonardo, L.; Domingo, A.O.; Pérez, M.A.B. Procedure for microbial identification based on matrix-assisted laser desorption/ionization-time of flight mass spectrometry from screening-positive urine samples. Apmis 2014, 122, 790–795. [Google Scholar] [CrossRef]
- Nyvang Hartmeyer, G.; Kvistholm Jensen, A.; Böcher, S.; Bartels, M.D.; Pedersen, M.; Clausen, M.E.; Abdul-Redha, R.; Dargis, R.; Schouenborg, P.; Højlyng, N.; et al. Mass spectrometry: Pneumococcal meningitis verified and Brucella species identified in less than half an hour. Scand. J. Infect. Dis. 2010, 42, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Oya, A.L. Direct Application of MALDI-TOF Mass Spectrometry to Cerebrospinal Fluid for Pathogen Identification. In The Use of Mass Spectrometry Technology (MALDI-TOF) in Clinical Microbiology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–165. [Google Scholar]
- Marinach-Patrice, C.; Fekkar, A.; Atanasova, R.; Gomes, J.; Djamdjian, L.; Brossas, J.-Y.; Meyer, I.; Buffet, P.; Snounou, G.; Datry, A.; et al. Rapid species diagnosis for invasive candidiasis using mass spectrometry. PLoS ONE 2010, 5, e8862. [Google Scholar] [CrossRef] [PubMed]
- Eigner, U.; Holfelder, M.; Oberdorfer, K.; Betz-Wild, U.; Bertsch, D.; Fahr, A.-M. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clin. Lab. 2009, 55, 289–296. [Google Scholar] [PubMed]
- Mellmann, A.; Cloud, J.; Maier, T.; Ramminger, I.; Iwen, P.; Dunn, J.; Hall, G.; Wilson, D.; Lasala, P.; Kostrzewa, M.; et al. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 2008, 46, 1946–1954. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- Tran, A.; Alby, K.; Kerr, A.; Jones, M.; Gilligan, P.H. Cost savings realized by implementation of routine microbiological identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2015, 53, 2473–2479. [Google Scholar] [CrossRef]




| Main Characteristics | N = 130 | % |
|---|---|---|
| Participant’s status | ||
| Reproductive age without pregnancy | 110 | 84.7% |
| Pregnancy | 17 | 13% |
| Postmenopausal | 3 | 2.3% |
| History of bacterial vaginosis | ||
| Yes | 34 | 26% |
| No | 96 | 74% |
| Medical contraceptive method | 39 | 30% |
| Estrogen/progesterone | 17 | 43.6% |
| Progesterone only | 5 | 12.8% |
| Copper intrauterine device | 14 | 35.9% |
| No data | 3 | 7.7% |
| Reason for consultation | ||
| Abnormal vaginal discharge | 62 | 47.7% |
| Gynecological follow-up | 31 | 23.9% |
| Pelvic pain | 16 | 12.3% |
| Others | 21 | 16.1% |
| Discharge + vpH £ | Amsel-Like Criteria | Nugent Score | Molecular Biology | MALDI-TOF | Selected Diagnosis (*) | Interpretation of Results |
|---|---|---|---|---|---|---|
| 5 diagnostic strategies applied to 84 women | ||||||
| NF | NF | NF | NF | NF | NF (28) | Concordance of the 5 diagnostic strategies |
| BV | BV | BV | BV | BV | BV (14) | Concordance of the 5 diagnostic strategies |
| NF | NF | IF | NF | NF | NF (14) | Difficulty to classify the IF identified with NS |
| BV | BV | IF | BV | BV | BV (1) | Difficulty to classify the IF identified with NS |
| BV | NF | NF | NF | NF | NF (4) | False positive of vaginal discharge + vpH |
| NF | NF | BV | NF | NF | NF (2) | False positive of NS |
| NF | BV | BV | BV | BV | BV (3) | False negative of vaginal discharge + vpH |
| BV | BV | BV | BV | NF | BV (1) | False negative of MALDI-TOF |
| BV | NF | IF | NF | NF | NF (3) | Difficulty to classify the IF identified with NS and false positive of discharge + vpH |
| NF | BV | IF | NF | NF | NF (3) | Difficulty to classify the IF identified with NS and false positive of Amsel-like criteria |
| BV | BV | NF | NF | NF | NF (2) | False positive of vaginal discharge + vpH and Amsel-like criteria |
| BV | NF | NF | NF | BV | NF (1) | False positive of vaginal discharge + vpH and MALDI-TOF |
| NF | NF | BV | BV | NF | NF (1) | False positive of NS and molecular biology |
| BV | BV | BV | NF | NF | BV (1) | False negative of molecular biology and MALDI-TOF |
| NF | NF | BV | BV | BV | BV (1) | False negative of vaginal discharge + vpH and Amsel-like criteria |
| BV | BV | IF | NF | NF | unconclusive (4) | Too many discrepancies between the different strategies to conclude |
| BV | NF | IF | NF | BV | unconclusive (1) | |
| 4 diagnostic strategies applied to 33 women | ||||||
| NF | NF | Np | NF | NF | NF (17) | Concordance of the 4 diagnostic strategies |
| BV | BV | Np | BV | BV | BV (4) | Concordance of the 4 diagnostic strategies |
| NF | BV | Np | NF | NF | NF (1) | False positive of Amsel-like criteria |
| NF | BV | Np | BV | BV | BV (2) | False negative of vaginal discharge + vpH |
| BV | BV | Np | NF | BV | BV (1) | False negative of molecular biology |
| BV | BV | Np | NF | NF | unconclusive (9) | Too many discrepancies between the different strategies to conclude |
| 3 diagnostic strategies applied to 12 women | ||||||
| NF | Np | Np | NF | NF | NF (2) | Concordance of the 3 diagnostic strategies |
| BV | Np | Np | BV | BV | BV (3) | Concordance of the 3 diagnostic strategies |
| BV | Np | Np | NF | NF | NF (5) | False positive of vaginal discharge + vpH |
| NF | Np | Np | BV | BV | BV (1) | False negative of vaginal discharge + vpH |
| 2 diagnostic strategies applied to 1 woman | ||||||
| Np | Np | Np | NF | NF | NF (1) | Concordance of the 2 diagnostic strategies |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Strategies | Bacterial vaginosis (with the Nugent score * as the reference tool) | ||||||||
| Sens % | 95% CI | Spec % | 95% CI | PPV % | 95% CI | NPV % | 95% CI | Accuracy % | |
| Discharge + vpH | 69.6 | (47.08–86.79) | 80 | (63.06–91.56) | 69.6 | (52.77–82.38) | 80 | (67.84–88.35) | 75.9 |
| Amsel-like criteria | 82.6 | (61.22–95.05) | 94.3 | (80.84–99.30) | 90.5 | (70.94–97.37) | 89.2 | (77.13–95.28) | 89.7 |
| Molecular biology | 87 | (66.41–97.22) | 100 | (90–100) | 100 | - | 92.1 | (80.24–97.10) | 94.8 |
| MALDI-TOF | 78.3 | (56.30–92.54) | 97.1 | (85.08–99.93) | 94.7 | (72.04–99.21) | 87.2 | (75.76–93.67) | 89.7 |
| (b) | |||||||||
| Diagnostic strategies | Bacterial vaginosis (with molecular biology as the reference tool) | ||||||||
| Sens % | 95% CI | Spec % | 95% CI | PPV % | 95% CI | NPV % | 95% CI | Accuracy % | |
| Discharge + vpH | 76.2 | (52.83–91.78) | 74.6 | (62.06–84.73) | 50 | (38.08–61.92) | 90.4 | (81.19–95.34) | 75 |
| Amsel-like criteria | 90.5 | (69.62–98.83) | 84.1 | (72.74–92.12) | 65.5 | (51.42–77.33) | 96.4 | (87.59–99.00) | 85.7 |
| Nugent score £ | 95.2 | (76.18–99.18) | 95.2 | (86.71–99.01) | 87 | (68.75–95.28) | 98.4 | (89.85–99.75) | 95.2 |
| MALDI-TOF | 90.5 | (69.62–98.83) | 96.8 | (89.00–99.61) | 90.5 | (70.69–97.40) | 96.8 | (89.08–99.13) | 95.2 |
| Variables | N | Sens % (95% CI) | Spec % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Accuracy % |
|---|---|---|---|---|---|---|
| Discharge + vpH | 129 | 46.3 (32.62–60.39) | 90.7 (81.71–96.16) | 78.1 (62.51–88.44) | 70.1 (64.43–75.22) | 72 |
| Amsel-like criteria | 118 | 54.3 (39.01–69.10) | 95.8 (88.30–99.13) | 89.3 (72.74–96.30) | 76.7 (70.49–81.88) | 79.7 |
| Nugent score £ | 84 | 78.7 (56.30–92.54) | 97.1 (85.08–99.93) | 94.7 (72.04–99.21) | 87.2 (75.76–93.67) | 89.7 |
| Molecular biology | 130 | 93.6 (78.58–99.21) | 97 (91.40–99.21) | 90.6 (75.96–96.73) | 98 (92.62–99.46) | 96.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Chacra, L.; Drouet, H.; Ly, C.; Bretelle, F.; Fenollar, F. Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry. Microorganisms 2024, 12, 111. https://doi.org/10.3390/microorganisms12010111
Abou Chacra L, Drouet H, Ly C, Bretelle F, Fenollar F. Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry. Microorganisms. 2024; 12(1):111. https://doi.org/10.3390/microorganisms12010111
Chicago/Turabian StyleAbou Chacra, Linda, Hortense Drouet, Claudia Ly, Florence Bretelle, and Florence Fenollar. 2024. "Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry" Microorganisms 12, no. 1: 111. https://doi.org/10.3390/microorganisms12010111
APA StyleAbou Chacra, L., Drouet, H., Ly, C., Bretelle, F., & Fenollar, F. (2024). Evaluation of Various Diagnostic Strategies for Bacterial Vaginosis, Including a New Approach Based on MALDI-TOF Mass Spectrometry. Microorganisms, 12(1), 111. https://doi.org/10.3390/microorganisms12010111







