Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses
Abstract
:1. Introduction
2. Materials and Methods
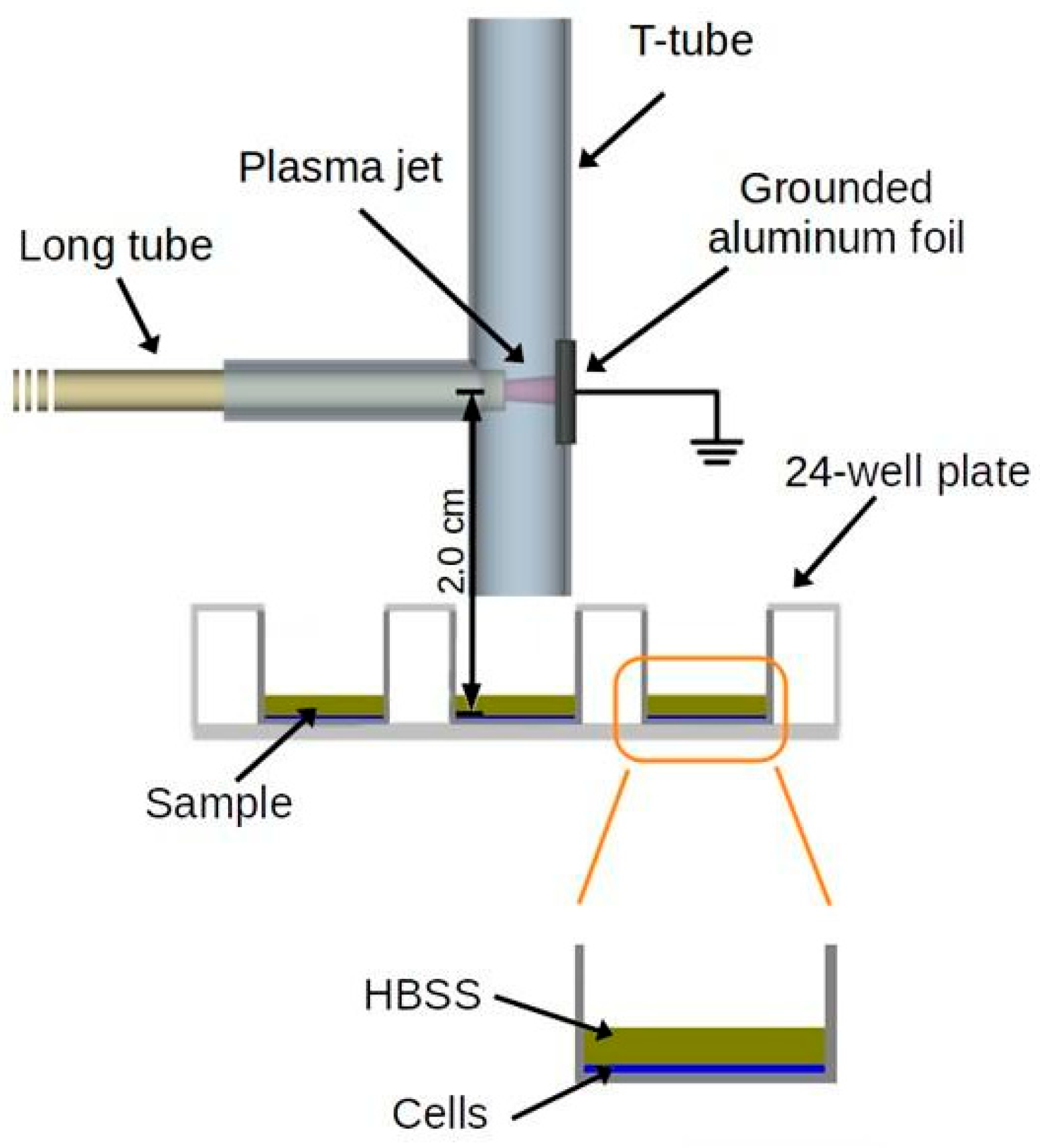
2.1. Characterization of CAP Interactions with the MGS Surface
2.2. Formation of Monospecies Biofilms on MGS Surfaces
2.3. Formation of Multispecies Biofilms on MGS Surfaces
2.4. CAP Treatment of the Biofilms Formed on MGS
2.5. Determination of Viable Cell Counts
2.6. Cytotoxicity Analysis
2.7. Statistical Analysis
3. Results
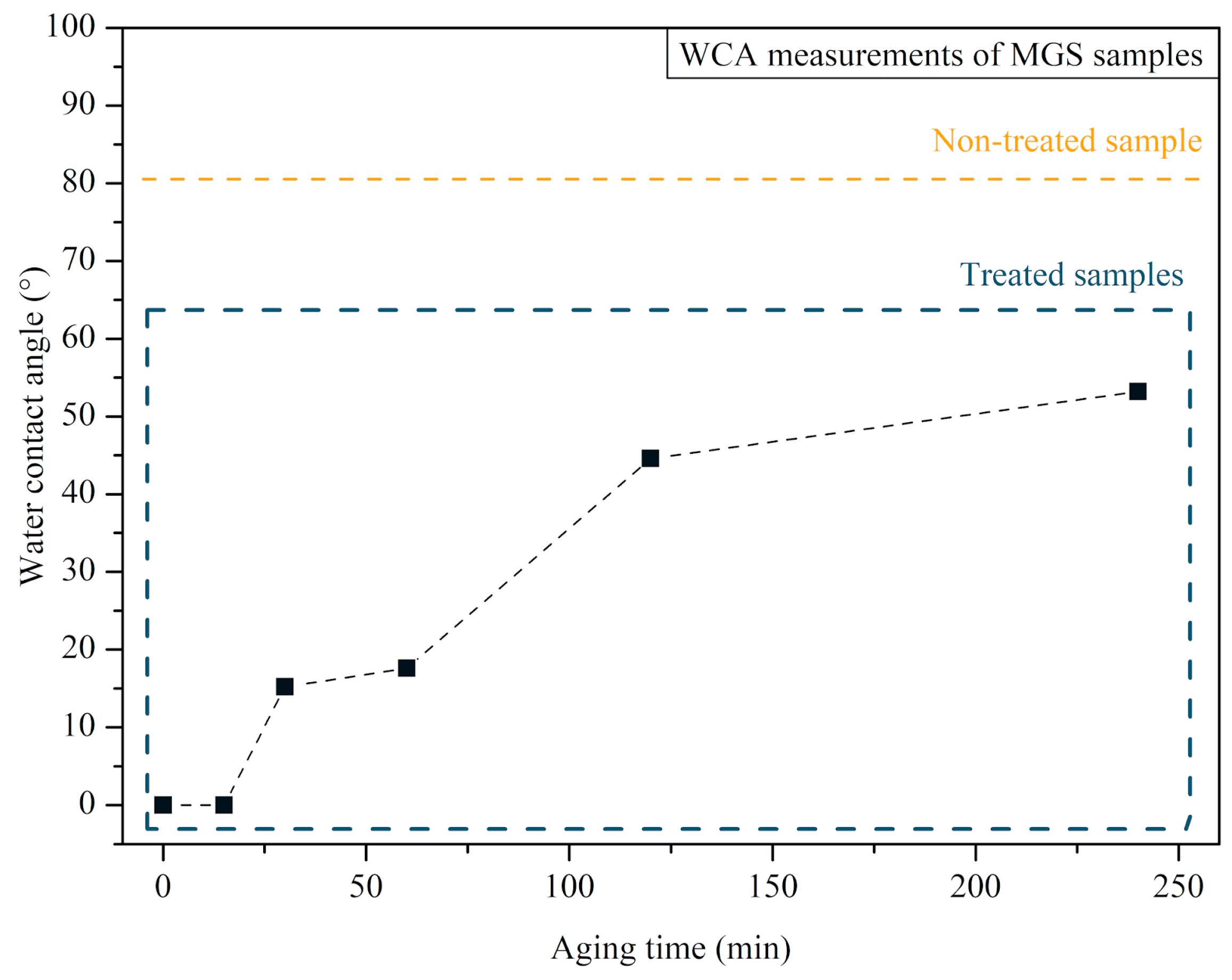
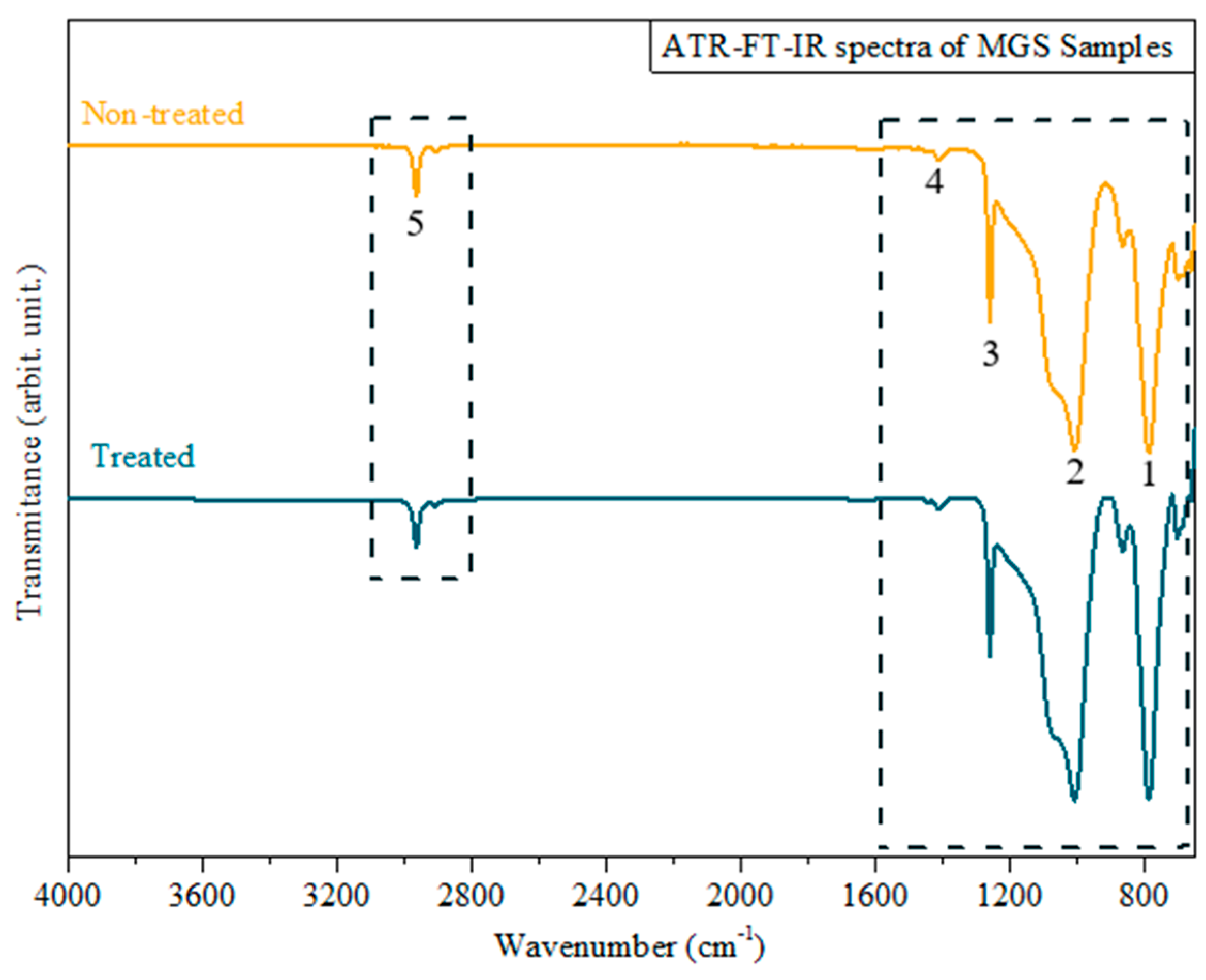
3.1. MGS Surface Characterization after CAP Exposure
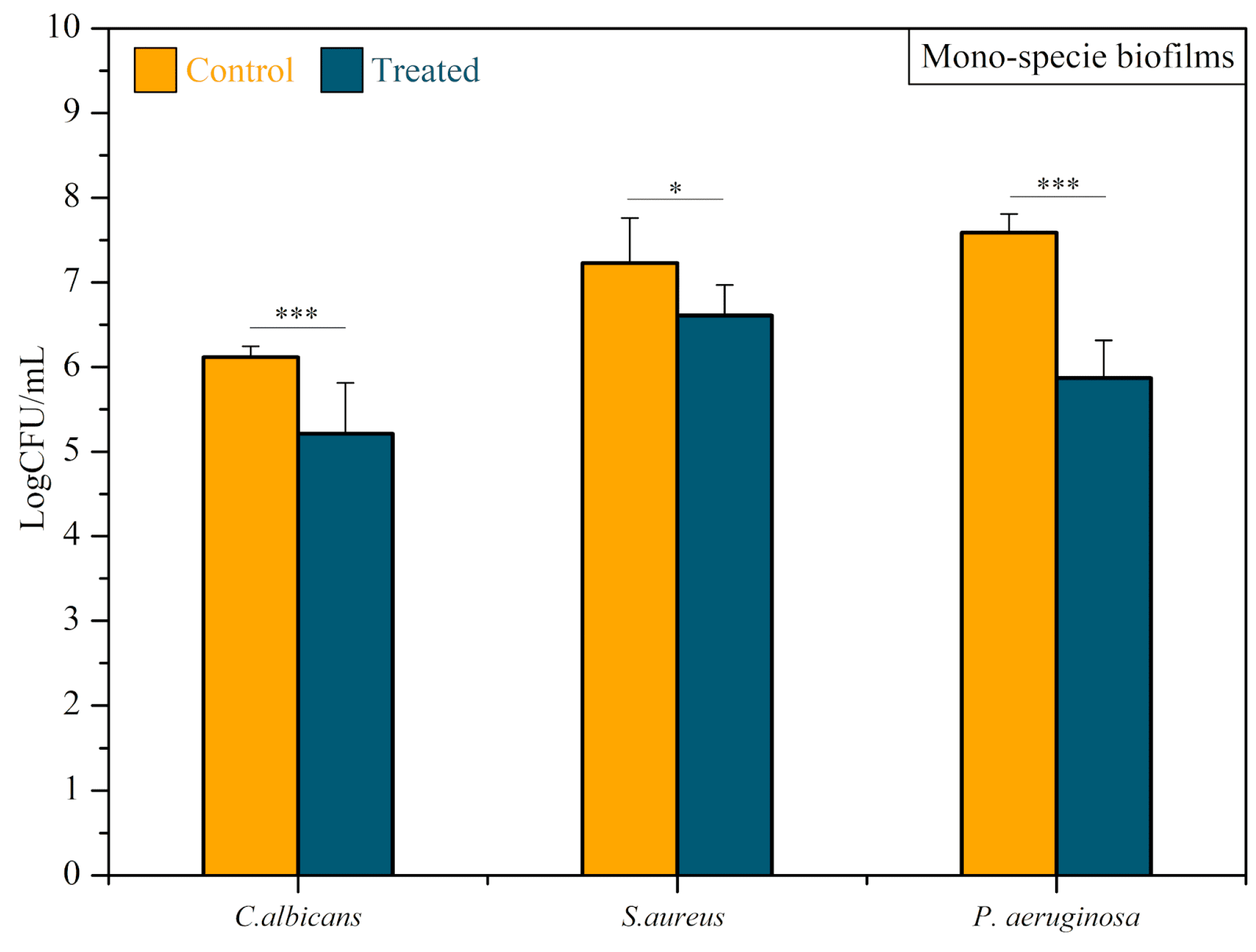
3.2. Antimicrobial Activity of CAP
3.3. Cytotoxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piazza, C.; Filauro, M.; Dikkers, F.G.; Nouraei, S.A.R.; Sandu, K.; Sittel, C.; Amin, M.R.; Campos, G.; Eckel, H.E.; Peretti, G. Long-Term Intubation and High Rate of Tracheostomy in COVID-19 Patients Might Determine an Unprecedented Increase of Airway Stenoses: A Call to Action from the European Laryngological Society. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1–7. [Google Scholar] [CrossRef]
- Esteller-Moré, E.; Ibañez, J.; Matiñó, E.; Ademà, J.M.; Nolla, M.; Quer, I.M. Prognostic Factors in Laryngotracheal Injury Following Intubation and/or Tracheotomy in ICU Patients. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2005, 262, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Marchioni, A.; Andreani, A.; Cappiello, G.; Fermi, M.; Presutti, L. Post-Intubation Tracheal Stenosis in COVID-19 Patients. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Alturk, A.; Bara, A.; Darwish, B. Post-Intubation Tracheal Stenosis after Severe COVID-19 Infection: A Report of Two Cases. Ann. Med. Surg. 2021, 67, 102468. [Google Scholar] [CrossRef]
- Auchincloss, H.G.; Wright, C.D. Complications after Tracheal Resection and Reconstruction: Prevention and Treatment. J. Thorac. Dis. 2016, 8, S160–S167. [Google Scholar] [PubMed]
- Rorris, F.P.; Chatzimichali, E.; Liverakou, E.; Antonopoulos, C.N.; Balis, E.; Kotsifas, C.; Stratakos, G.; Koutsoukou, A.; Zisis, C. Tracheal Resection in Patients Post–COVID-19 Is Associated with High Reintervention Rate and Early Restenosis. JTCVS Tech. 2023, 18, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ershadi, R.; Rafieian, S.; Sarbazzadeh, J.; Vahedi, M. Tracheal Stenosis Following Mild-to-Moderate COVID-19 Infection without History of Tracheal Intubation: A Case Report. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 303–307. [Google Scholar] [CrossRef]
- Montgomery, W.W. T-Tube Tracheal Stent. Arch. Otolaryngol. 1965, 82, 320–321. [Google Scholar] [CrossRef]
- Dumon, J.-F. A Dedicated Tracheobronchial Stent. Chest 1990, 97, 328–332. [Google Scholar] [CrossRef]
- Bibas, B.J.; Terra, R.M.; Oliveira Junior, A.L.; Tamagno, M.F.L.; Minamoto, H.; Cardoso, P.F.G.; Pêgo-Fernandes, P.M. Predictors for Postoperative Complications After Tracheal Resection. Ann. Thorac. Surg. 2014, 98, 277–282. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Liu, S.; Wang, S.; Mi, J. Preparation of Medical Hydrophilic and Antibacterial Silicone Rubberviasurface Modification. RSC Adv. 2021, 11, 39950–39957. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Hashmi, N.; Bloom, J.D.; Tamashiro, E.; Doghramji, L.; Sarani, B.; Palmer, J.N.; Cohen, N.A.; Mirza, N. Biofilm Accumulation on Endotracheal Tubes Following Prolonged Intubation. J. Laryngol. Otol. 2012, 126, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Fusconi, M.; lo Vasco, V.R.; Delfini, A.; de Virgilio, A.; Taddei, A.R.; Vassalli, C.; Conte, M.; del Sette, F.; Benincasa, A.T.; de Vincentiis, M. Is Montgomery Tracheal Safe-T-Tube Clinical Failure Induced by Biofilm? Otolaryngol.-Head Neck Surg. 2013, 149, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nouraei, S.A.R.; Petrou, M.A.; Randhawa, P.S.; Singh, A.; Howard, D.J.; Sandhu, G.S. Bacterial Colonization of Airway Stents. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1086. [Google Scholar] [CrossRef] [PubMed]
- Raveendra, N.; Rathnakara, S.H.; Haswani, N.; Subramaniam, V. Bacterial Biofilms on Tracheostomy Tubes. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 4995–4999. [Google Scholar] [CrossRef] [PubMed]
- Bibas, B.J.; Cardoso, P.F.G.; Salati, M.; Minamoto, H.; Luiz Tamagno, M.F.; Terra, R.M.; Pêgo-Fernandes, P.M. Health-Related Quality of Life Evaluation in Patients with Non-Surgical Benign Tracheal Stenosis. J. Thorac. Dis. 2018, 10, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, K.; Gunawardana, M.; Webster, P.; Hochstim, C.; Koempel, J.; Kokot, N.; Sinha, U.; Rice, D.; Baum, M. Bacterial Biofilms and Increased Bacterial Counts Are Associated with Airway Stenosis. Otolaryngol.-Head Neck Surg. 2014, 150, 834–840. [Google Scholar] [CrossRef]
- Chen, X.; Ling, X.; Liu, G.; Xiao, J. Antimicrobial Coating: Tracheal Tube Application. Int. J. Nanomed. 2022, 17, 1483–1494. [Google Scholar] [CrossRef]
- Martusevich, A.K.; Surovegina, A.V.; Bocharin, I.V.; Nazarov, V.V.; Minenko, I.A.; Artamonov, M.Y. Cold Argon Athmospheric Plasma for Biomedicine: Biological Effects, Applications and Possibilities. Antioxidants 2022, 11, 1262. [Google Scholar] [CrossRef]
- Miebach, L.; Poschkamp, B.; van der Linde, J.; Bekeschus, S. Medical Gas Plasma—A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications. Appl. Sci. 2022, 12, 3800. [Google Scholar] [CrossRef]
- Bekeschus, S.; Saadati, F.; Emmert, S. The Potential of Gas Plasma Technology for Targeting Breast Cancer. Clin. Transl. Med. 2022, 12, e1022. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Panariello, B.H.D. Comprehensive Biomedical Applications of Low Temperature Plasmas. Arch. Biochem. Biophys. 2020, 693, 108560. [Google Scholar] [CrossRef] [PubMed]
- Kostov; Borges, A.C.; Koga-Ito, C.Y.; Nishime, T.M.C.; Prysiazhnyi, V.; Honda, R.Y. Inactivation of Candida Albicans by Cold Atmospheric Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2015, 43, 770–775. [Google Scholar] [CrossRef]
- Kostov; Nishime, T.M.C.; Machida, M.; Borges, A.C.; Prysiazhnyi, V.; Koga-Ito, C.Y. Study of Cold Atmospheric Plasma Jet at the End of Flexible Plastic Tube for Microbial Decontamination. Plasma Process. Polym. 2015, 12, 1383–1391. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.D.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-Modulated Cold Atmospheric Pressure Plasma Jet for Treatment of Oral Candidiasis: In Vivo Study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef] [PubMed]
- Bastin, O.; Thulliez, M.; Servais, J.; Nonclercq, A.; Delchambre, A.; Hadefi, A.; Devière, J.; Reniers, F. Optical and Electrical Characteristics of an Endoscopic DBD Plasma Jet. Plasma Med. 2020, 10, 71–90. [Google Scholar] [CrossRef]
- Decauchy, H.; Pavy, A.; Camus, M.; Fouassier, L.; Dufour, T. Cold Plasma Endoscopy Applied to Biliary Ducts: Feasibility Risk Assessment on Human-like and Porcine Models for the Treatment of Cholangiocarcinoma. J. Phys. D Appl. Phys. 2022, 55, 455401. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-Lived and Short-Lived Reactive Species Produced by a Cold Atmospheric Pressure Plasma Jet for the Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Free Radic. Biol. Med. 2018, 124, 275–287. [Google Scholar] [CrossRef]
- Mrochen, D.M.; Miebach, L.; Skowski, H.; Bansemer, R.; Drechsler, C.A.; Hoffmann, U.; Hein, M.; Mamat, U.; Gerling, T.; Schaible, U.; et al. Toxicity and Virucidal Activity of a Neon-Driven Micro Plasma Jet on Eukaryotic Cells and a Coronavirus. Free Radic. Biol. Med. 2022, 191, 105–118. [Google Scholar] [CrossRef]
- ISO 10993-5 2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test Conditions Can Significantly Affect the Results of in Vitro Cytotoxicity Testing of Degradable Metallic Biomaterials. Sci. Rep. 2021, 11, 6628. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.I.; Oleiwi, J.K.; Ali, H.M. Study the Mechanical Properties of Polymeric Blends (SR/PMMA) Using for Maxillofacial Prosthesis Application. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2018; Volume 454. [Google Scholar]
- Beyoglu, M.A.; Sahin, M.F.; Turkkan, S.; Yazicioglu, A.; Yekeler, E. Complex Post-Intubation Tracheal Stenosis in Covid-19 Patients. Indian J. Surg. 2022, 84, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shi, F.; Wang, Q.; Zhang, Z.; Guo, J.; Zhuang, J. Penetration Effect of the KINPen Plasma Jet Investigated with a 3D Agar-Entrapped Bacteria Model. Microchem. J. 2022, 183, 107973. [Google Scholar] [CrossRef]
- Tiernan, H.; Byrne, B.; Kazarian, S.G. ATR-FTIR Spectroscopy and Spectroscopic Imaging for the Analysis of Biopharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118636. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.C.D.; Lima, G.D.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Menezes, B.R.C.D.; Caliari, M.V.; Kostov, K.G.; Koga-Ito, C.Y. Inhibitory Effect of Cold Atmospheric Plasma on Chronic Wound-Related Multispecies Biofilms. Appl. Sci. 2021, 11, 5441. [Google Scholar] [CrossRef]
- Borges, A.C.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Yumi Koga-Ito, C. Cold Atmospheric Pressure Plasma Jet Modulates Candida Albicans Virulence Traits. Clin. Plasma Med. 2017, 7–8, 9–15. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas Aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]





| Microorganism | Reference Number | Optical Density | Wavelength (nm) |
|---|---|---|---|
| Candida albicans | ATCC 18804 | 0.760 | 530 |
| Pseudomonas aeruginosa | ATCC 27853 | 0.130 | 600 |
| Staphylococcus aureus | ATCC 6538 | 0.249 | 490 |
| Microorganism | Culture Medium | |
|---|---|---|
| Monospecies biofilm | C. albicans | Sabouraud agar |
| P. aeruginosa | Brain Heart Infusion (BHI) agar | |
| S. aureus | Brain Heart Infusion (BHI) agar | |
| Multispecies biofilm | C. albicans | CHROMagar |
| P. aeruginosa | Cetrimide agar | |
| S. aureus | Mannitol agar |
| Functional Group | Wavenumber (cm−1) | Peak # | Reference |
|---|---|---|---|
| –CH3 of SiCH3 | 1258 and 1412 | 1, 2 | [33] |
| Si–O–Si | 800–1000 | 3, 4 | |
| C–H | 2962.3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.M.d.; Do Nascimento, F.; Milhan, N.V.M.; Oliveira, M.A.C.d.; Cardoso, P.F.G.; Legendre, D.; Aoki, F.G.; Kostov, K.G.; Koga-Ito, C.Y. Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses. Microorganisms 2024, 12, 130. https://doi.org/10.3390/microorganisms12010130
Silva DMd, Do Nascimento F, Milhan NVM, Oliveira MACd, Cardoso PFG, Legendre D, Aoki FG, Kostov KG, Koga-Ito CY. Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses. Microorganisms. 2024; 12(1):130. https://doi.org/10.3390/microorganisms12010130
Chicago/Turabian StyleSilva, Diego Morais da, Fellype Do Nascimento, Noala Vicensoto Moreira Milhan, Maria Alcionéia Carvalho de Oliveira, Paulo Francisco Guerreiro Cardoso, Daniel Legendre, Fabio Gava Aoki, Konstantin Georgiev Kostov, and Cristiane Yumi Koga-Ito. 2024. "Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses" Microorganisms 12, no. 1: 130. https://doi.org/10.3390/microorganisms12010130
APA StyleSilva, D. M. d., Do Nascimento, F., Milhan, N. V. M., Oliveira, M. A. C. d., Cardoso, P. F. G., Legendre, D., Aoki, F. G., Kostov, K. G., & Koga-Ito, C. Y. (2024). Cold Atmospheric Helium Plasma in the Post-COVID-19 Era: A Promising Tool for the Disinfection of Silicone Endotracheal Prostheses. Microorganisms, 12(1), 130. https://doi.org/10.3390/microorganisms12010130





