Effectiveness of Volatiles Emitted by Streptomyces abikoensis TJGA-19 for Managing Litchi Downy Blight Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Plant Materials
2.2. Screening Antagonistic Actinomycetes for Volatile Mediated Antifungal Activity against P. litchii
2.3. Production of S. abikoensis TJGA-19 Volatile Substances
2.4. The Effect of Cultivation Time on the Antifungal Activity of Volatiles Produced by S. abikoensis TJGA-19
2.5. Testing Antifungal Spectrum of Volatiles of S. abikoensisTJGA-19
2.6. Preparation of P. litchii Sporangia Suspension
2.7. Inhibitory Activity of Volatiles from S. abikoensisTJGA-19 In Vitro
2.8. Observation of Morphology by Electron Microscopy
2.9. Inoculation Bioassay In Vivo
2.10. Collection and Analysis of Volatiles Produced by S. abikoensis TJGA-19
2.11. Statistical Analysis
3. Results
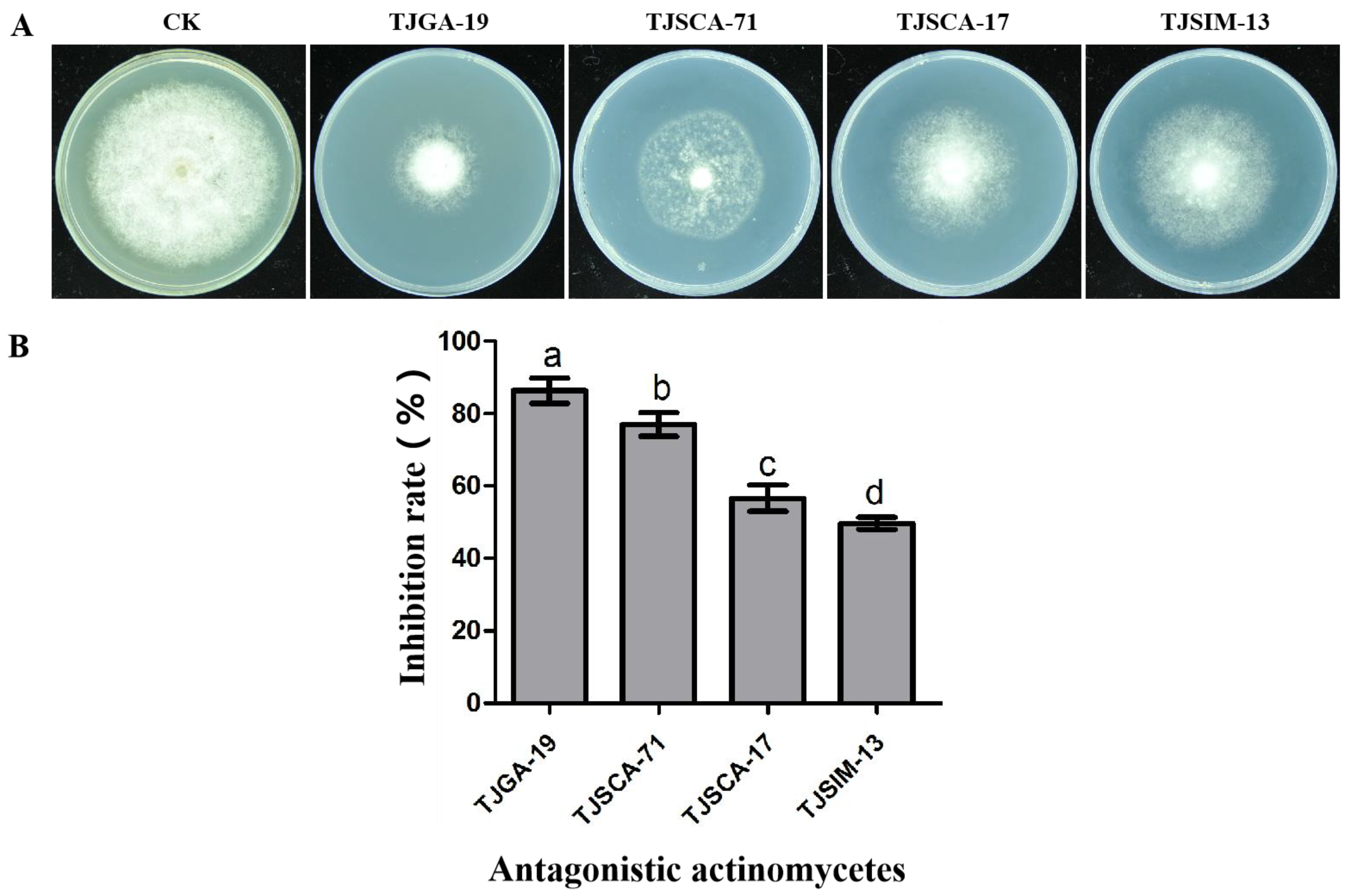
3.1. Screening Actinomycetes for Antagonistic Activity
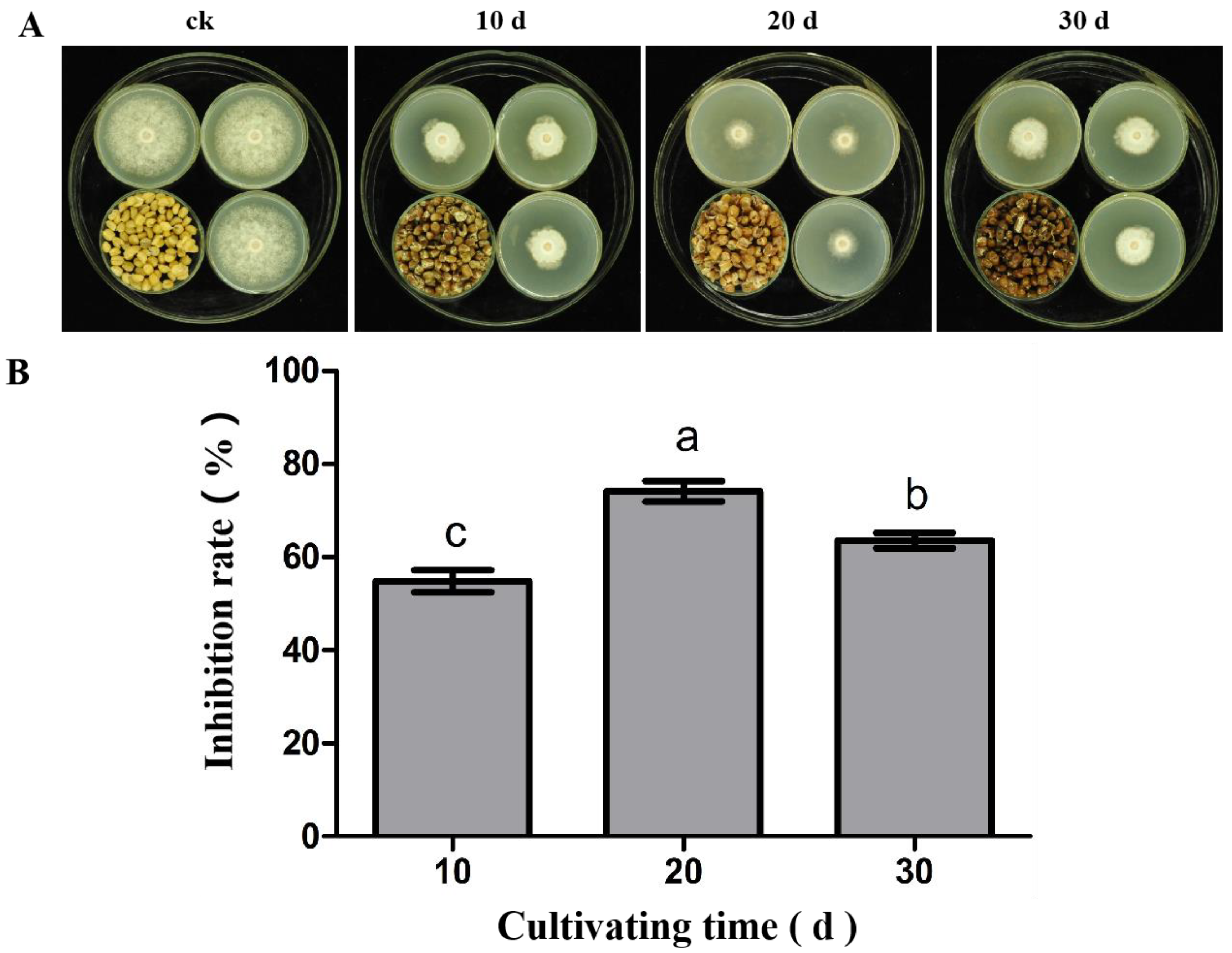
3.2. The Antifungal Activity of Volatile Substances from S. abikoensis TJGA-19 at Different Cultivation Times against P. litchii
3.3. Determination of the Antifungal Spectrum of S. abikoensis TJGA-19 Volatiles
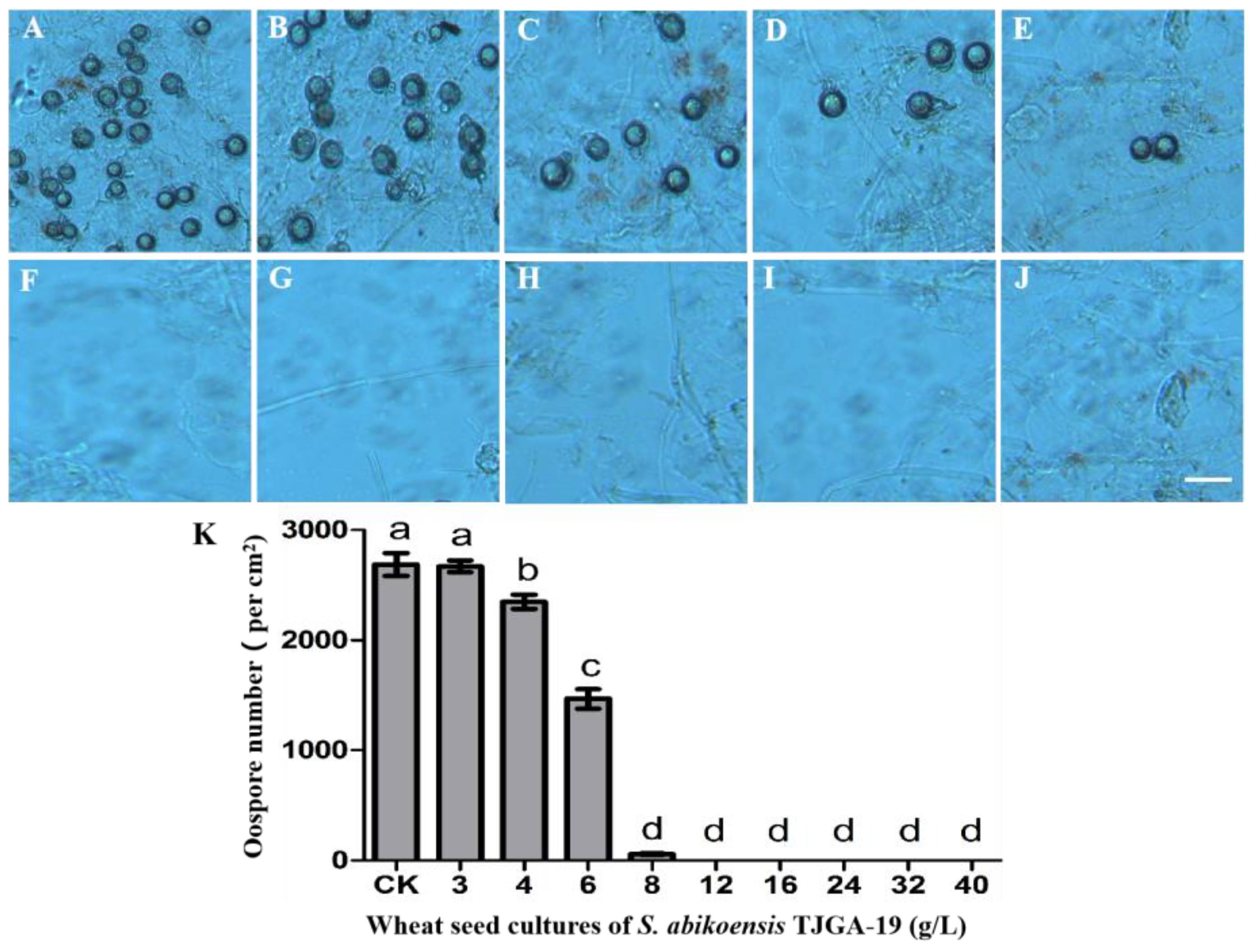
3.4. Antifungal Activity of Volatiles from S. abikoensis TJGA-19 on P. litchii
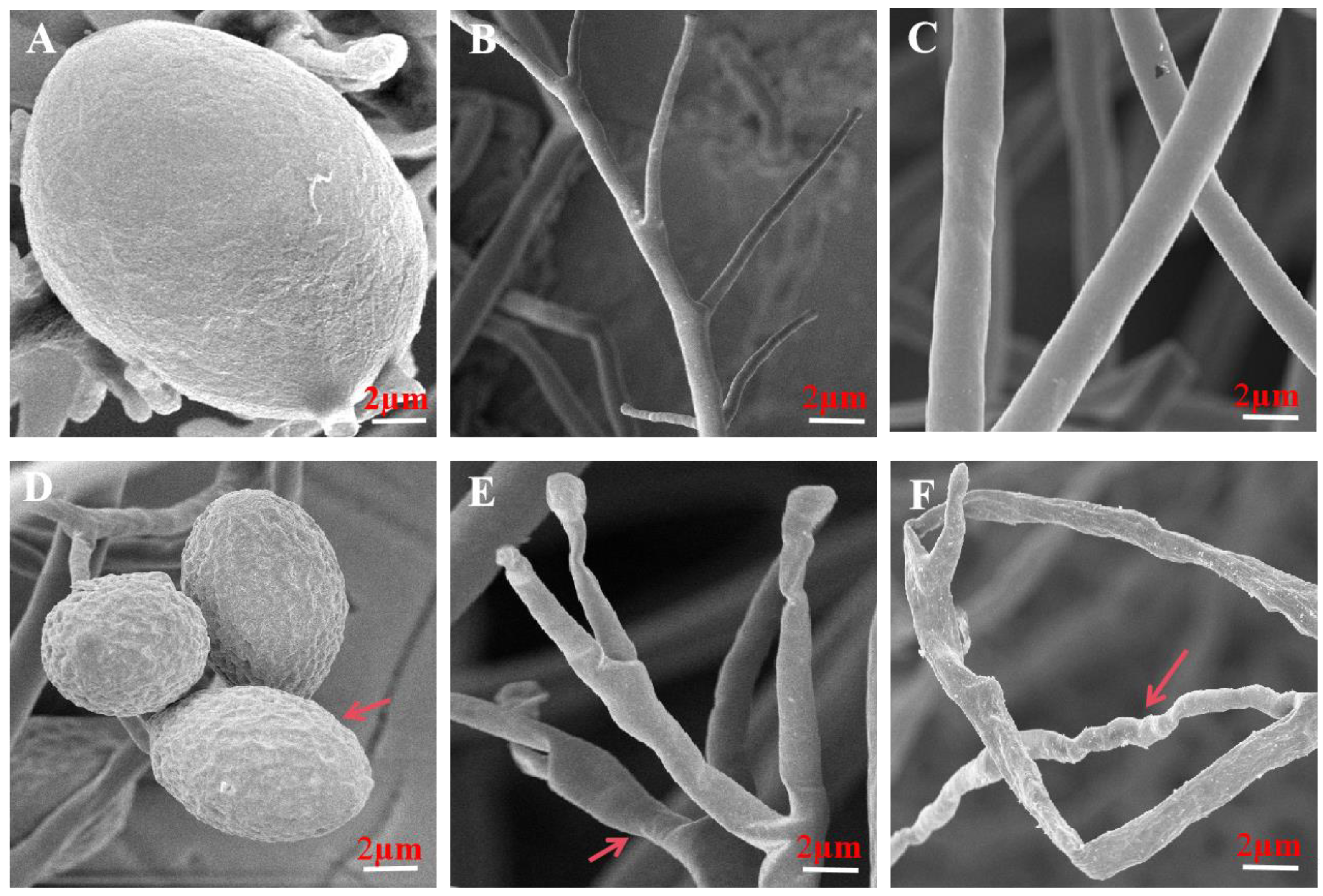
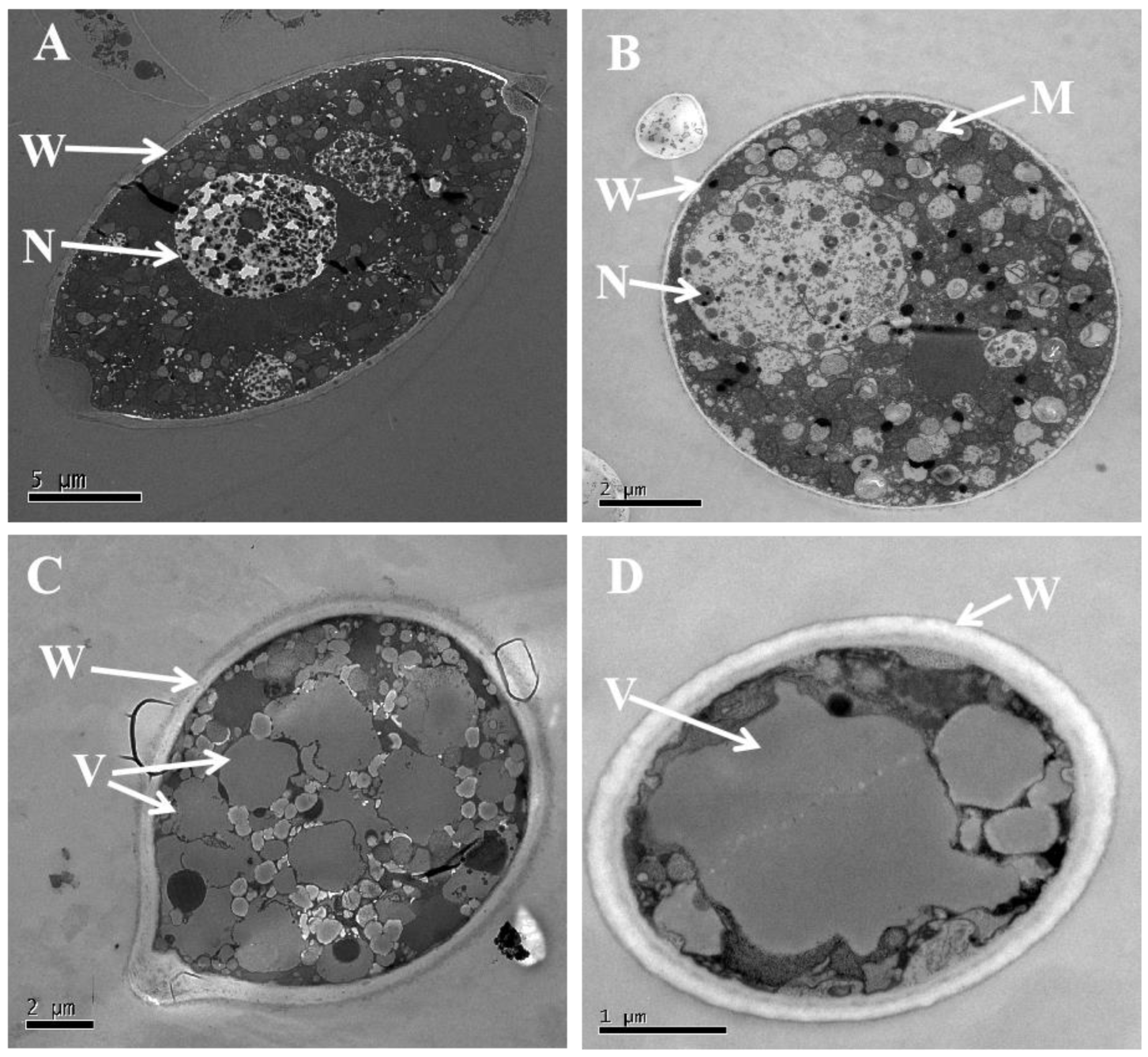
3.5. Volatiles Resulted in Morphological and Ultrastructural Changes in P. litchii
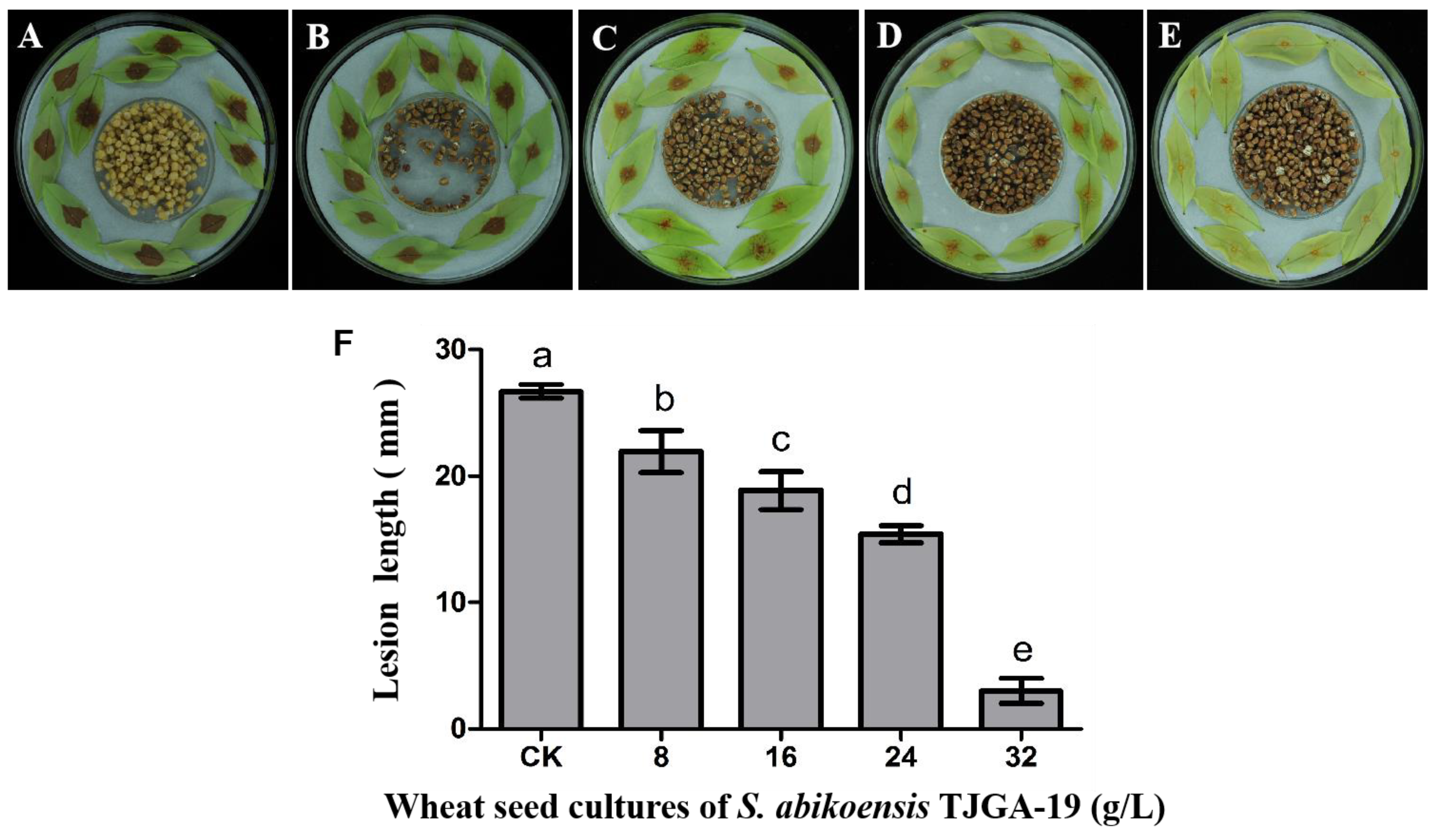
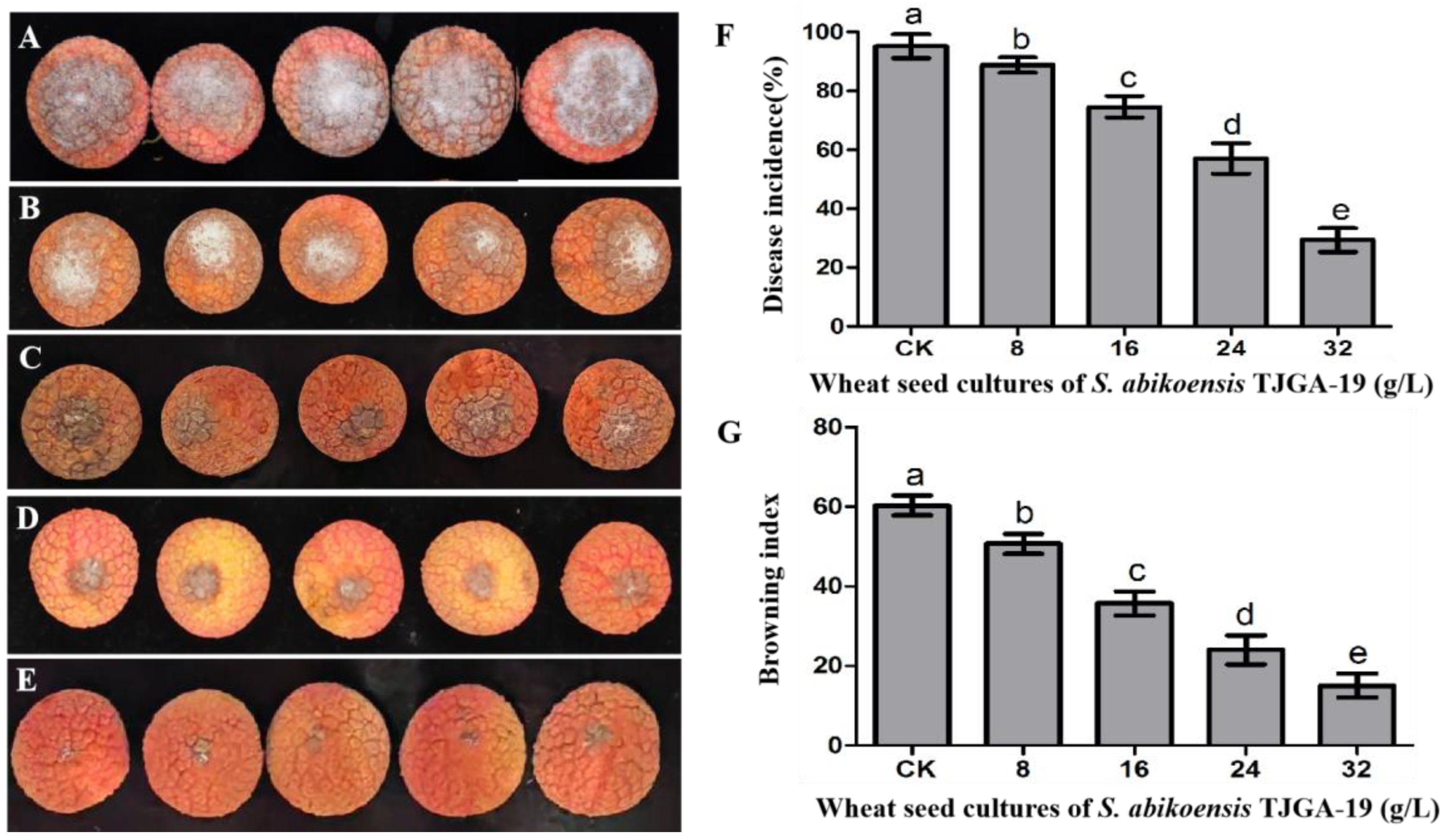
3.6. The Inhibitory Effects of S. abikoensis TJGA-19 Volatiles on Litchi Downy Blight in Litchi Leaves and Fruits
3.7. Analysis of Volatiles Produced by S. abikoensis TJGA-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef] [PubMed]
- Situ, J.; Zheng, L.; Xu, D.; Gu, C.; Xi, P.; Deng, Y.; Hsiang, T.; Jiang, Z. Screening of effective biocontrol agents against postharvest litchi downy blight caused by Peronophythora litchii. Postharvest Biol. Technol. 2023, 198, 112249. [Google Scholar] [CrossRef]
- Wang, H.; Qian, Z.; Ma, S.; Zhou, Y.; Qu, H. Energy status of ripening and postharvest senescent fruit of litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, H.; Ma, J.; Stammler, G.; Zhou, M. Fungicide Effectiveness during the Various Developmental Stages of Peronophythora litchii In Vitro. J. Phytopathol. 2009, 157, 407–412. [Google Scholar] [CrossRef]
- Peikun, C.; Sueping, P. On downy blight of litchi chinensis sonn.i.the pathogen and its infection process. Acta Phytopathol. Sin. 1984, 14, 113–119. [Google Scholar]
- Niazian, M.; Sabbatini, P. Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Planta 2021, 254, 111. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Microbial Secondary Metabolism: A New Theoretical Frontier for Academia, a New Opportunity for Industry. In Ciba Foundation Symposium 171-Secondary Metabolites: Their Function and Evolution: Secondary Metabolites: Their Function and Evolution: Ciba Foundation Symposium 171; John Wiley & Sons: Chichester, UK, 2007; pp. 3–23. [Google Scholar]
- Kim, T.; Jang, J.; Yu, N.; Chi, W.; Bae, C.; Yeo, J.; Park, A.; Hur, J.; Park, H.; Park, J. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest Manag. Sci. 2018, 74, 384–391. [Google Scholar] [CrossRef]
- Barthélemy, M.; Elie, N.; Genta-Jouve, G.; Stien, D.; Eparvier, V. Identification of Antagonistic Compounds between the Palm Tree Xylariale Endophytic Fungi and the Phytopathogen Fusarium oxysporum. J. Agric. Food Chem. 2021, 69, 10893–10906. [Google Scholar] [CrossRef]
- Strobel, G. Muscodor albus and its biological promise. J. Ind. Microbiol. Biotechnol. 2006, 33, 514. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Ryu, C.; Farag, M.A.; Hu, C.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal Activity of Bacillus amyloliquefaciens NJN-6 Volatile Compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological control and plant growth promotion by volatile organic compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Delgado, N.; Olivera, M.; Cádiz, F.; Bravo, G.; Montenegro, I.; Madrid, A.; Fuentealba, C.; Pedreschi, R.; Salgado, E.; Besoain, X. Volatile Organic Compounds (VOCs) Produced by Gluconobacter cerinus and Hanseniaspora osmophila Displaying Control Effect against Table Grape-Rot Pathogens. Antibiotics 2021, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, L.; Álvarez-García, S.; Salas, J.A.; Méndez, C.; Olano, C.; Malmierca, M.G. The Volatile Organic Compounds of Streptomyces spp.: An In-Depth Analysis of Their Antifungal Properties. Microorganisms 2023, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Campos-Avelar, I.; Colas De La Noue, A.; Durand, N.; Cazals, G.; Martinez, V.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Aspergillus flavus growth inhibition and aflatoxin B1 decontamination by Streptomyces isolates and their metabolites. Toxins 2021, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, T.; Jensen, B.; Jørgensen, N.O. Volatiles produced by Streptomyces spp. delay rot in apples caused by Colletotrichum acutatum. Curr. Res. Microb. Sci. 2022, 3, 100121. [Google Scholar] [CrossRef]
- Corral, D.A.P.; Paz, J.D.J.O.; Orozco, G.I.O.; Muñiz, C.H.A.; Marina, M.Á.S.; Cisneros, M.F.R.; Corral, F.J.M.; Pavía, S.P.F.; Velasco, C.R. Antagonistic effect of volatile and non-volatile compounds from Streptomyces strains on cultures of several phytopathogenic fungi. Emir. J. Food Agric. 2020, 32, 879–889. [Google Scholar] [CrossRef]
- Wan, M.; Li, G.; Zhang, J.; Jiang, D.; Huang, H. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 2008, 46, 552–559. [Google Scholar] [CrossRef]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol. Control 2012, 61, 113–120. [Google Scholar] [CrossRef]
- Danaei, M.; Baghizadeh, A.; Shahram, P.; Mohammadmehdi, Y. Effect of volatile substances of Streptomyces coelicolor on control of Botrytis cinerea and Penicillium chrysogenum. Casp. J. Appl. Sci. Res. 2013, 2, 45–51. [Google Scholar]
- Wang, Z.; Wang, C.; Li, F.; Li, Z.; Chen, M.; Wang, Y.; Qiao, X.; Zhang, H. Fumigant activity of volatiles from Streptomyces alboflavus TD-1 against Fusarium moniliforme Sheldon. J. Microbiol. 2013, 51, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Situ, J.; Zhu, Q.; Xi, P.; Zheng, Y.; Liu, H.; Zhou, X.; Jiang, Z. Identification of volatile organic compounds for the biocontrol of postharvest litchi fruit pathogen Peronophythora litchii. Postharvest Biol. Technol. 2019, 155, 37–46. [Google Scholar] [CrossRef]
- Xing, M.; Zhao, J.; Zhang, J.; Wu, Y.; Khan, R.A.A.; Li, X.; Wang, R.; Li, T.; Liu, T. 6-Pentyl-2H-pyran-2-one from Trichoderma erinaceum Is Fungicidal against Litchi Downy Blight Pathogen Peronophythora litchii and Preservation of Litchi. J. Agric. Food Chem. 2023, 71, 19488–19500. [Google Scholar] [CrossRef]
- Xing, M.; Zheng, L.; Deng, Y.; Xu, D.; Xi, P.; Li, M.; Kong, G.; Jiang, Z. Antifungal Activity of Natural Volatile Organic Compounds against Litchi Downy Blight Pathogen Peronophythora litchii. Molecules 2018, 23, 358. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Xu, D.; Wu, Y.; Liu, T.; Xi, P.; Wang, R.; Zhao, J.; Jiang, Z. Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii. Fermentation 2023, 9, 1011. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Khan, R.A.A.; Zhang, J.; Chen, J.; Yin, Y.; Tang, Z.; Wang, R.; Lu, B.; Liu, T. Control of postharvest stem-end rot on mango by antifungal metabolites of Trichoderma pinnatum LS029-3. Sci. Hortic. 2023, 310, 111696. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef]
- Boukaew, S.; Petlamul, W.; Bunkrongcheap, R.; Chookaew, T.; Kabbua, T.; Thippated, A.; Prasertsan, P. Fumigant activity of volatile compounds of Streptomyces philanthi RM-1-138 and pure chemicals (acetophenone and phenylethyl alcohol) against anthracnose pathogen in postharvest chili fruit. Crop Prot. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Xu, M.; Zhang, C.; Gao, J.; Li, C.; Xing, K.; Qin, S. Antifungal Volatile Organic Compounds from Streptomyces setonii WY228 Control Black Spot Disease of Sweet Potato. Appl. Environ. Microbiol. 2022, 88, e2317–e2321. [Google Scholar] [CrossRef] [PubMed]
- Boukaew, S.; Cheirsilp, B.; Prasertsan, P.; Yossan, S. Antifungal effect of volatile organic compounds produced by Streptomyces salmonis PSRDC-09 against anthracnose pathogen Colletotrichum gloeosporioides PSU-03 in postharvest chili fruit. J. Appl. Microbiol. 2021, 131, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Boukaew, S.; Plubrukam, A.; Prasertsan, P. Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. Biocontrol 2013, 58, 471–482. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Zhu, Z.; Kong, X.; Wan, L.; Situ, J.; Li, M.; Gao, L.; Jiang, Z. Biological activity of pterostilbene against Peronophythora litchii, the litchi downy blight pathogen. Postharvest Biol. Technol. 2018, 144, 29–35. [Google Scholar] [CrossRef]
- Liao, L.; Zhou, J.; Wang, H.; He, F.; Liu, S.; Jiang, Z.; Chen, S.; Zhang, L. Control of litchi downy blight by zeamines produced by Dickeya zeae. Sci. Rep. 2015, 5, 15719. [Google Scholar] [CrossRef]
- Luo, J.; Li, Z.; Wang, J.; Weng, Q.; Chen, S.; Hu, M. Antifungal activity of isoliquiritin and its inhibitory effect against Peronophythora litchii Chen through a membrane damage mechanism. Molecules 2016, 21, 237. [Google Scholar] [CrossRef]
- Xu, L.; Xue, J.; Wu, P.; Wang, D.; Wei, X. Antifungal Activity of Hypothemycin against Peronophythora litchii In Vitro and In Vivo. J. Agric. Food Chem. 2013, 61, 10091–10095. [Google Scholar] [CrossRef]


| Volatile Organic Compounds a | RT b (min) | Area (%) |
|---|---|---|
| Arsenous acid, tris(trimethylsilyl) ester | 14.9147 | 3.5529 |
| Benzonitrile, 2-(2-hydroxy-5-nitrobenzylidenamino)- | 18.1902 | 3.2558 |
| 2-Methylisoborneol | 18.9705 | 25.9287 |
| 4-(2-Methyl-cyclohex-1-enyl)-but-3-en-2-one | 20.9163 | 1.5504 |
| Cyclotetrasiloxane, octamethyl- | 21.8342 | 2.8193 |
| Tridecane | 24.4551 | 1.6461 |
| Naphthalene,1,2,4a,5,8,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1.alpha.,4a.beta.,8a.alpha.)- | 25.7243 | 5.2184 |
| 1,3,5,7,9-Pentaethylcyclopentasiloxane | 25.9002 | 2.1199 |
| 2-Methyltetracosane | 27.2091 | 1.9879 |
| Benzoic acid, hexyl ester | 27.585 | 2.3067 |
| Dodecane, 2,6,10-trimethyl- | 27.7489 | 9.9877 |
| 4-(1-methyl-1-cyclobutyl)phenol | 28.0618 | 4.9239 |
| (2Z,4E)-3,7,11-Trimethyl-2,4,10-dodecatriene | 28.3478 | 3.0363 |
| Tetradecane | 28.7473 | 7.8256 |
| (1R,4aS,8aR)-1,4a-Dimethyl-7-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,8a-octahydronaphthalene | 28.8855 | 2.9132 |
| Disparlure | 29.1225 | 2.3421 |
| 2,6,10-Trimethyltridecane | 31.3051 | 6.2293 |
| 1H-Cyclopropa[a]naphthalene, 1a,2,3,3a,4,5,6,7b-octahydro-1,1,3a,7-tetramethyl-, [1aR-(1a.alpha.,3a.alpha.,7b.alpha.)]- | 31.4971 | 3.8272 |
| Octacosane | 32.6604 | 2.1236 |
| Pentadecane | 32.8364 | 2.234 |
| Hexadecane | 34.4358 | 1.2136 |
| Longifolene | 36.7445 | 2.9574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, M.; Sun, T.; Liu, T.; Jiang, Z.; Xi, P. Effectiveness of Volatiles Emitted by Streptomyces abikoensis TJGA-19 for Managing Litchi Downy Blight Disease. Microorganisms 2024, 12, 184. https://doi.org/10.3390/microorganisms12010184
Xing M, Sun T, Liu T, Jiang Z, Xi P. Effectiveness of Volatiles Emitted by Streptomyces abikoensis TJGA-19 for Managing Litchi Downy Blight Disease. Microorganisms. 2024; 12(1):184. https://doi.org/10.3390/microorganisms12010184
Chicago/Turabian StyleXing, Mengyu, Tao Sun, Tong Liu, Zide Jiang, and Pinggen Xi. 2024. "Effectiveness of Volatiles Emitted by Streptomyces abikoensis TJGA-19 for Managing Litchi Downy Blight Disease" Microorganisms 12, no. 1: 184. https://doi.org/10.3390/microorganisms12010184





