Host–Microbiome Interactions in a Changing Sea: The Gill Microbiome of an Invasive Oyster under Drastic Temperature Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. DNA Extraction, Library Preparation, and Deep Sequencing
2.3. Microbiome Data Processing
2.4. Statistical Analysis
3. Results
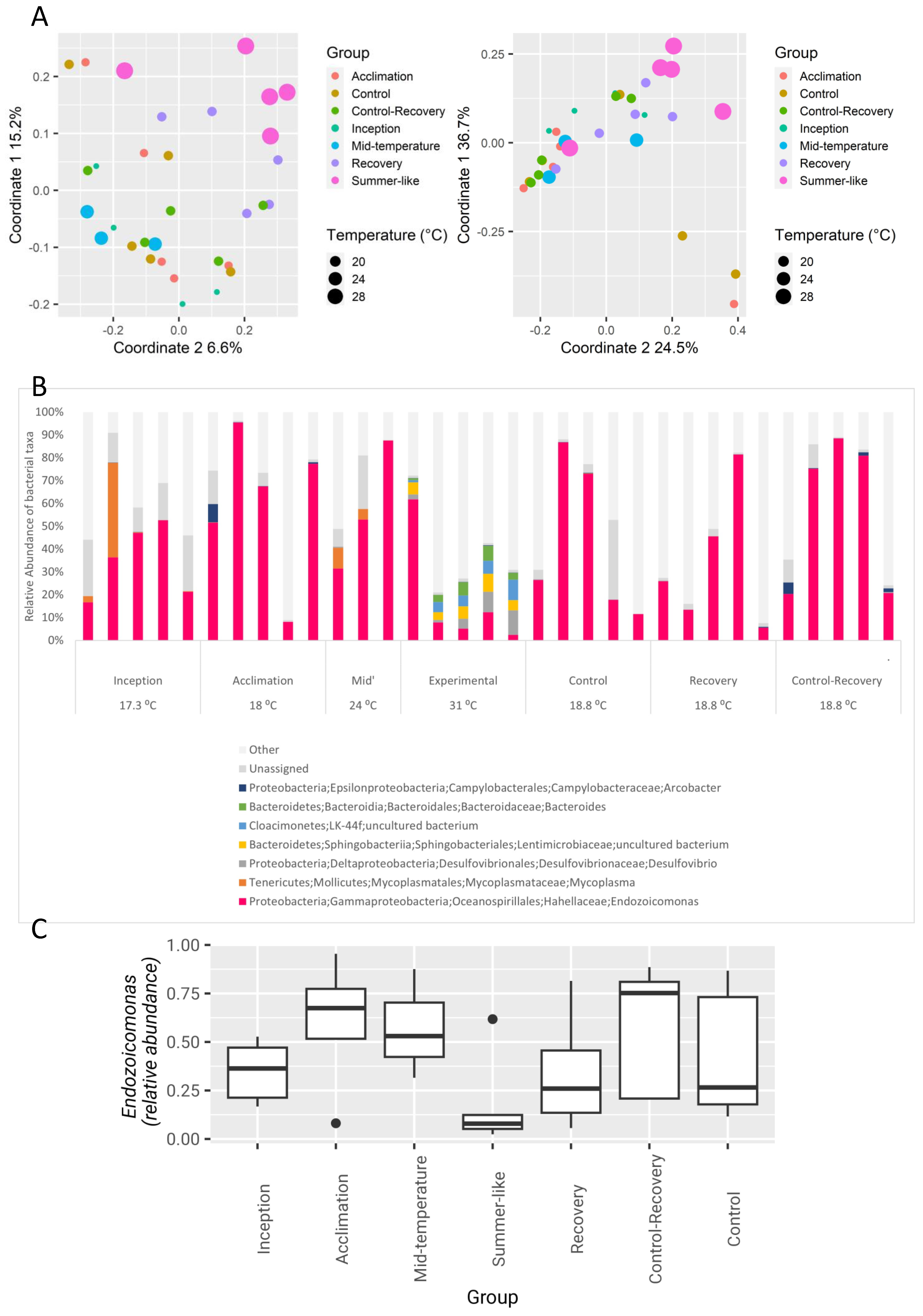
3.1. The Effect of Seasonal Temperature Patterns on the Gill Microbiome
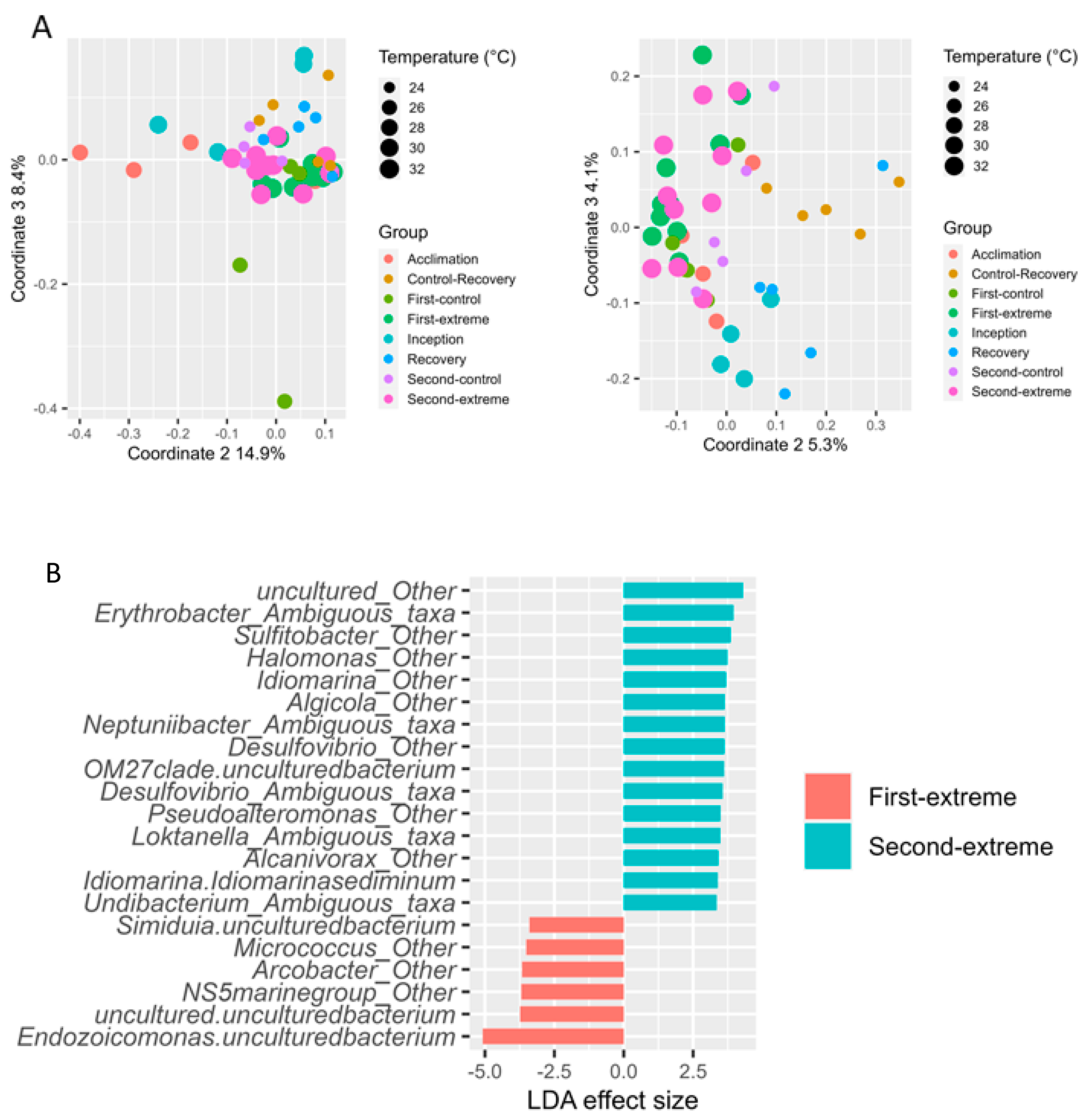
3.2. The Effect of Extreme Temperature Scenarios on the Gill Microbiome
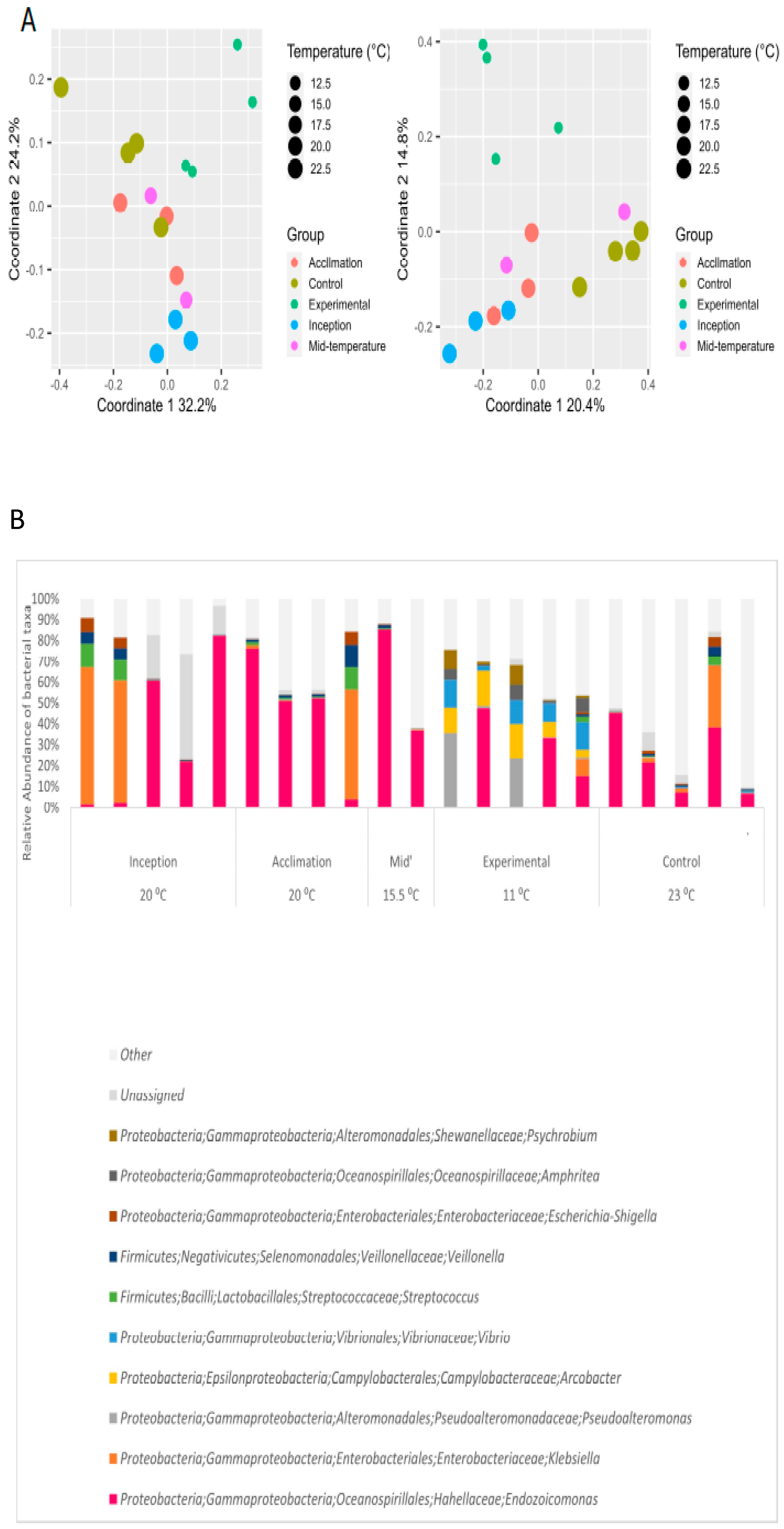
3.3. Effect of Extreme Cold on Oysters and Their Gill Microbiome
4. Discussion
4.1. Bacterial Gill Composition Is Strongly Affected by Temperature
4.2. Oyster Symbionts in an Era of Climate Change
4.3. Oysters and Endozoicomonas Bacteria Are Strongly Impacted by Cold Temperatures Prevailing in the Western Mediterranean Sea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Breithaupt, H. Aliens on the shores: Biodiversity and national economies are being threatened by the invasion of non-native species. EMBO Rep. 2003, 4, 547–550. [Google Scholar] [PubMed]
- Bianchi, C.; Morri, C. Global sea warming and ‘tropicalization’ of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeographia 2003, 24, 319–329. [Google Scholar] [CrossRef]
- Por, F.D. One Hundred Years of Suez Canal-A Century of Lessepsian Migration: Retrospect And Viewpoints. Syst. Zool. 1971, 20, 138–159. [Google Scholar] [CrossRef]
- Golik, A.; Golik, A.; Oceanographic, I. Indirect Evidence for Sediment Transport on the Continental Shelf off Israel; Springer: Berlin/Heidelberg, Germany, 1993; pp. 159–164. [Google Scholar]
- Mienis, H.; Galili, E.; Rapoport, J. The spiny oyster, Spondylus spinosus, a well-established Indo-Pacific bivalve in the Eastern Mediterranean off Israel (Mollusca, Bivalvia, Spondylidae). Zool. Middle East 1993, 9, 83–92. [Google Scholar]
- Engl, W.; Çeviker, D. New migrant species from southeast Turkey Psammotreta praerupta (Salisbury, 1934) and Antigona lamellaris Schumacher, 1817. La Conchiglia 1999, 290, 17–20. [Google Scholar]
- Çeviker, D.; Albayrak, S. Three alien molluscs from Iskenderun Bay (SE Turkey). Aquat. Invasions 2006, 1, 76–79. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsiamis, K.; Ioannou, G.; Michailidis, N.; Zenetos, A. Inventory of alien marine species of Cyprus (2009). Mediterr. Mar. Sci. 2009, 10, 109–133. [Google Scholar] [CrossRef]
- Crocetta, F.; Bitar, G.; Zibrowius, H.; Oliverio, M. Biogeographical homogeneity in the eastern Mediterranean Sea. II. Temporal variation in Lebanese bivalve biota. Aquat. Biol. 2013, 19, 75–84. [Google Scholar] [CrossRef]
- Shabtay, A.; Tikochinski, Y.; Benayahu, Y.; Rilov, G. Preliminary data on the genetic structure of a highly successful invading population of oyster suggesting its establishment dynamics in the Levant. Mar. Biol. Res. 2014, 10, 407–415. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien Marine Species in the Mediterranean—The 100 ‘Worst Invasives’ and their Impact. Mediterr. Mar. Sci. 2006, 7, 87–117. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Bradford, C.; Turner, R.D. A Cellulolytic Nitrogen-Fixing Bacterium Cultured from the Gland of Deshayes in Shipworms ( Bivalvia: Teredinidae). Science 1983, 221, 1401–1403. [Google Scholar]
- Prieur, D.; Mevel, G.; Nicolas, J.L.; Plusquellec, A.; Vigneulle, M. Interactions between bivalve molluscs and bacteria in the marine environment. Ocean. Mar. Biol. Annu. Rev. 1990, 28, 277–352. [Google Scholar]
- Zurel, D.; Benayahu, Y.; Or, A.; Kovacs, A.; Gophna, U. Composition and dynamics of the gill microbiota of an invasive Indo-Pacific oyster in the eastern Mediterranean Sea. Environ. Microbiol. 2011, 13, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Wentrup, C.; Wendeberg, A.; Huang, J.Y.; Borowski, C.; Dubilier, N. Shift from widespread symbiont infection of host tissues to specific colonization of gills in juvenile deep-sea mussels. ISME J. 2013, 7, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Roterman, Y.R.; Benayahu, Y.; Reshef, L.; Gophna, U. The gill microbiota of invasive and indigenous Spondylus oysters from the Mediterranean Sea and northern Red Sea. Environ. Microbiol. Rep. 2015, 7, 860–867. [Google Scholar] [CrossRef]
- Zerebecki, R.A.; Sorte, C.J.B. Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS ONE 2011, 6, e14806. [Google Scholar] [CrossRef]
- Labiosa, R.G.; Arrigo, K.R.; Genin, A.; Monismith, S.G.; Van Dijken, G. The interplay between upwelling and deep convective mixing in determining the seasonal phytoplankton dynamics in the Gulf of Aqaba: Evidence from SeaWiFS and MODIS. Limnol. Oceanogr. 2003, 48, 2355–2368. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Payne, A.; Pidcock, R.; et al. Summary for Policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018; Available online: https://www.ipcc.ch/sr15/ (accessed on 1 April 2019).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontolia Electron. 2001, 4, 1. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Similarity-based testing for community pattern: The two-way layout with no replication. Mar. Biol. 1994, 118, 167–176. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Palomera, I.; Olivar, M.; Salat, J.; Sabatés, A.; Coll, M.; García, A.; Morales-Nin, B. Small pelagic fish in the NW Mediterranean Sea: An ecological review. Prog. Oceanogr. 2007, 74, 377–396. [Google Scholar] [CrossRef]
- Bernardello, R.; Serrano, E.; Coma, R.; Ribes, M.; Bahamon, N. A comparison of remote-sensing SST and in situ seawater temperature in near-shore habitats in the western Mediterra nean Sea. Mar. Ecol. Prog. Ser. 2016, 559, 21–34. [Google Scholar] [CrossRef]
- van de Water, J.A.J.M.; Voolstra, C.R.; Rottier, C.; Cocito, S.; Peirano, A.; Allemand, D.; Ferrier-Pagès, C. Seasonal Stability in the Microbiomes of Temperate Gorgonians and the Red Coral Corallium rubrum Across the Mediterranean Sea. Microb. Ecol. 2018, 75, 274–288. [Google Scholar] [CrossRef]
- Yang, C.-S.; Chen, M.-H.; Arun, A.B.; Chen, C.A.; Wang, J.-T.; Chen, W.-M. Endozoicomonas montiporae sp. nov., isolated from the encrusting pore coral Montipora aequituberculata. Int. J. Syst. Evol. Microbiol. 2010, 60 Pt 5, 1158–1162. [Google Scholar] [CrossRef]
- Kurahashi, M.; Yokota, A. Endozoicomonas elysicola gen. nov., sp. nov., a gamma-proteobacterium isolated from the sea slug Elysia ornata. Syst. Appl. Microbiol. 2007, 30, 202–206. [Google Scholar] [CrossRef]
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Terwin, J.R.; Whitlatch, R.B.; Osman, R.W. Linking climate change and biological invasions: Ocean warming facilitates nonindigenous species invasions. Proc. Natl. Acad. Sci. USA 2002, 99, 15497–15500. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Metaxas, A. Temperature explains settlement patterns of the introduced bryozoan Membranipora membranacea in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 2007, 344, 95–106. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J. Potential effects of climate change on a marine invasion: The importance of current context 2 Predicted Effect of Warming Tem-perature on Lionfish Pelagic Larval Duration and Dispersal. Curr. Zool. 2012, 58, 1–8. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Carlton, J.T. Marine range shifts and species introductions: Comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 2010, 19, 303–316. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Zerebecki, R.A. Ocean warming increases threat of invasive species in a marine fouling community R eports R eports. Ecology 2010, 91, 2198–2204. [Google Scholar] [CrossRef]
- Collado, L.; Jara, R.; Vásquez, N.; Telsaint, C. Antimicrobial resistance and virulence genes of Arcobacter isolates recovered from edible bivalve molluscs. Food Control 2014, 46, 508–512. [Google Scholar] [CrossRef]
- Kayman, T.; Abay, S.; Hizlisoy, H.; Atabay, H.I.; Diker, K.S.; Aydin, F. Emerging pathogen Arcobacter spp. in acute gastroenteritis: Molecular identification, antibiotic susceptibilities and genotyping of the isolated arcobacters. J. Med. Microbiol. 2012, 61 Pt 10, 1439–1444. [Google Scholar] [CrossRef]
- Snelling, W.J.; Matsuda, M.; Moore, J.E.; Dooley, J.S.G. Under the microscope: Arcobacter. Lett. Appl. Microbiol. 2006, 42, 7–14. [Google Scholar] [CrossRef]
- Sunagawa, S.; DeSantis, T.Z.; Piceno, Y.M.; Brodie, E.L.; DeSalvo, M.K.; Voolstra, C.R.; Weil, E.; Andersen, G.L.; Medina, M. Bacterial diversity and white Plague disease-associated community changes in the caribbean coral montastraea faveolata. ISME J. 2009, 3, 512–521. [Google Scholar] [CrossRef]
- Ramsby, B.D.; Hoogenboom, M.O.; Whalan, S.; Webster, N.S. Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol. Ecol. 2018, 27, 2124–2137. [Google Scholar] [CrossRef]
- Toren, A.; Landau, L.; Kushmaro, A.; Loya, Y.; Rosenberg, E. Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl. Environ. Microbiol. 1998, 64, 1379–1384. [Google Scholar] [CrossRef]
- Ben-haim, Y.; Zicherman-keren, M.; Rosenberg, E. Temperature-Regulated Bleaching and Lysis of the Coral Pocillopora damicornis by the Novel Pathogen Vibrio coralliilyticus Temperature-Regulated Bleaching and Lysis of the Coral Pocillopora damicornis by the Novel Pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 2003, 69, 4236–4241. [Google Scholar] [CrossRef] [PubMed]
- Bally, M.; Garrabou, J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: A new case of emerging disease linked to climate change. Glob. Chang. Biol. 2007, 13, 2078–2088. [Google Scholar] [CrossRef]
- Thiel, V.; Leininger, S.; Schmaljohann, R.; Brümmer, F.; Imhoff, J.F. Sponge-specific bacterial associations of the Mediterranean sponge Chondrilla nucula (Demospongiae, Tetractinomorpha). Microb. Ecol. 2007, 54, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, M.; Adachi, K.; Katsuta, A.; Shizuri, Y.; Yamasato, K. Endozoicomonas numazuensis sp. nov., a gammaproteobacterium isolated from marine sponges, and emended description of the genus Endozoicomonas Kurahashi and Yokota 2007. Int. J. Syst. Evol. Microbiol. 2013, 63, 709–714. [Google Scholar] [PubMed]
- Bayer, T.; Arif, C.; Ferrier-Pagès, C.; Zoccola, D.; Aranda, M.; Voolstra, C. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar. Ecol. Prog. Ser. 2013, 479, 75–84. [Google Scholar] [CrossRef]
- Roder, C.; Bayer, T.; Aranda, M.; Kruse, M.; Voolstra, C.R. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Mol. Ecol. 2015, 24, 3501–3511. [Google Scholar] [CrossRef]
- Zielinski, F.U.; Pernthaler, A.; Duperron, S.; Raggi, L.; Giere, O.; Borowski, C.; Dubilier, N. Widespread occurrence of an intranuclear bacterial parasite in vent and seep bathymodiolin mussels. Environ. Microbiol. 2009, 11, 1150–1167. [Google Scholar] [CrossRef] [PubMed]
- Beinart, R.A.; Nyholm, S.V.; Dubilier, N.; Girguis, P.R. Intracellular Oceanospirillales inhabit the gills of the hydrothermal vent snail A lviniconcha with chemosynthetic, γ-Proteobacterial symbionts. Environ. Microbiol. Rep. 2014, 6, 656–664. [Google Scholar] [CrossRef]
- van de Water, J.A.J.M.; De Mares, M.C.; Dixon, G.B.; Raina, J.; Willis, B.L.; Bourne, D.G.; van Oppen, M.J.H. Antimicrobial and stress responses to increased temperature and bacterial pathogen challenge in the holobiont of a reef-building coral. Mol. Ecol. 2018, 27, 1065–1080. [Google Scholar] [CrossRef]
- Glasl, B.; Herndl, G.J.; Frade, P.R. The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. ISME J. 2016, 10, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; Paul, V.J.; Teplitski, M. Community shifts in the surface microbiomes of the coral Porites astreoides with unusual lesions. PLoS ONE 2014, 9, e100316. [Google Scholar] [CrossRef]
- Morrow, K.M.; Bromhall, K.; Motti, C.A.; Munn, C.B.; Bourne, D.G. Allelochemicals produced by brown macroalgae of the Lobophora genus are active against coral larvae and associated bacteria, supporting pathogenic shifts to Vibrio dominance. Appl. Environ. Microbiol. 2017, 83, e02391-16. [Google Scholar] [CrossRef]
- Roder, C.; Arif, C.; Bayer, T.; Aranda, M.; Daniels, C.; Shibl, A.; Chavanich, S.; Voolstra, C.R. Bacterial profiling of White Plague Disease in a comparative coral species framework. ISME J. 2014, 8, 31–39. [Google Scholar] [CrossRef]
- Choudhury, J.D.; Pramanik, A.; Webster, N.S.; Llewellyn, L.E.; Gachhui, R.; Mukherjee, J. The Pathogen of the Great Barrier Reef Sponge Rhopaloeides odorabile Is a New Strain of Pseudoalteromonas agarivorans Containing Abundant and Diverse Virulence-Related Genes. Mar. Biotechnol. 2015, 17, 463–478. [Google Scholar] [CrossRef]
- Zurel, D.; Gophna, U.; Benayahu, Y. Parity and disparity between two Chama oysters: The reproductive biology of the Indo-Pacific C. pacifica Broderip, invasive to the Mediterranean Sea; and C. savignyi Lamy, indigenous to the Red Sea. Mar. Ecol. 2012, 33, 261–271. [Google Scholar] [CrossRef]
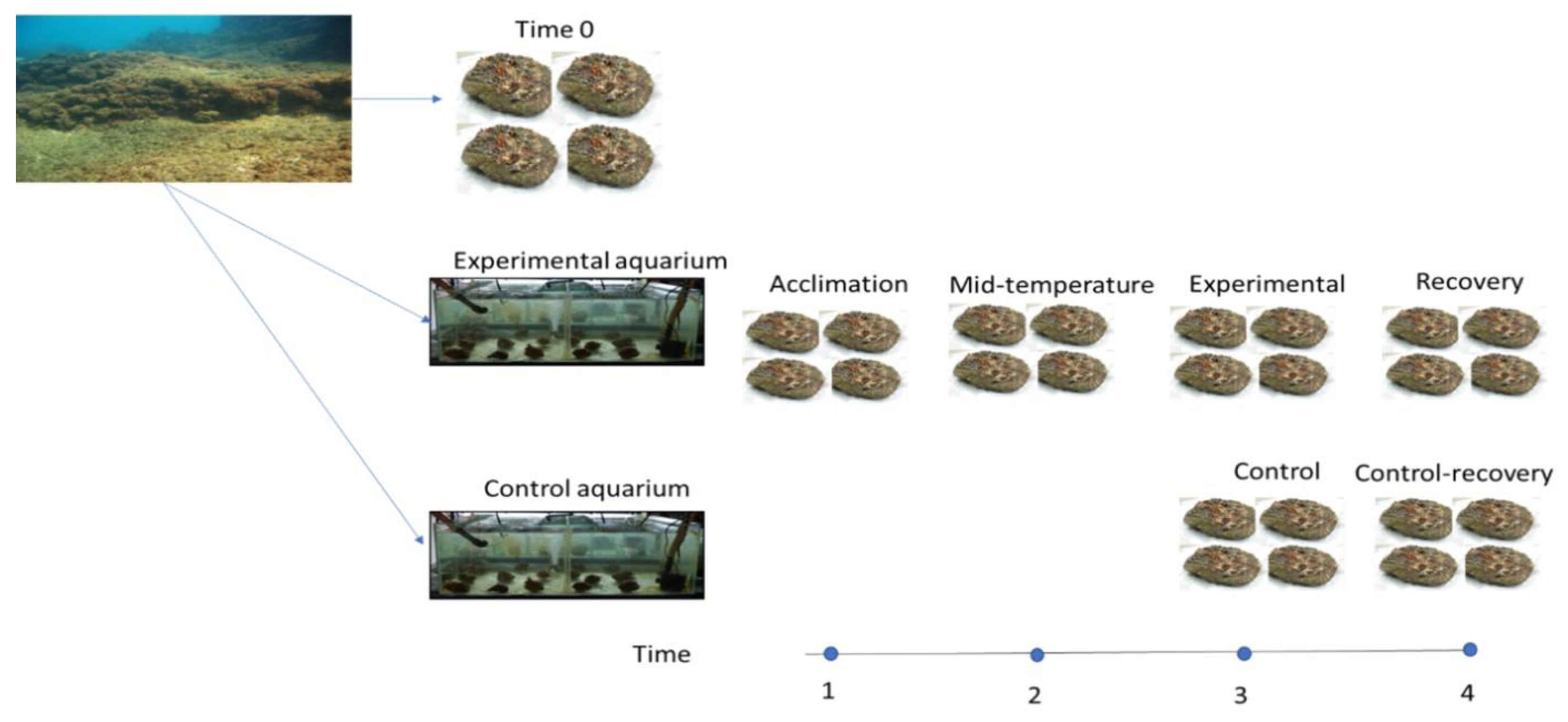
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment | Warming Experiment | Global Warming Experiment | Extreme Cold Experiment | ||||||
| Inception date | 4 Feburary 2016 | 5 October 2016 | 5 April 2016 | ||||||
| Number of oysters | 33 | 49 | 22 | ||||||
| Onset temperature (°C) | 17 | 29 | 20 | ||||||
| Target temperature (°C) | 31 | 32 & 33 | 11 | ||||||
| B. | |||||||||
| Group | Days since Inception | Temperature (°C) | Sample Size | Days since Inception | Temperature (°C) | Sample Size | Days since Inception | Temperature (°C) | Sample Size |
| Inception | 0 | 17 | 5 | 0 | 29 | 5 | 0 | 20 | 5 |
| Acclimation | 10 | 18 | 5 | 8 | 27 | 4 | 7 | 20 | 4 |
| Mid-temperature | 13 | 24 | 3 | - | - | - | 9 | 15 | 2 |
| Experimental | 28 | 31 | 5 | 23 | 32 | 10 | 23 | 11 | 5 |
| 36 | 33 | 10 | |||||||
| Control | 28 | 18 | 4 | 23 | 26 | 5 | 23 | 23 | 5 |
| 36 | 24 | 5 | |||||||
| Recovery | 47 | 18 | 5 | 53 | 24 | 5 | - | - | - |
| Control- recovery | 47 | 18 | 5 | 53 | 24 | 5 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dor-Roterman, Y.R.; Benayahu, Y.; Reshef, L.; Gophna, U. Host–Microbiome Interactions in a Changing Sea: The Gill Microbiome of an Invasive Oyster under Drastic Temperature Changes. Microorganisms 2024, 12, 197. https://doi.org/10.3390/microorganisms12010197
Dor-Roterman YR, Benayahu Y, Reshef L, Gophna U. Host–Microbiome Interactions in a Changing Sea: The Gill Microbiome of an Invasive Oyster under Drastic Temperature Changes. Microorganisms. 2024; 12(1):197. https://doi.org/10.3390/microorganisms12010197
Chicago/Turabian StyleDor-Roterman, Yahala Rina, Yehuda Benayahu, Leah Reshef, and Uri Gophna. 2024. "Host–Microbiome Interactions in a Changing Sea: The Gill Microbiome of an Invasive Oyster under Drastic Temperature Changes" Microorganisms 12, no. 1: 197. https://doi.org/10.3390/microorganisms12010197





