Immunomodulating Enzymes from Streptococcus pyogenes—In Pathogenesis, as Biotechnological Tools, and as Biological Drugs
Abstract
:1. Introduction
2. Main Functional Categories of S. pyogenes Immunomodulating Enzymes
2.1. Immunoglobulin Degrading and Modifying Enzymes
2.1.1. Immunoglobulin Cysteine Proteinases
The Broad-Spectrum Cysteine Proteinase SpeB
The Immunoglobulin G-Degrading Cysteine Proteinases IdeS/Mac-1/Mac-2

2.1.2. Immunoglobulin Glycan Hydrolases
The Endo-β-N-acetylglucosaminidase EndoS
The Endo-β-N-acetylglucosaminidase EndoS2
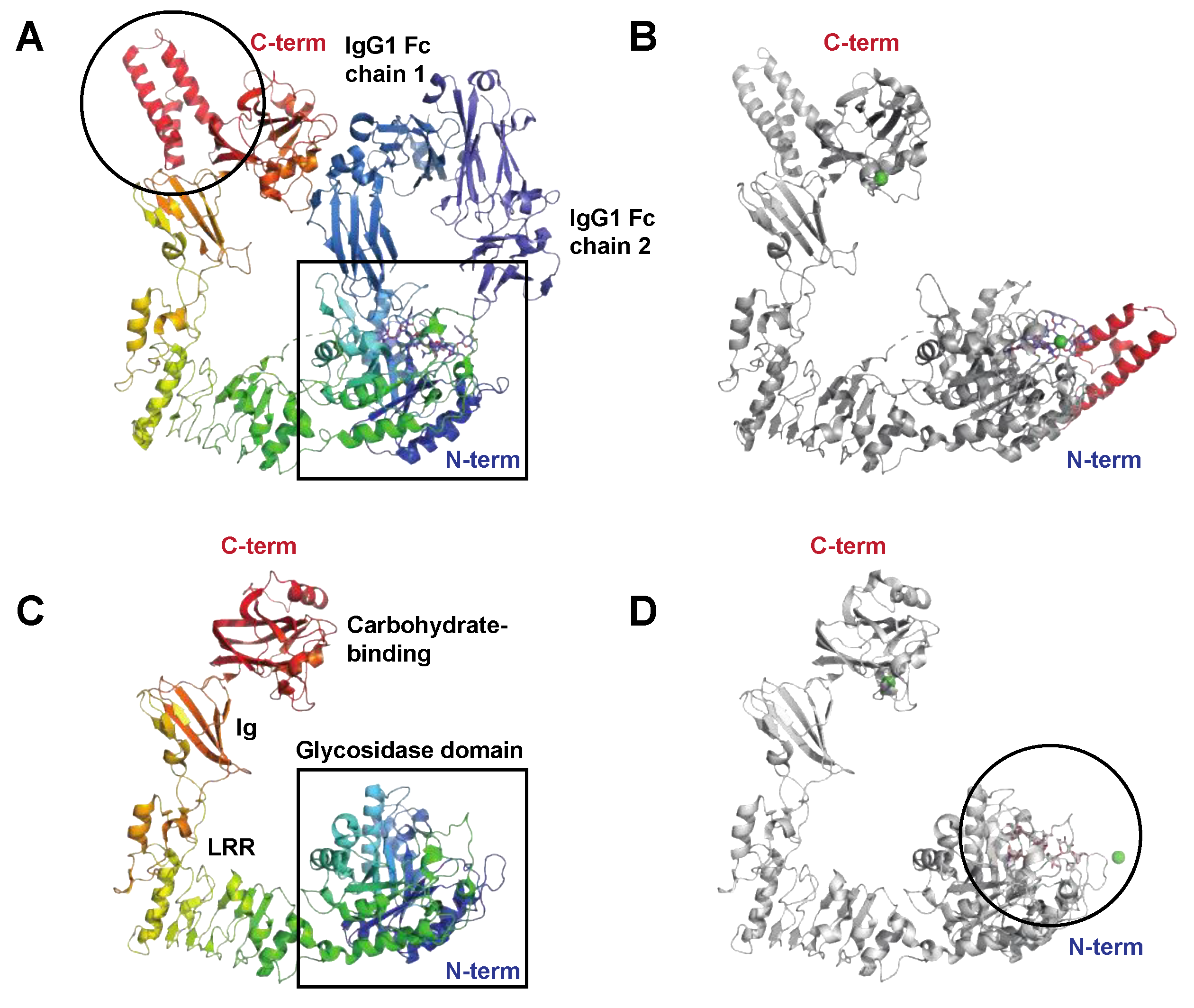
2.2. Enzymes Interfering with Innate Immunity
2.2.1. Complement and Antimicrobial Peptide Degrading Enzymes
The Broad-Spectrum Cysteine Proteinase SpeB
The Serine Endopeptidase C5a, ScpA
2.2.2. Chemokine, Cytokine, and Kinin Active Enzymes
SpeB Activity on Immunologically Active Peptides
The Serine Proteinase SpyCEP
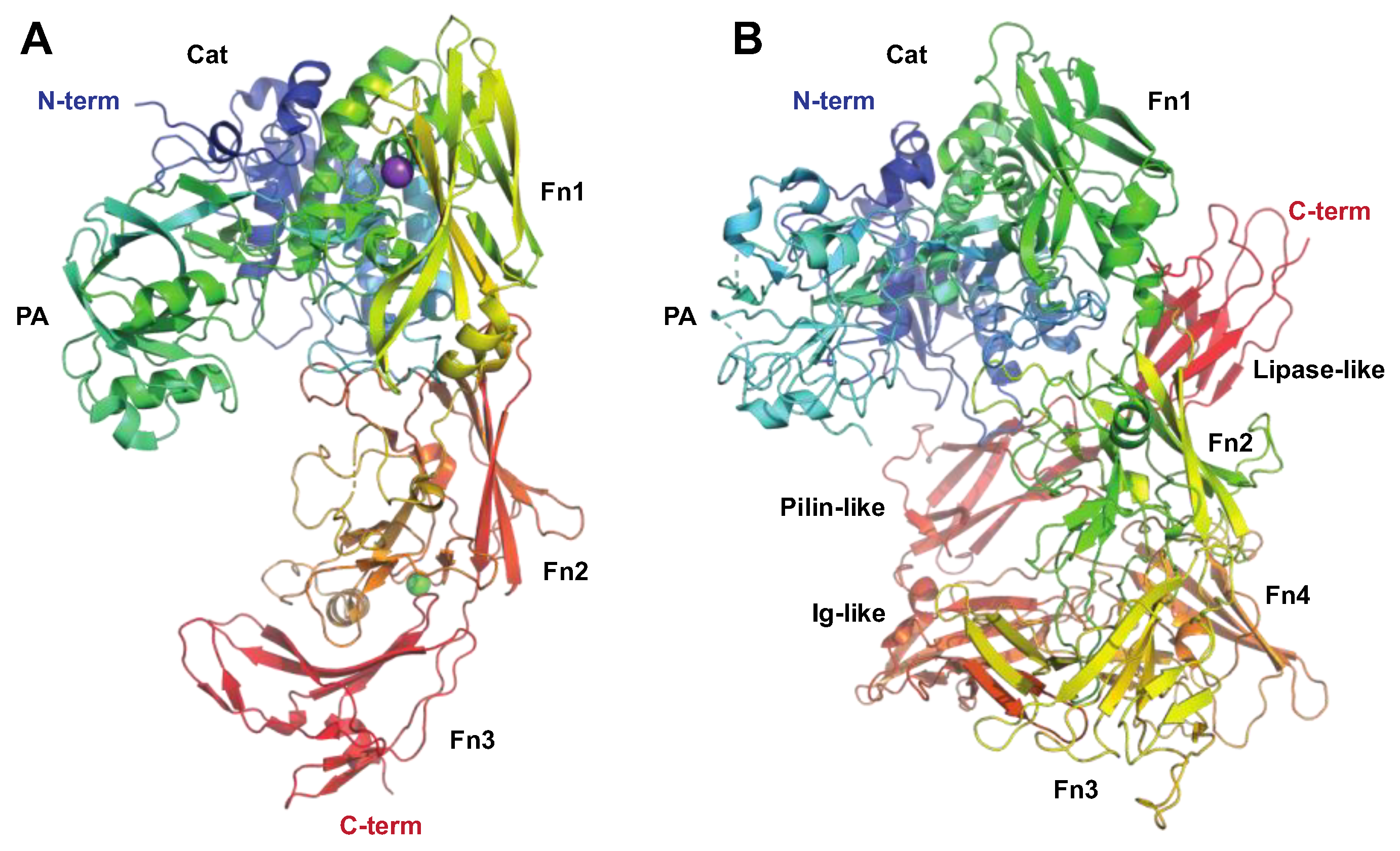
2.3. Enzymes Acting on Chromatin and Cellular Processes
2.3.1. Enzymes Acting on DNA and Nucleotides
The Streptococcal Nuclease Sda1
The Streptococcus pyogenes Nuclease A, SpnA
The Streptococcal 5′-Nucleotidase A, S5nA
The Streptococcal NAD(+)-Glycohydrolase, NADase
The C-di-AMP Synthase DacA
2.3.2. Enzymatic Effects on Pyroptosis and Autophagy
SpeB Activity on Gasdermins
SpeB Activity on Ubiquitin-Binding Proteins
2.3.3. Enzymes Acting on Other Cellular Processes
The Streptococcal Arginine Deiminase SAGP
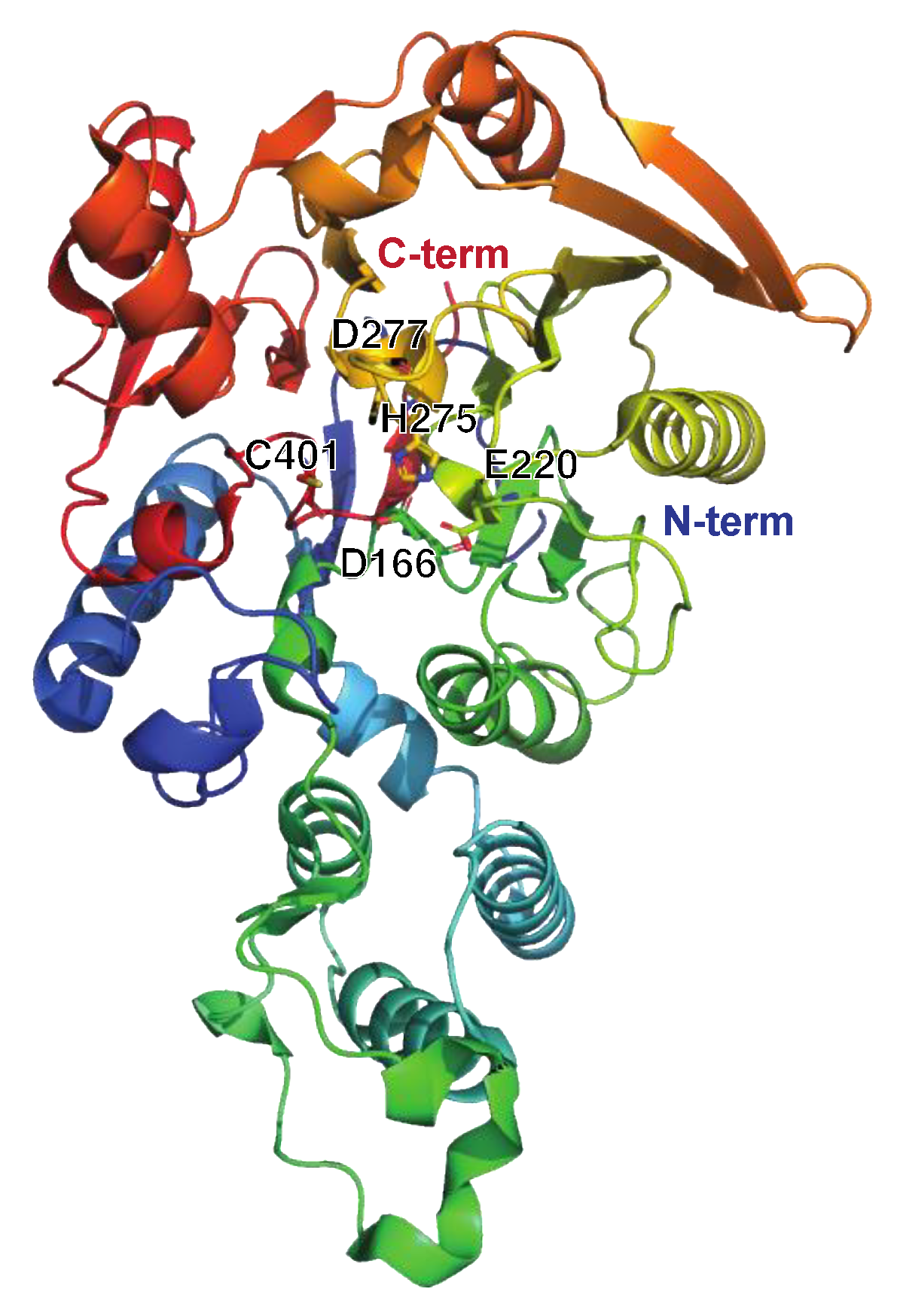
| Enzyme | Type of Enzyme, Designations, and Structure Used for Illustrations | Occurrence, Immunomodulating Activities, and Role in Pathogenesis | References |
|---|---|---|---|
| SpeB | Protease (cysteine proteinase) Streptopain, streptococcal cysteine proteinase PDB: 4RKX, 4D8B, 6UKD (w smi), 4D8E (w smi), 4D8I (w smi), 1DKI (zymogen), 1PVJ (w smi), 6UQD (w smi), 2JTC, 2UZJ Figure 2A |
| [15,18,48,86,87,93,94,95,96] |
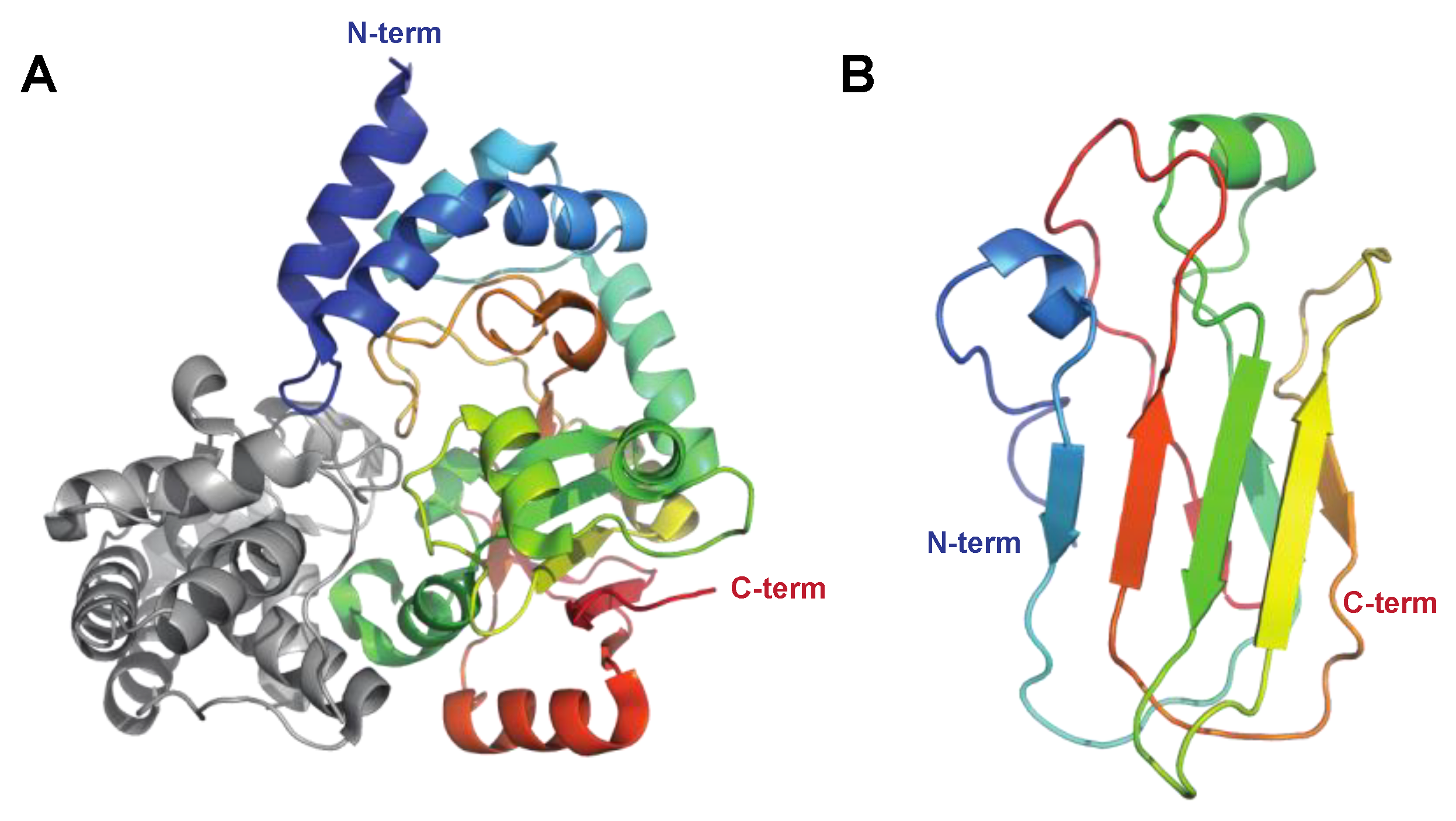
| IdeS/Mac-1 | Protease (cysteine proteinase) Immunoglobulin-G-degrading enzyme of S. pyogenes PDB: IY08, 2AU1, 2AVW Figure 2B |
| [19,20,23,25,97,98] |
| Mac-2 | Protease (cysteine proteinase) 8A47 (IgG Fc) Figure 2B |
| [21,22,99] |
| SpyCEP | Protease (serine proteinase) ScpC, CepA PDB: 5XYA, 6VJB (mut), 5XXZ, 5XYR Figure 4B |
| [6,53,59,61,100,101,102] |
| ScpA | Protease (serine proteinase) C5a peptidase PDB: 3EIF, 7BJ3 (mut), 7YZX (mut), 1XF1 Figure 4A |
| [43,103,104,105] |
| EndoS | Endoglycosidase (endo-β-N-acetylglucosaminidase, GH18) PDB: 4NUY, 4NUZ (mut), 6EN3 (G2 oligo), 8A64 (IgG Fc), 8A49 (IgG Fc) Figure 3A,B |
| [14,15,106,107,108] |
| EndoS2 | Endoglycosidase (endo-β-N-acetylglucosaminidase, GH18) PDB: 6E58, 6MDS (bia glyc), 6MDV (hm glyc) Figure 3C,D |
| [30,34] |
| SodA | Superoxide dismutase No experimental structure. |
| Not covered in depth here; for a review, see [39] |
| AhpC | Alkyl hydroperoxidase No experimental structure. |
| Not covered in depth here; for a review, see [39] |
| GpoA | Glutathione peroxidase No experimental structure. |
| Not covered in depth here; for a review, see [39] |
| NoxA | NADH oxidase A reductase No experimental structure. |
| Not covered in depth here; for a review, see [39] |
| Sda1 | Nuclease Streptococcal nuclease Sda1, Streptodornase PDB: 5FGU, 5FGW Figure 5 |
| [68,70,109,110,111,112] |
| SpnA | Nuclease Streptococcal nuclease A No experimental structure. |
| [67,72,73,113,114,115] |
| S5nA | Nucleotidase Streptococcal 5′-nucleotidase A No experimental structure. |
| [74,78] |
| NADase | NAD glycohydrolase PDB: 4KT6, 7JI1, 7WVH (w SLO) Figure 6 |
| [82,84,116,117,118,119,120] |
| DacA | C-di-AMP synthase No experimental structure. |
| [83,84,121] |
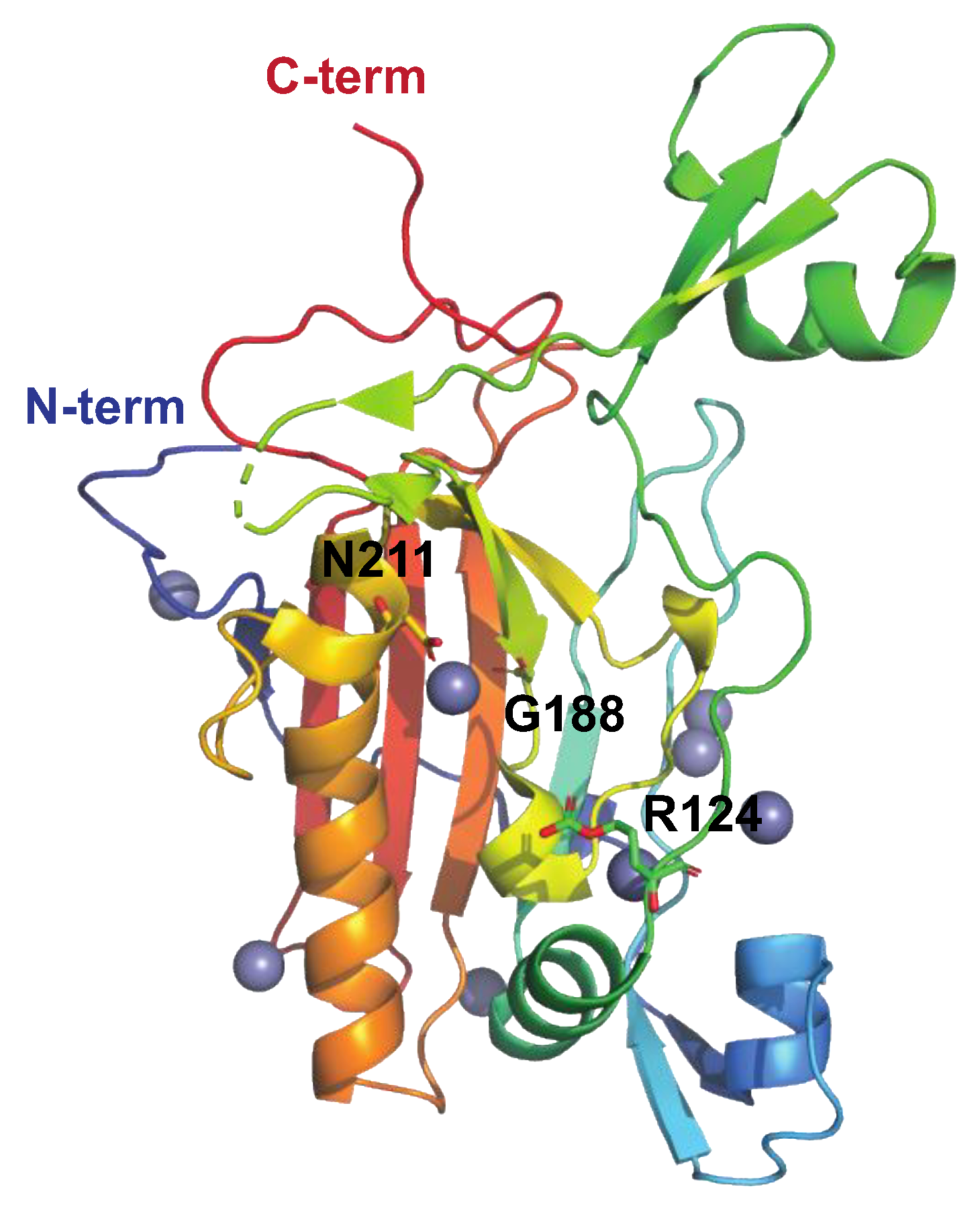
| SAGP | Arginine deiminase Streptococcal acid glycoprotein (SAGP) Arginine deiminase (ADI) PDB: 4BOF Figure 7. |
| [88,89,91,92,122] |
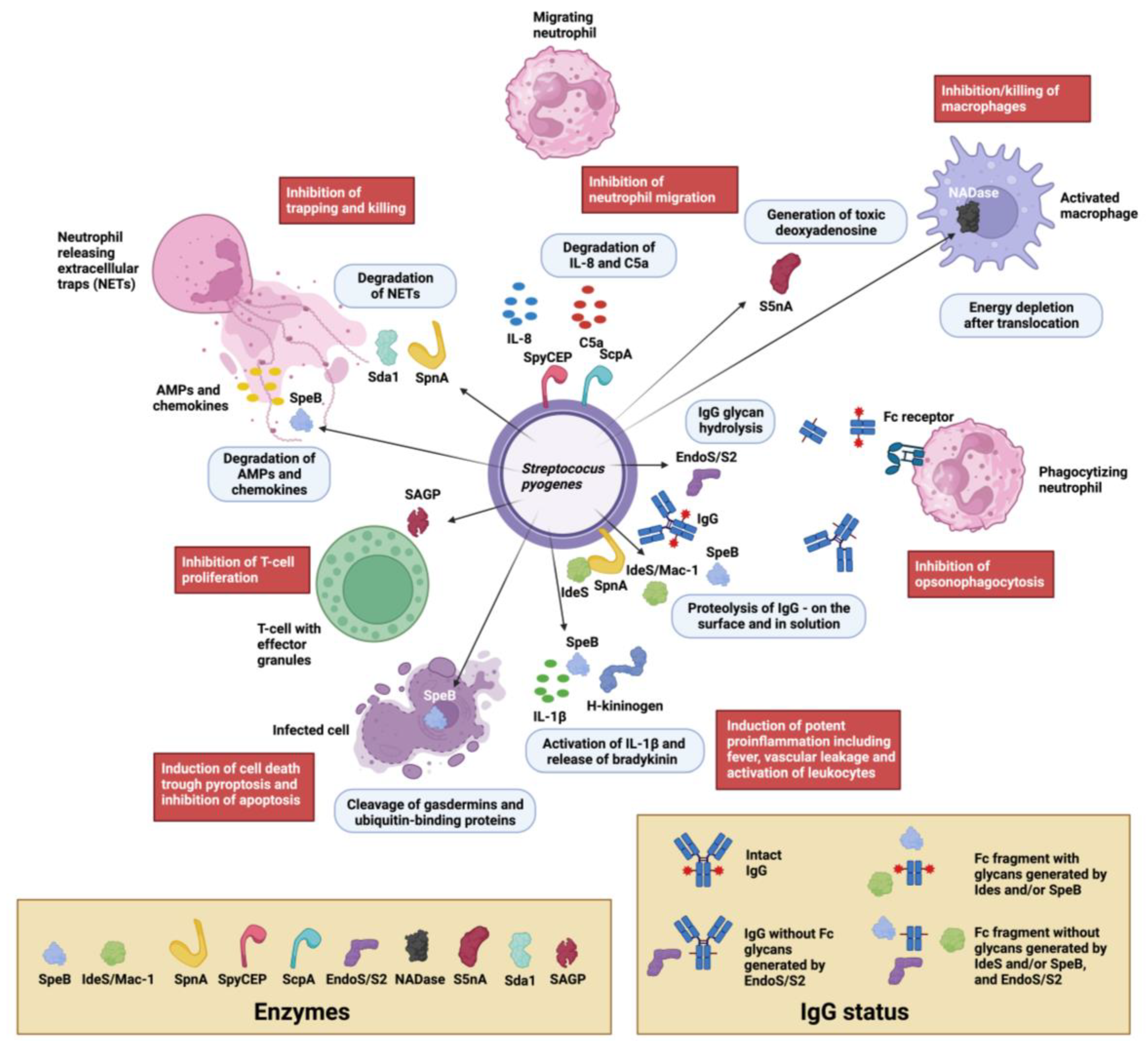
3. Immunomodulating Enzymes in S. pyogenes Pathogenesis
4. Immunomodulating Enzymes as Biotechnological Tools
| Enzyme | Application | References |
|---|---|---|
| SpeB |
| [144] |
| IdeS |
| [145,146,147,148,149,150,151] |
| EndoS |
| [150,152,153] |
| EndoS2 |
| [30,150,154,155] |
| SpyCEP |
| [122,156] |
| ScpA |
| [105,122] |
| SAGP |
| [122] |

5. Immunomodulating Enzymes as Biological Drugs and Vaccines
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Bisno, A.L. Acute pharyngitis. N. Engl. J. Med. 2001, 344, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bisno, A.L.; Stevens, D.L. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 1996, 334, 240–245. [Google Scholar] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Reglinski, M.; Sriskandan, S. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence 2014, 5, 127–136. [Google Scholar] [PubMed]
- Cole, J.N.; Barnett, T.C.; Nizet, V.; Walker, M.J. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 2011, 9, 724–736. [Google Scholar] [PubMed]
- Barnett, T.C.; Cole, J.N.; Rivera-Hernandez, T.; Henningham, A.; Paton, J.C.; Nizet, V.; Walker, M.J. Streptococcal toxins: Role in pathogenesis and disease. Cell. Microbiol. 2015, 17, 1721–1741. [Google Scholar] [CrossRef]
- Okumura, C.Y.M.; Nizet, V. Subterfuge and sabotage: Evasion of host innate defenses by invasive gram-positive bacterial pathogens. Annu. Rev. Microbiol. 2014, 68, 439–458. [Google Scholar]
- Collin, M.; Olsén, A. Extracellular enzymes with immunomodulating activities: Variations on a theme in Streptococcus pyogenes. Infect. Immun. 2003, 71, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef]
- Collin, M.; Kilian, M. Bacterial modulation of Fc effector functions. In Antibody Fc; Elsevier: Amsterdam, The Netherlands, 2014; pp. 317–332. [Google Scholar]
- Nelson, D.C.; Garbe, J.; Collin, M. Cysteine proteinase SpeB from Streptococcus pyogenes—A potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 2011, 392, 1077–1088. [Google Scholar] [CrossRef]
- Carroll, R.K.; Musser, J.M. From transcription to activation: How group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol. Microbiol. 2011, 81, 588–601. [Google Scholar]
- Collin, M.; Olsén, A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001, 20, 3046–3055. [Google Scholar] [PubMed]
- Collin, M.; Olsén, A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 2001, 69, 7187–7189. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Norgren, M. Cleavage of antigen-bound immunoglobulin G by SpeB contributes to streptococcal persistence in opsonizing blood. Infect. Immun. 2003, 71, 211–217. [Google Scholar] [CrossRef]
- Collin, M.; Svensson, M.D.; Sjöholm, A.G.; Jensenius, J.C.; Sjöbring, U.; Olsén, A. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 2002, 70, 6646–6651. [Google Scholar] [CrossRef]
- Persson, H.; Vindebro, R.; von Pawel-Rammingen, U. The streptococcal cysteine protease SpeB is not a natural immunoglobulin-cleaving enzyme. Infect. Immun. 2013, 81, 2236–2241. [Google Scholar] [PubMed]
- Lei, B.; DeLeo, F.R.; Hoe, N.P.; Graham, M.R.; Mackie, S.M.; Cole, R.L.; Liu, M.; Hill, H.R.; Low, D.E.; Federle, M.J.; et al. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 2001, 7, 1298–1305. [Google Scholar] [CrossRef]
- von Pawel-Rammingen, U.; Johansson, B.P.; Björck, L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002, 21, 1607–1615. [Google Scholar] [CrossRef]
- Agniswamy, J.; Lei, B.; Musser, J.M.; Sun, P.D. Insight of host immune evasion mediated by two variants of group A Streptococcus Mac protein. J. Biol. Chem. 2004, 279, 52789–52796. [Google Scholar] [CrossRef]
- Söderberg, J.J.; Engström, P.; von Pawel-Rammingen, U. The intrinsic immunoglobulin g endopeptidase activity of streptococcal Mac-2 proteins implies a unique role for the enzymatically impaired Mac-2 protein of M28 serotype strains. Infect. Immun. 2008, 76, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Vincents, B.; von Pawel-Rammingen, U.; Björck, L.; Abrahamson, M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 2004, 43, 15540–15549. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, J.J.; von Pawel-Rammingen, U. The streptococcal protease IdeS modulates bacterial IgGFc binding and generates 1/2Fc fragments with the ability to prime polymorphonuclear leucocytes. Mol. Immunol. 2008, 45, 3347–3353. [Google Scholar] [CrossRef]
- Wenig, K.; Chatwell, L.; von Pawel-Rammingen, U.; Björck, L.; Huber, R.; Sondermann, P. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc. Natl. Acad. Sci. USA 2004, 101, 17371–17376. [Google Scholar] [CrossRef]
- Sudol, A.S.L.; Butler, J.; Ivory, D.P.; Tews, I.; Crispin, M. Extensive substrate recognition by the streptococcal antibody-degrading enzymes IdeS and EndoS. Nat. Commun. 2022, 13, 7801. [Google Scholar] [PubMed]
- Dardenne, L.E.; Werneck, A.S.; de Oliveira Neto, M.; Bisch, P.M. Electrostatic properties in the catalytic site of papain: A possible regulatory mechanism for the reactivity of the ion pair. Proteins 2003, 52, 236–253. [Google Scholar] [PubMed]
- González-Páez, G.E.; Wolan, D.W. Ultrahigh and high resolution structures and mutational analysis of monomeric Streptococcus pyogenes SpeB reveal a functional role for the glycine-rich C-terminal loop. J. Biol. Chem. 2012, 287, 24412–24426. [Google Scholar] [CrossRef]
- Trimble, R.B.; Tarentino, A.L. Identification of distinct endoglycosidase (endo) activities in Flavobacterium meningosepticum: Endo F1, Endo F2, and Endo F3. Endo F1 and Endo H hydrolyze only high mannose and hybrid glycans. J. Biol. Chem. 1991, 266, 1646–1651. [Google Scholar] [CrossRef]
- Sjögren, J.; Cosgrave, E.F.J.; Allhorn, M.; Nordgren, M.; Björk, S.; Olsson, F.; Fredriksson, S.; Collin, M. EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology 2015, 25, 1053–1063. [Google Scholar]
- Du, J.J.; Klontz, E.H.; Guerin, M.E.; Trastoy, B.; Sundberg, E.J. Structural insights into the mechanisms and specificities of IgG-active endoglycosidases. Glycobiology 2019, 30, 268–279. [Google Scholar]
- Klontz, E.H.; Trastoy, B.; Deredge, D.; Fields, J.K.; Li, C.; Orwenyo, J.; Marina, A.; Beadenkopf, R.; Günther, S.; Flores, J.; et al. Molecular basis of broad spectrum N-Glycan specificity and processing of therapeutic IgG monoclonal antibodies by Endoglycosidase S2. ACS Cent. Sci. 2019, 5, 524–538. [Google Scholar] [CrossRef]
- Trastoy, B.; Lomino, J.V.; Pierce, B.G.; Carter, L.G.; Günther, S.; Giddens, J.P.; Snyder, G.A.; Weiss, T.M.; Weng, Z.; Wang, L.-X.; et al. Crystal structure of Streptococcus pyogenes EndoS, an immunomodulatory endoglycosidase specific for human IgG antibodies. Proc. Natl. Acad. Sci. USA 2014, 111, 6714–6719. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Struwe, W.B.; Cosgrave, E.F.J.; Rudd, P.M.; Stervander, M.; Allhorn, M.; Hollands, A.; Nizet, V.; Collin, M. EndoS2 is a unique and conserved enzyme of serotype M49 group A Streptococcus that hydrolyses N-linked glycans on IgG and α1-acid glycoprotein. Biochem. J. 2013, 455, 107–118. [Google Scholar] [CrossRef]
- Sudol, A.S.L.; Tews, I.; Crispin, M. Bespoke conformation and antibody recognition distinguishes the streptococcal immune evasion factors EndoS and EndoS2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Trastoy, B.; Klontz, E.; Orwenyo, J.; Marina, A.; Wang, L.-X.; Sundberg, E.J.; Guerin, M.E. Structural basis for the recognition of complex-type N-glycans by Endoglycosidase S. Nat. Commun. 2018, 9, 1874. [Google Scholar] [CrossRef] [PubMed]
- Aghababa, H.; Ting, Y.T.; Pilapitiya, D.; Loh, J.M.S.; Young, P.G.; Proft, T. Complement evasion factor (CEF), a novel immune evasion factor of Streptococcus pyogenes. Virulence 2022, 13, 225–240. [Google Scholar] [PubMed]
- Åkesson, P.; Sjöholm, A.G.; Björck, L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 1996, 271, 1081–1088. [Google Scholar]
- Henningham, A.; Döhrmann, S.; Nizet, V.; Cole, J.N. Mechanisms of group A Streptococcus resistance to reactive oxygen species. FEMS Microbiol. Rev. 2015, 39, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Mori, Y.; Yamaguchi, M.; Shimizu, Y.; Ooe, K.; Hamada, S.; Kawabata, S. Group A streptococcal cysteine protease degrades C3 (C3b) and contributes to evasion of innate immunity. J. Biol. Chem. 2008, 283, 6253–6260. [Google Scholar] [CrossRef]
- Kuo, C.-F.; Lin, Y.-S.; Chuang, W.-J.; Wu, J.-J.; Tsao, N. Degradation of complement 3 by streptococcal pyrogenic exotoxin B inhibits complement activation and neutrophil opsonophagocytosis. Infect. Immun. 2008, 76, 1163–1169. [Google Scholar]
- Tsao, N.; Tsai, W.-H.; Lin, Y.-S.; Chuang, W.-J.; Wang, C.-H.; Kuo, C.-F. Streptococcal pyrogenic exotoxin B cleaves properdin and inhibits complement-mediated opsonophagocytosis. Biochem. Biophys. Res. Commun. 2006, 339, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Cleary, P.P.; Prahbu, U.; Dale, J.B.; Wexler, D.E.; Handley, J. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 1992, 60, 5219–5223. [Google Scholar] [PubMed]
- Wexler, D.E.; Cleary, P.P. Purification and characteristics of the streptococcal chemotactic factor inactivator. Infect. Immun. 1985, 50, 757–764. [Google Scholar] [PubMed]
- DeMaster, E.; Schnitzler, N.; Cheng, Q.; Cleary, P. M+ group a streptococci are phagocytized and killed in whole blood by C5a-activated polymorphonuclear leukocytes. Infect. Immun. 2002, 70, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.; Björck, L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 1995, 270, 9862–9867. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Hoover, D.; Staley, P.; Tucker, K.; Lubkowski, J.; Oppenheim, J. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J. Leukoc. Biol. 2003, 74, 448–455. [Google Scholar] [PubMed]
- Egesten, A.; Olin, A.I.; Linge, H.M.; Yadav, M.; Mörgelin, M.; Karlsson, A.; Collin, M. SpeB of Streptococcus pyogenes differentially modulates antibacterial and receptor activating properties of human chemokines. PLoS ONE 2009, 4, e4769. [Google Scholar] [CrossRef]
- Kapur, V.; Majesky, M.W.; Li, L.L.; Black, R.A.; Musser, J.M. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 1993, 90, 7676–7680. [Google Scholar] [CrossRef]
- Herwald, H.; Collin, M.; Müller-Esterl, W.; Björck, L. Streptococcal cysteine proteinase releases kinins: A novel virulence mechanism. J. Exp. Med. 1996, 184, 665–673. [Google Scholar] [CrossRef]
- Watanabe, Y.; Todome, Y.; Ohkuni, H.; Sakurada, S.; Ishikawa, T.; Yutsudo, T.; Fischetti, V.A.; Zabriskie, J.B. Cysteine protease activity and histamine release from the human mast cell line HMC-1 stimulated by recombinant streptococcal pyrogenic exotoxin B/streptococcal cysteine protease. Infect. Immun. 2002, 70, 3944–3947. [Google Scholar] [CrossRef]
- Hidalgo-Grass, C.; Dan-Goor, M.; Maly, A.; Eran, Y.; Kwinn, L.A.; Nizet, V.; Ravins, M.; Jaffe, J.; Peyser, A.; Moses, A.E.; et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet 2004, 363, 696–703. [Google Scholar]
- Turner, C.E.; Kurupati, P.; Jones, M.D.; Edwards, R.J.; Sriskandan, S. Emerging role of the interleukin-8 cleaving enzyme SpyCEP in clinical Streptococcus pyogenes infection. J. Infect. Dis. 2009, 200, 555–563. [Google Scholar] [PubMed]
- Zingaretti, C.; Falugi, F.; Nardi-Dei, V.; Pietrocola, G.; Mariani, M.; Liberatori, S.; Gallotta, M.; Tontini, M.; Tani, C.; Speziale, P.; et al. Streptococcus pyogenes SpyCEP: A chemokine-inactivating protease with unique structural and biochemical features. FASEB J. 2010, 24, 2839–2848. [Google Scholar] [CrossRef]
- Chiappini, N.; Seubert, A.; Telford, J.L.; Grandi, G.; Serruto, D.; Margarit, I.; Janulczyk, R. Streptococcus pyogenes SpyCEP influences host-pathogen interactions during infection in a murine air pouch model. PLoS ONE 2012, 7, e40411. [Google Scholar] [CrossRef]
- Lawrenson, R.A.; Sriskandan, S. Cell Envelope Proteinase A (Streptococcus). Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3195–3202. [Google Scholar]
- Edwards, R.J.; Taylor, G.W.; Ferguson, M.; Murray, S.; Rendell, N.; Wrigley, A.; Bai, Z.; Boyle, J.; Finney, S.J.; Jones, A.; et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 2005, 192, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Sumby, P.; Zhang, S.; Whitney, A.R.; Falugi, F.; Grandi, G.; Graviss, E.A.; Deleo, F.R.; Musser, J.M. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect. Immun. 2008, 76, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, A.S.; Timmer, A.M.; Pence, M.A.; Locke, J.B.; Buchanan, J.T.; Turner, C.E.; Mishalian, I.; Sriskandan, S.; Hanski, E.; Nizet, V. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe 2008, 4, 170–178. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Barnett, T.C.; Korn, O.; Rivera-Hernandez, T.; Seymour, L.M.; Schulz, B.L.; Nizet, V.; Wells, C.A.; Sweet, M.J.; Walker, M.J. Group A Streptococcus M1T1 intracellular infection of primary tonsil epithelial cells dampens levels of secreted IL-8 through the action of SpyCEP. Front. Cell. Infect. Microbiol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Biswas, D.; Ambalavanan, P.; Ravins, M.; Anand, A.; Sharma, A.; Lim, K.X.Z.; Tan, R.Y.M.; Lim, H.Y.; Sol, A.; Bachrach, G.; et al. LL-37-mediated activation of host receptors is critical for defense against group A streptococcal infection. Cell Rep. 2021, 34, 108766. [Google Scholar] [CrossRef]
- Jobichen, C.; Tan, Y.C.; Prabhakar, M.T.; Nayak, D.; Biswas, D.; Pannu, N.S.; Hanski, E.; Sivaraman, J. Structure of ScpC, a virulence protease from Streptococcus pyogenes, reveals the functional domains and maturation mechanism. Biochem. J. 2018, 475, 2847–2860. [Google Scholar]
- Kagawa, T.F.; O’Connell, M.R.; Mouat, P.; Paoli, M.; O’Toole, P.W.; Cooney, J.C. Model for substrate interactions in C5a peptidase from Streptococcus pyogenes: A 1.9 Å crystal structure of the active form of ScpA. J. Mol. Biol. 2009, 386, 754–772. [Google Scholar]
- Liao, C.; Mao, F.; Qian, M.; Wang, X. Pathogen-derived nucleases: An effective weapon for escaping extracellular traps. Front. Immunol. 2022, 13, 899890. [Google Scholar]
- Remmington, A.; Turner, C.E. The DNases of pathogenic Lancefield streptococci. Microbiology 2018, 164, 242–250. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.K.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar]
- Chang, A.; Khemlani, A.; Kang, H.; Proft, T. Functional analysis of Streptococcus pyogenes nuclease A (SpnA), a novel group A streptococcal virulence factor. Mol. Microbiol. 2011, 79, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Ismail, S.A.; Park, H.-W.; Kotb, M. Post-proteomic identification of a novel phage-encoded streptodornase, Sda1, in invasive M1T1 Streptococcus pyogenes. Mol. Microbiol. 2004, 54, 184–197. [Google Scholar] [CrossRef]
- Aziz, R.K.; Edwards, R.A.; Taylor, W.W.; Low, D.E.; McGeer, A.; Kotb, M. Mosaic prophages with horizontally acquired genes account for the emergence and diversification of the globally disseminated M1T1 clone of Streptococcus pyogenes. J. Bacteriol. 2005, 187, 3311–3318. [Google Scholar] [PubMed]
- Uchiyama, S.; Andreoni, F.; Schuepbach, R.A.; Nizet, V.; Zinkernagel, A.S. DNase Sda1 allows invasive M1T1 Group A Streptococcus to prevent TLR9-dependent recognition. PLoS Pathog. 2012, 8, e1002736. [Google Scholar] [CrossRef]
- Moon, A.F.; Krahn, J.M.; Lu, X.; Cuneo, M.J.; Pedersen, L.C. Structural characterization of the virulence factor Sda1 nuclease from Streptococcus pyogenes. Nucleic Acids Res. 2016, 44, 3946–3957. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Minami, M.; Okamoto, A.; Tatsuno, I.; Isaka, M.; Ohta, M. Characterization of a virulence-associated and cell-wall-located DNase of Streptococcus pyogenes. Microbiology 2010, 156 Pt 1, 184–190. [Google Scholar] [CrossRef]
- Frick, I.-M.; Happonen, L.; Wrighton, S.; Nordenfelt, P.; Björck, L. IdeS, a secreted proteinase of Streptococcus pyogenes, is bound to a nuclease at the bacterial surface where it inactivates opsonizing IgG antibodies. J. Biol. Chem. 2023, 105345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Khemlani, A.; Lorenz, N.; Loh, J.M.S.; Langley, R.J.; Proft, T. Streptococcal 5′-Nucleotidase A (S5nA), a novel Streptococcus pyogenes virulence factor that facilitates immune evasion. J. Biol. Chem. 2015, 290, 31126–31137. [Google Scholar]
- Firon, A.; Dinis, M.; Raynal, B.; Poyart, C.; Trieu-Cuot, P.; Kaminski, P.A. Extracellular nucleotide catabolism by the Group B Streptococcus ectonucleotidase NudP increases bacterial survival in blood. J. Biol. Chem. 2014, 289, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Schneewind, O.; Missiakas, D.M. Enzymatic properties of Staphylococcus aureus adenosine synthase (AdsA). BMC Biochem. 2011, 12, 56. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Chuang-Smith, O.N.; Frank, K.L.; Guenther, B.D.; Kern, M.; Schlievert, P.M.; Herzberg, M.C. Ecto-5′-nucleotidase: A candidate virulence factor in Streptococcus sanguinis experimental endocarditis. PLoS ONE 2012, 7, e38059. [Google Scholar]
- Dangel, M.-L.; Dettmann, J.-C.; Haßelbarth, S.; Krogull, M.; Schakat, M.; Kreikemeyer, B.; Fiedler, T. The 5′-nucleotidase S5nA is dispensable for evasion of phagocytosis and biofilm formation in Streptococcus pyogenes. PLoS ONE 2019, 14, e0211074. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C.; Ruiz, N.; Caparon, M. Cytolysin-mediated translocation (CMT): A functional equivalent of type III secretion in gram-positive bacteria. Cell 2001, 104, 143–152. [Google Scholar] [CrossRef]
- Meehl, M.A.; Pinkner, J.S.; Anderson, P.J.; Hultgren, S.J.; Caparon, M.G. A novel endogenous inhibitor of the secreted streptococcal NAD-glycohydrolase. PLoS Pathog. 2005, 1, e35. [Google Scholar] [CrossRef]
- Yoon, J.Y.; An, D.R.; Yoon, H.J.; Kim, H.S.; Lee, S.J.; Im, H.N.; Jang, J.Y.; Suh, S.W. High-resolution crystal structure of Streptococcus pyogenes β-NAD+ glycohydrolase in complex with its endogenous inhibitor IFS reveals a highly water-rich interface. J. Synchrotron. Radiat. 2013, 20 Pt 6, 962–967. [Google Scholar] [CrossRef]
- Velarde, J.J.; Piai, A.; Lichtenstein, I.J.; Lynskey, N.N.; Chou, J.J.; Wessels, M.R. Structure of the Streptococcus pyogenes NAD+ glycohydrolase translocation domain and its essential role in toxin binding to oropharyngeal keratinocytes. J. Bacteriol. 2022, 204, e0036621. [Google Scholar] [CrossRef]
- Fahmi, T.; Faozia, S.; Port, G.; Cho, K.H. The second messenger c-di-AMP regulates diverse cellular pathways involved in stress response, biofilm formation, cell wall Homeostasis, SpeB expression and virulence in Streptococcus pyogenes. Infect. Immun. 2019, 87, e00147-19. [Google Scholar] [CrossRef]
- Movert, E.; Bolarin, J.S.; Valfridsson, C.; Velarde, J.; Skrede, S.; Nekludov, M.; Hyldegaard, O.; Arnell, P.; Svensson, M.; Norrby-Teglund, A.; et al. Interplay between human STING genotype and bacterial NADase activity regulates inter-individual disease variability. Nat. Commun. 2023, 14, 4008. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- LaRock, D.L.; Johnson, A.F.; Wilde, S.; Sands, J.S.; Monteiro, M.P.; LaRock, C.N. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 2022, 605, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.C.; Liebl, D.; Seymour, L.M.; Gillen, C.M.; Lim, J.Y.; Larock, C.N.; Davies, M.R.; Schulz, B.L.; Nizet, V.; Teasdale, R.D.; et al. The globally disseminated M1T1 clone of group A Streptococcus evades autophagy for intracellular replication. Cell Host Microbe 2013, 14, 675–682. [Google Scholar] [CrossRef]
- Kanaoka, M.; Fukita, Y.; Taya, K.; Kawanaka, C.; Negoro, T.; Agui, H. Antitumor activity of streptococcal acid glycoprotein produced by Streptococcus pyogenes Su. Jpn. J. Cancer Res. 1987, 78, 1409–1414. [Google Scholar] [PubMed]
- Degnan, B.A.; Palmer, J.M.; Robson, T.; Jones, C.E.; Fischer, M.; Glanville, M.; Mellor, G.D.; Diamond, A.G.; Kehoe, M.A.; Goodacre, J.A. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect. Immun. 1998, 66, 3050–3058. [Google Scholar] [PubMed]
- Starikova, E.A.; Mammedova, J.T.; Ozhiganova, A.; Leveshko, T.A.; Lebedeva, A.M.; Sokolov, A.V.; Isakov, D.V.; Karaseva, A.B.; Burova, L.A.; Kudryavtsev, I.V. Streptococcal arginine deiminase inhibits T lymphocyte differentiation in vitro. Microorganisms 2023, 11, 2585. [Google Scholar] [CrossRef]
- Degnan, B.A.; Fontaine, M.C.; Doebereiner, A.H.; Lee, J.J.; Mastroeni, P.; Dougan, G.; Goodacre, J.A.; Kehoe, M.A. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 2000, 68, 2441–2448. [Google Scholar] [CrossRef]
- Henningham, A.; Ericsson, D.J.; Langer, K.; Casey, L.W.; Jovcevski, B.; Chhatwal, G.S.; Aquilina, J.A.; Batzloff, M.R.; Kobe, B.; Walker, M.J. Structure-informed design of an enzymatically inactive vaccine component for group A Streptococcus. MBio 2013, 4, e00509-13. [Google Scholar]
- LaRock, C.N.; Todd, J.; LaRock, D.L.; Olson, J.; O’Donoghue, A.J.; Robertson, A.A.B.; Cooper, M.A.; Hoffman, H.M.; Nizet, V. IL-1β is an innate immune sensor of microbial proteolysis. Sci. Immunol. 2016, 1, eaah3539. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.J.; Raghuram, A.; Cantu, C.; Hartman, M.H.; Jimenez, F.E.; Lee, S.; Ngo, A.; Rice, K.A.; Saddington, D.; Spillman, H.; et al. The majority of 9,729 Group A Streptococcus strains causing disease secrete SpeB cysteine protease: Pathogenesis implications. Infect. Immun. 2015, 83, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Frick, I.-M.; Nordin, S.L.; Baumgarten, M.; Mörgelin, M.; Sørensen, O.E.; Olin, A.I.; Egesten, A. Constitutive and inflammation-dependent antimicrobial peptides produced by epithelium are differentially processed and inactivated by the commensal Finegoldia magna and the pathogen Streptococcus pyogenes. J. Immunol. 2011, 187, 4300–4309. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Frick, I.-M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.A.Q.; Järnum, S.; Winstedt, L.; Kjellman, C.; Björck, L.; Linder, A.; Malmström, J.A. Streptococcus pyogenes infection and the human proteome with a special focus on the immunoglobulin G-cleaving enzyme IdeS. Mol. Cell. Proteom. 2018, 17, 1097–1111. [Google Scholar]
- Okumura, C.Y.M.; Anderson, E.L.; Döhrmann, S.; Tran, D.N.; Olson, J.; von Pawel-Rammingen, U.; Nizet, V. IgG protease Mac/IdeS is not essential for phagocyte resistance or mouse virulence of M1T1 group A Streptococcus. MBio 2013, 4, e00499-13. [Google Scholar]
- Lei, B.; DeLeo, F.R.; Reid, S.D.; Voyich, J.M.; Magoun, L.; Liu, M.; Braughton, K.R.; Ricklefs, S.; Hoe, N.P.; Cole, R.L.; et al. Opsonophagocytosis-inhibiting mac protein of group A Streptococcus: Identification and characteristics of two genetic complexes. Infect. Immun. 2002, 70, 6880–6890. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Grass, C.; Mishalian, I.; Dan-Goor, M.; Belotserkovsky, I.; Eran, Y.; Nizet, V.; Peled, A.; Hanski, E. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 2006, 25, 4628–4637. [Google Scholar] [CrossRef]
- Dale, J.B.; Walker, M.J. Update on group A streptococcal vaccine development. Curr. Opin. Infect. Dis. 2020, 33, 244–250. [Google Scholar] [CrossRef]
- Kurupati, P.; Turner, C.E.; Tziona, I.; Lawrenson, R.A.; Alam, F.M.; Nohadani, M.; Stamp, G.W.; Zinkernagel, A.S.; Nizet, V.; Edwards, R.J.; et al. Chemokine-cleaving Streptococcus pyogenes protease SpyCEP is necessary and sufficient for bacterial dissemination within soft tissues and the respiratory tract. Mol. Microbiol. 2010, 76, 1387–1397. [Google Scholar] [CrossRef]
- Wexler, D.E.; Chenoweth, D.E.; Cleary, P.P. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 1985, 82, 8144–8148. [Google Scholar] [PubMed]
- Ji, Y.; McLandsborough, L.; Kondagunta, A.; Cleary, P.P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 1996, 64, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Carlson, B.; Kondagunta, A.; Cleary, P.P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect. Immun. 1997, 65, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Okumura, C.Y.M.; Collin, M.; Nizet, V.; Hollands, A. Study of the IgG endoglycosidase EndoS in group A streptococcal phagocyte resistance and virulence. BMC Microbiol. 2011, 11, 120. [Google Scholar]
- Allhorn, M.; Olin, A.I.; Nimmerjahn, F.; Collin, M. Human IgG/Fc gamma R interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS ONE 2008, 3, e1413. [Google Scholar] [CrossRef]
- Naegeli, A.; Bratanis, E.; Karlsson, C.; Shannon, O.; Kalluru, R.; Linder, A.; Malmström, J.; Collin, M. Streptococcus pyogenes evades adaptive immunity through specific IgG glycan hydrolysis. J. Exp. Med. 2019, 216, 1615–1629. [Google Scholar]
- Podbielski, A.; Zarges, I.; Flosdorff, A.; Weber-Heynemann, J. Molecular characterization of a major serotype M49 group A streptococcal DNase gene (sdaD). Infect. Immun. 1996, 64, 5349–5356. [Google Scholar] [CrossRef]
- Barnett, T.C.; Bugrysheva, J.V.; Scott, J.R. Role of mRNA stability in growth phase regulation of gene expression in the group A Streptococcus. J. Bacteriol. 2007, 189, 1866–1873. [Google Scholar] [CrossRef]
- Aziz, R.K.; Kansal, R.; Aronow, B.J.; Taylor, W.L.; Rowe, S.L.; Kubal, M.; Chhatwal, G.S.; Walker, M.J.; Kotb, M. Microevolution of group A streptococci in vivo: Capturing regulatory networks engaged in sociomicrobiology, niche adaptation, and hypervirulence. PLoS ONE 2010, 5, e9798. [Google Scholar] [CrossRef]
- Keller, N.; Woytschak, J.; Heeb, L.E.M.; Marques Maggio, E.; Mairpady Shambat, S.; Snäll, J.; Hyldegaard, O.; Boyman, O.; Norrby-Teglund, A.; Zinkernagel, A.S. Group A streptococcal dnase sda1 impairs plasmacytoid dendritic cells’ type 1 interferon response. J. Investig. Dermatol. 2019, 139, 1284–1293. [Google Scholar] [CrossRef]
- Radcliff, F.J.; Fraser, J.D.; Proft, T. Vaccination with Streptococcus pyogenes nuclease A stimulates a high antibody response but no protective immunity in a mouse model of infection. Med. Microbiol. Immunol. 2015, 204, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, C.; Khemlani, A.H.J.; Sohn, C.R.; Loh, J.M.S.; Tsai, C.J.-Y.; Proft, T. Streptococcus pyogenes nuclease A (SpnA) mediated virulence does not exclusively depend on nuclease activity. J. Microbiol. Immunol. Infect. 2020, 53, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, A.L.; McGregor, R.; Bennett, J.; Gurney, J.K.; Williamson, D.A.; Baker, M.G.; Moreland, N.J. Increased breadth of Group A Streptococcus antibody responses in children with acute rheumatic fever compared to precursor pharyngitis and skin infections. J. Infect. Dis. 2022, 226, 167–176. [Google Scholar] [PubMed]
- Kimoto, H.; Fujii, Y.; Yokota, Y.; Taketo, A. Molecular characterization of NADase-streptolysin O operon of hemolytic streptococci. Biochim. Biophys. Acta 2005, 1681, 134–149. [Google Scholar] [PubMed]
- Michos, A.; Gryllos, I.; Håkansson, A.; Srivastava, A.; Kokkotou, E.; Wessels, M.R. Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J. Biol. Chem. 2006, 281, 8216–8223. [Google Scholar] [PubMed]
- Magassa, N.; Chandrasekaran, S.; Caparon, M.G. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 2010, 11, 400–405. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, M.; Wessels, M.R. Streptolysin O and its co-toxin NAD-glycohydrolase protect group A Streptococcus from xenophagic killing. PLoS Pathog. 2013, 9, e1003394. [Google Scholar]
- Sharma, O.; O’Seaghdha, M.; Velarde, J.J.; Wessels, M.R. NAD+-glycohydrolase promotes intracellular survival of Group A Streptococcus. PLoS Pathog. 2016, 12, e1005468. [Google Scholar] [CrossRef]
- Faozia, S.; Fahmi, T.; Port, G.C.; Cho, K.H. c-di-AMP-regulated K+ importer KtrAB affects biofilm formation, stress response, and SpeB expression in Streptococcus pyogenes. Infect. Immun. 2021, 89, e00317-20. [Google Scholar] [CrossRef]
- Rivera-Hernandez, T.; Carnathan, D.G.; Jones, S.; Cork, A.J.; Davies, M.R.; Moyle, P.M.; Toth, I.; Batzloff, M.R.; McCarthy, J.; Nizet, V.; et al. An experimental group A Streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. MBio 2019, 10, e00693-19. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar]
- Yu, C.E.; Ferretti, J.J. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A streptococci. Infect. Immun. 1991, 59, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Luca-Harari, B.; Darenberg, J.; Neal, S.; Siljander, T.; Strakova, L.; Tanna, A.; Creti, R.; Ekelund, K.; Koliou, M.; Tassios, P.T.; et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 2009, 47, 1155–1165. [Google Scholar] [CrossRef]
- Darenberg, J.; Luca-Harari, B.; Jasir, A.; Sandgren, A.; Pettersson, H.; Schalén, C.; Norgren, M.; Romanus, V.; Norrby-Teglund, A.; Normark, B.H. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 2007, 45, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Gubba, S.; Low, D.E.; Musser, J.M. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect. Immun. 1998, 66, 765–770. [Google Scholar] [CrossRef]
- Holm, S.E.; Norrby, A.; Bergholm, A.M.; Norgren, M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J. Infect. Dis. 1992, 166, 31–37. [Google Scholar] [PubMed]
- Talkington, D.F.; Schwartz, B.; Black, C.M.; Todd, J.K.; Elliott, J.; Breiman, R.F.; Facklam, R.R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect. Immun. 1993, 61, 3369–3374. [Google Scholar]
- Kansal, R.G.; McGeer, A.; Low, D.E.; Norrby-Teglund, A.; Kotb, M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 2000, 68, 6362–6369. [Google Scholar] [CrossRef] [PubMed]
- Raeder, R.; Harokopakis, E.; Hollingshead, S.; Boyle, M.D. Absence of SpeB production in virulent large capsular forms of group A streptococcal strain 64. Infect. Immun. 2000, 68, 744–751. [Google Scholar] [CrossRef]
- Husmann, L.K.; Yung, D.L.; Hollingshead, S.K.; Scott, J.R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 1997, 65, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Nasser, W.; Beres, S.B.; Olsen, R.J.; Dean, M.A.; Rice, K.A.; Long, S.W.; Kristinsson, K.G.; Gottfredsson, M.; Vuopio, J.; Raisanen, K.; et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc. Natl. Acad. Sci. USA 2014, 111, E1768–E1776. [Google Scholar] [PubMed]
- Bricker, A.L.; Carey, V.J.; Wessels, M.R. Role of NADase in virulence in experimental invasive group A streptococcal infection. Infect. Immun. 2005, 73, 6562–6566. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, A.; Sjöquist, J. “Protein A” from S. aureus. I. Pseudo-immune reaction with human ɣ-globulin. J. Immunol. 1966, 97, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Björck, L.; Kronvall, G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J. Immunol. 1984, 133, 969–974. [Google Scholar] [PubMed]
- Nilson, B.H.; Solomon, A.; Björck, L.; Åkerström, B. Protein L from Peptostreptococcus magnus binds to the κ light chain variable domain. J. Biol. Chem. 1992, 267, 2234–2239. [Google Scholar] [PubMed]
- Lindahl, G.; Åkerström, B.; Vaerman, J.P.; Stenberg, L. Characterization of an IgA receptor from group B streptococci: Specificity for serum IgA. Eur. J. Immunol. 1990, 20, 2241–2247. [Google Scholar] [CrossRef]
- Åkesson, P.; Cooney, J.; Kishimoto, F.; Björck, L. Protein H—A novel IgG binding bacterial protein. Mol. Immunol. 1990, 27, 523–531. [Google Scholar] [CrossRef]
- Mulks, M.H.; Plaut, A.G. IgA protease production as a characteristic distinguishing pathogenic from harmless Neisseriaceae. N. Engl. J. Med. 1978, 299, 973–976. [Google Scholar] [CrossRef]
- Mulks, M.H.; Kornfeld, S.J.; Plaut, A.G. Specific proteolysis of human IgA by Streptococcus pneumoniae and Haemophilus influenzae. J. Infect. Dis. 1980, 141, 450–456. [Google Scholar] [CrossRef]
- Plaut, A.G. The IgA1 proteases of pathogenic bacteria. Annu. Rev. Microbiol. 1983, 37, 603–622. [Google Scholar] [CrossRef]
- Sjögren, J.; Andersson, L.; Mejàre, M.; Olsson, F. Generating and purifying Fab fragments from human and mouse IgG using the bacterial enzymes IdeS, SpeB and Kgp. Methods Mol. Biol. 2017, 1535, 319–329. [Google Scholar] [PubMed]
- Huang, E.; Maldonado, A.Q.; Kjellman, C.; Jordan, S.C. Imlifidase for the treatment of anti-HLA antibody-mediated processes in kidney transplantation. Am. J. Transplant. 2022, 22, 691–697. [Google Scholar] [CrossRef]
- Lonze, B.E.; Tatapudi, V.S.; Weldon, E.P.; Min, E.S.; Ali, N.M.; Deterville, C.L.; Gelb, B.E.; Benstein, J.A.; Dagher, N.N.; Wu, M.; et al. IdeS (Imlifidase): A novel agent that cleaves human IgG and permits successful kidney transplantation across high-strength donor-specific antibody. Ann. Surg. 2018, 268, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T. Imlifidase: First Approval. Drugs 2020, 80, 1859–1864. [Google Scholar]
- Bou-Jaoudeh, M.; Delignat, S.; Daventure, V.; Astermark, J.; Lévesque, H.; Dimitrov, J.D.; Deligne, C.; Proulle, V.; Lacroix-Desmazes, S. The IgG-degrading enzyme, Imlifidase, restores the therapeutic activity of FVIII in inhibitor-positive hemophilia A mice. Haematologica 2023, 108, 1322–1334. [Google Scholar] [CrossRef]
- Tyrberg, L.; Andersson, F.; Uhlin, F.; Hellmark, T.; Segelmark, M. Using imlifidase to elucidate the characteristics and importance of anti-GBM antibodies produced after start of treatment. Nephrol. Dial. Transplant. 2023, 39, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Lood, R.; Nägeli, A. On enzymatic remodeling of IgG glycosylation; unique tools with broad applications. Glycobiology 2020, 30, 254–267. [Google Scholar] [PubMed]
- Shaw, H.A.; Ozanne, J.; Burns, K.; Mawas, F. Multicomponent vaccines against Group A Streptococcus can effectively target broad disease presentations. Vaccines 2021, 9, 1025. [Google Scholar] [CrossRef]
- Huang, W.; Giddens, J.; Fan, S.-Q.; Toonstra, C.; Wang, L.-X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 2012, 134, 12308–12318. [Google Scholar]
- Wang, M.; Wang, Y.; Liu, K.; Dou, X.; Liu, Z.; Zhang, L.; Ye, X.-S. Engineering a bacterial sialyltransferase for di-sialylation of a therapeutic antibody. Org. Biomol. Chem. 2020, 18, 2886–2892. [Google Scholar] [PubMed]
- Toftevall, H.; Nyhlén, H.; Olsson, F.; Sjögren, J. Antibody conjugations via glycosyl remodeling. Methods Mol. Biol. 2020, 2078, 131–145. [Google Scholar] [PubMed]
- Wu, H.; Owen, C.D.; Juge, N. Structure and function of microbial α-l-fucosidases: A mini review. Essays Biochem. 2023, 67, 399–414. [Google Scholar] [PubMed]
- Goldblatt, J.; Lawrenson, R.A.; Muir, L.; Dattani, S.; Hoffland, A.; Tsuchiya, T.; Kanegasaki, S.; Sriskandan, S.; Pease, J.E. A requirement for neutrophil glycosaminoglycans in chemokine:receptor interactions is revealed by the streptococcal protease spycep. J. Immunol. 2019, 202, 3246–3255. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Olsson, F.; Beck, A. Rapid and improved characterization of therapeutic antibodies and antibody related products using IdeS digestion and subunit analysis. Analyst 2016, 141, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, J.; Collin, M. Bacterial glycosidases in pathogenesis and glycoengineering. Future Microbiol. 2014, 9, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Duivelshof, B.L.; Deslignière, E.; Hernandez-Alba, O.; Ehkirch, A.; Toftevall, H.; Sjögren, J.; Cianférani, S.; Beck, A.; Guillarme, D.; D’Atri, V. Glycan-mediated technology for obtaining homogeneous site-specific conjugated antibody-drug conjugates: Synthesis and analytical characterization by using complementary middle-up LC/HRMS analysis. Anal. Chem. 2020, 92, 8170–8177. [Google Scholar] [CrossRef]
- Walsh, S.J.; Rau, L.M. Autoimmune diseases: A leading cause of death among young and middle-aged women in the United States. Am. J. Public Health 2000, 90, 1463–1466. [Google Scholar]
- Lim, P.-L.; Zouali, M. Pathogenic autoantibodies: Emerging insights into tissue injury. Immunol. Lett. 2006, 103, 17–26. [Google Scholar]
- Colvin, R.B.; Smith, R.N. Antibody-mediated organ-allograft rejection. Nat. Rev. Immunol. 2005, 5, 807–817. [Google Scholar] [CrossRef]
- Collin, M.; Shannon, O.; Björck, L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 4265–4270. [Google Scholar] [CrossRef]
- Johansson, B.P.; Shannon, O.; Björck, L. IdeS: A bacterial proteolytic enzyme with therapeutic potential. PLoS ONE 2008, 3, e1692. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Boon, L.; Bockermann, R.; Robertson, A.-K.; Kjellman, C.; Anderson, C.C. Desensitization using imlifidase and EndoS enables chimerism induction in allo-sensitized recipient mice. Am. J. Transplant. 2020, 20, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Allhorn, M.; Briceño, J.G.; Baudino, L.; Lood, C.; Olsson, M.L.; Izui, S.; Collin, M. The IgG-specific endoglycosidase EndoS inhibits both cellular and complement-mediated autoimmune hemolysis. Blood 2010, 115, 5080–5088. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, M.M.; van der Veen, B.S.; Stegeman, C.A.; Petersen, A.H.; Hellmark, T.; Collin, M.; Heeringa, P. IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Vafia, K.; Kalies, K.; Groth, S.; Westermann, J.; Zillikens, D.; Ludwig, R.J.; Collin, M.; Schmidt, E. Enzymatic autoantibody glycan hydrolysis alleviates autoimmunity against type VII collagen. J. Autoimmun. 2012, 39, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Molnarfi, N.; Santiago-Raber, M.-L.; Weber, M.S.; Merkler, D.; Collin, M.; Lalive, P.H. IgG glycan hydrolysis by EndoS inhibits experimental autoimmune encephalomyelitis. J. Neuroinflammation 2012, 9, 209. [Google Scholar] [CrossRef]
- Lood, C.; Allhorn, M.; Lood, R.; Gullstrand, B.; Olin, A.I.; Rönnblom, L.; Truedsson, L.; Collin, M.; Bengtsson, A.A. IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: A possible new treatment? Arthritis Rheum. 2012, 64, 2698–2706. [Google Scholar]
- Collin, M.; Björck, L. Toward clinical use of the IgG specific enzymes IdeS and EndoS against antibody-mediated diseases. Methods Mol. Biol. 2017, 1535, 339–351. [Google Scholar]
- Yu, X.; Zheng, J.; Collin, M.; Schmidt, E.; Zillikens, D.; Petersen, F. EndoS reduces the pathogenicity of anti-mCOL7 IgG through reduced binding of immune complexes to neutrophils. PLoS ONE 2014, 9, e85317. [Google Scholar]
- Nandakumar, K.S.; Collin, M.; Happonen, K.E.; Lundström, S.L.; Croxford, A.M.; Xu, B.; Zubarev, R.A.; Rowley, M.J.; Blom, A.M.; Kjellman, C.; et al. Streptococcal Endo-β-N-acetylglucosaminidase suppresses antibody-mediated inflammation in vivo. Front. Immunol. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, K.S.; Holmdahl, R. Therapeutic cleavage of IgG: New avenues for treating inflammation. Trends Immunol. 2008, 29, 173–178. [Google Scholar] [PubMed]
- Nandakumar, K.S.; Johansson, B.P.; Björck, L.; Holmdahl, R. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo. Arthritis Rheum. 2007, 56, 3253–3260. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, K.S.; Collin, M.; Olsén, A.; Nimmerjahn, F.; Blom, A.M.; Ravetch, J.V.; Holmdahl, R. Endoglycosidase treatment abrogates IgG arthritogenicity: Importance of IgG glycosylation in arthritis. Eur. J. Immunol. 2007, 37, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Bockermann, R.; Järnum, S.; Runström, A.; Lorant, T.; Winstedt, L.; Palmqvist, N.; Kjellman, C. Imlifidase-generated Single-cleaved IgG: Implications for Transplantation. Transplantation 2021, 106, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E. A review of imlifidase in solid organ transplantation. Expert Opin. Biol. Ther. 2020, 21, 135–143. [Google Scholar] [PubMed]
- Mahmoud, A.; Toth, I.; Stephenson, R. Developing an effective glycan-based vaccine for Streptococcus pyogenes. Angew. Chem. Int Ed. 2022, 61, e202115342. [Google Scholar] [CrossRef]
- Burns, K.; Dorfmueller, H.C.; Wren, B.W.; Mawas, F.; Shaw, H.A. Progress towards a glycoconjugate vaccine against Group A Streptococcus. NPJ Vaccines 2023, 8, 48. [Google Scholar] [CrossRef]
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar]
- Cheng, Q.; Debol, S.; Lam, H.; Eby, R.; Edwards, L.; Matsuka, Y.; Olmsted, S.B.; Cleary, P.P. Immunization with C5a peptidase or peptidase-type III polysaccharide conjugate vaccines enhances clearance of group B Streptococci from lungs of infected mice. Infect. Immun. 2002, 70, 6409–6415. [Google Scholar]
- Flock, M.; Frykberg, L.; Sköld, M.; Guss, B.; Flock, J.-I. Antiphagocytic function of an IgG glycosyl hydrolase from Streptococcus equi subsp. equi and its use as a vaccine component. Infect. Immun. 2012, 80, 2914–2919. [Google Scholar] [CrossRef]
- Silva, J.W.; Droppa-Almeida, D.; Borsuk, S.; Azevedo, V.; Portela, R.W.; Miyoshi, A.; Rocha, F.S.; Dorella, F.A.; Vivas, W.L.; Padilha, F.F.; et al. Corynebacterium pseudotuberculosis cp09 mutant and cp40 recombinant protein partially protect mice against caseous lymphadenitis. BMC Vet. Res. 2014, 10, 965. [Google Scholar] [CrossRef]
- de Pinho, R.B.; Barbosa, T.N.; Dall’Agno, L.; da Rocha Fonseca, B.; Bezerra, F.S.B.; Sousa, F.S.S.; Seixas, F.K.; Collares, T.; Borsuk, S. Mycobacterium bovis BCG expressing the proteins CP40 or CP09720 of Corynebacterium pseudotuberculosis promotes protection in mice after challenge. Vaccine 2023, 42, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shadnezhad, A.; Naegeli, A.; Collin, M. CP40 from Corynebacterium pseudotuberculosis is an endo-β-N-acetylglucosaminidase. BMC Microbiol. 2016, 16, 261. [Google Scholar] [CrossRef]
- Droppa-Almeida, D.; Vivas, W.L.P.; Silva, K.K.O.; Rezende, A.F.S.; Simionatto, S.; Meyer, R.; Lima-Verde, I.B.; Delagostin, O.; Borsuk, S.; Padilha, F.F. Recombinant CP40 from Corynebacterium pseudotuberculosis confers protection in mice after challenge with a virulent strain. Vaccine 2016, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Happonen, L.; Collin, M. Immunomodulating Enzymes from Streptococcus pyogenes—In Pathogenesis, as Biotechnological Tools, and as Biological Drugs. Microorganisms 2024, 12, 200. https://doi.org/10.3390/microorganisms12010200
Happonen L, Collin M. Immunomodulating Enzymes from Streptococcus pyogenes—In Pathogenesis, as Biotechnological Tools, and as Biological Drugs. Microorganisms. 2024; 12(1):200. https://doi.org/10.3390/microorganisms12010200
Chicago/Turabian StyleHapponen, Lotta, and Mattias Collin. 2024. "Immunomodulating Enzymes from Streptococcus pyogenes—In Pathogenesis, as Biotechnological Tools, and as Biological Drugs" Microorganisms 12, no. 1: 200. https://doi.org/10.3390/microorganisms12010200
APA StyleHapponen, L., & Collin, M. (2024). Immunomodulating Enzymes from Streptococcus pyogenes—In Pathogenesis, as Biotechnological Tools, and as Biological Drugs. Microorganisms, 12(1), 200. https://doi.org/10.3390/microorganisms12010200







