Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies
Abstract
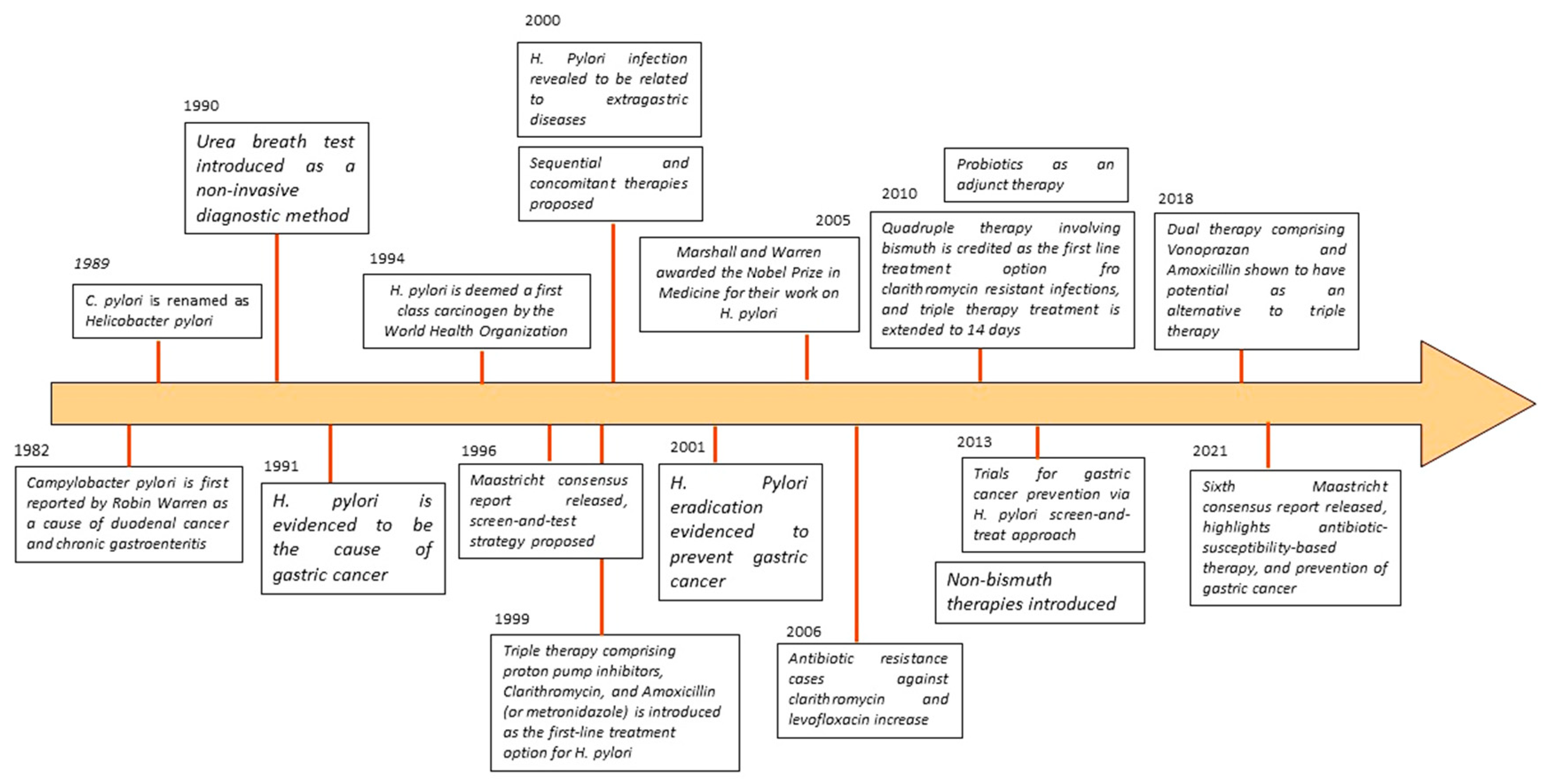
1. Introduction
1.1. H. pylori Infection: The Global Scenario
1.2. Significance of Study
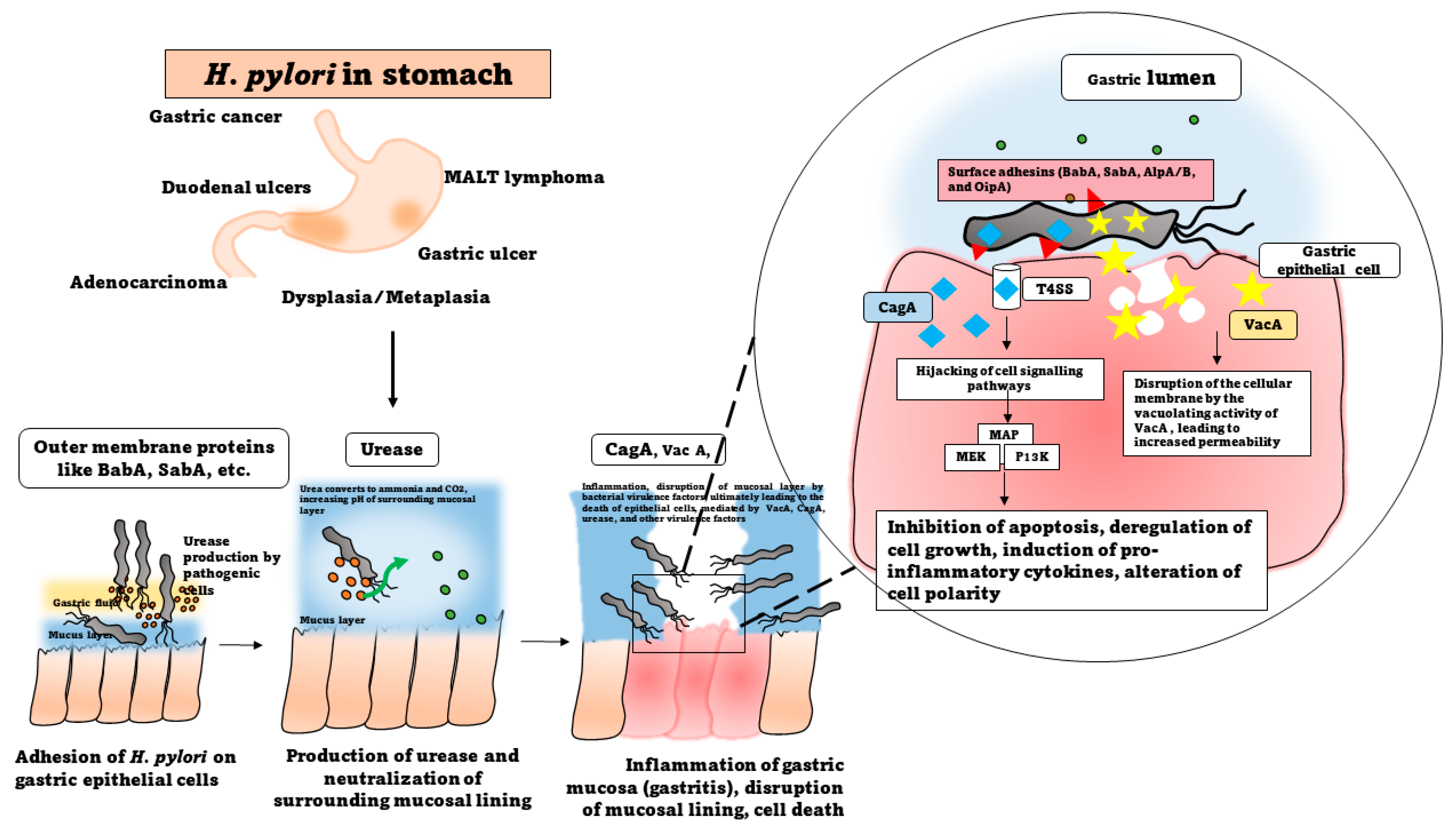
2. Pathogenesis of Helicobacter pylori
2.1. Dispersal and Routes of Infection
2.2. Molecular Mechanisms of Infection
2.2.1. Attachment and Colonization
2.2.2. Production of Virulence Factors
2.2.3. CagA and VacA
2.2.4. Urease Production and Survival at Low pH
2.3. Immune System Modulation and Induction of Inflammatory Responses
2.4. Modulation of Mucin Production
3. Disease Associations
4. Diagnosis of Helicobacter pylori Infections
4.1. Non-Invasive Tests
4.1.1. Serological Assays
4.1.2. Urea Breath Test (UBT)
4.1.3. Stool Antigen Test (SAT)
4.2. Invasive Tests
4.2.1. Histology
4.2.2. Culture Examinations
| Diagnostic Test | Sensitivity | Specificity | Advantages/Disadvantages | References |
|---|---|---|---|---|
| Urea breath test (UBT) | >95% | >95% | Advantages: Gold standard in many clinical diagnoses, cost-effective, reliable, simple, non-invasive, and can be used for confirming eradication of infection. Disadvantages: May give false negatives in the presence of other urease-producing bacteria and Helicobacter species. Low accuracy under conditions of gastritis and gastric malignancies, requires a high load of bacteria in the specimen. Requires expensive equipment. | [96,109] |
| Stool antigen test (SAT) | 96% | 97% | Advantages: Cost-effective, simple, rapid, and does not require expensive instruments. Disadvantages: It may give false negatives under low bacterial count, and accuracy is affected by recent intake of PPI and CAM; it is not useful for post-eradication confirmation. | [99,100] |
| Serological tests | 85% | >80% | Advantages: Inexpensive and can be employed for patients who have recently undergone triple therapy. Only tests not affected by PPI intake or use of antibiotics. Disadvantages: Unreliable for ongoing infections and cannot be used to confirm eradication. | [87] |
| Rapid urease test (RUT) | 80–90% | 93–100% | Advantages: Rapid, inexpensive, and simple. Disadvantages: Invasive, requires additional confirmatory tests, and accuracy affected by intake of PPI and antibiotics. | [61] |
| Culture | 70–90% | 100% | Advantages: Gold standard for confirmation and can be used to ascertain antibiotic sensitivity. Disadvantages: Elaborate, time-consuming, expensive, and requires specific expertise in microbiology. | [110] |
| Histopathology | >95% | 99% | Advantages: Gold standard in routine clinical diagnostics, provides additional information about associated pathologies, and extremely sensitive and specific. | [101] |
| Molecular methods (PCR) | 96% | 98% | Advantage: Sensitive even at very low bacterial counts. Disadvantages: Expensive, requires sophisticated equipment, and may give false positive results. | [88] |
4.2.3. Molecular and Genetic Markers
5. Treatment Strategies
5.1. Antibiotic Therapy and Selection of Antibiotics
5.2. Proton Pump Inhibitors
5.3. The Rising Issue of Antibiotic Resistance
5.4. Maastricht VI/Florence Consensus
| Treatment Option | Drugs Employed | Duration of Therapy | References |
|---|---|---|---|
| Triple therapy (PPI+ two antibiotics) | PPI, Clarithromycin, Amoxicillin (or Metronidazole) | 7 days | [134] |
| Bismuth Quadruple therapy (BQT) | PPI, bismuth, tetracycline, and metronidazole | 14 days | [40] |
| Levofloxacin-containing triple therapy | PPI, levofloxacin, amoxicillin | 14 days | [135] |
| Levofloxacin-amoxicillin quadruple therapy | PPI, bismuth, levofloxacin, amoxicillin | 10 days | [136] |
| Tetracycline-levofloxacin quadruple therapy | PPI, bismuth, levofloxacin, tetracycline | 10 days | [137] |
| Concomitant therapy (non-bismuth therapy) | PPI, amoxicillin, clarithromycin, and a nitrimidazole | [134] | |
| Sequential therapy (dual) | PPI and amoxicillin for 5 days, followed by triple therapy (PPI, clarithromycin, and tinidazole) for next 5 days | 10 days | [138] |
6. Future Directions
6.1. Challenges and Opportunities
6.2. Advancements in Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robin Warren, J.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Moayyedi, P. Helicobacter pylori Infection in Functional Dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Fong, I.W. Helicobacter pylori Infection: When Should It Be Treated? In Current Trends and Concerns in Infectious Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 81–102. ISBN 978-3-030-36966-8. [Google Scholar]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and Extragastric Diseases: A Review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection—The Maastricht IV/ Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer; World Health Organization. Schistosomes, Liver Flukes and Helicobacter Pylori; IARC: Lyon, France, 1994; ISBN 9283212614. [Google Scholar]
- Al Mutawa, O.A.; Izhari, M.A.; Alharbi, R.A.; Sindi, A.A.A.; Alqarni, A.M.; Alotaibi, F.E.; Gosady, A.R.A.; Dardari, D.M.M.; Almutairi, A.M.; Alshehri, M.; et al. Helicobacter pylori (H. pylori) Infection-Associated Anemia in the Asir Region, Saudi Arabia. Diagnostics 2023, 13, 2404. [Google Scholar] [CrossRef]
- Lu, C.; Yu, Y.; Li, L.; Yu, C.; Xu, P. Systematic Review of the Relationship of Helicobacter pylori Infection with Geographical Latitude, Average Annual Temperature and Average Daily Sunshine. BMC Gastroenterol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Usarov, K.; Ahmedov, A.; Abasiyanik, M.F.; Ku Khalif, K.M.N. Forecasting of Infection Prevalence of Helicobacter pylori (H. pylori) Using Regression Analysis. IIUM Eng. J. 2022, 23, 183–192. [Google Scholar] [CrossRef]
- Lehours, P. Actual Diagnosis of Helicobacter pylori Infection. Minerva Gastroenterol. Dietol. 2018, 64, 267–279. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global Prevalence of Helicobacter pylori Infection between 1980 and 2022: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer between 1980 and 2022. Gastroenterology 2024, in press. [Google Scholar] [CrossRef]
- Butt, J.; Epplein, M. How Do Global Trends in Helicobacter pylori Prevalence Inform Prevention Planning? Lancet Gastroenterol. Hepatol. 2023, 8, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Öztekin, M.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Overview of Helicobacter pylori Infection: Clinical Features, Treatment, and Nutritional Aspects. Diseases 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Farinha, P.; Gascoyne, R.D. Helicobacter pylori and MALT Lymphoma. Gastroenterology 2005, 128, 1579–1605. [Google Scholar] [CrossRef]
- Ishikawa, E.; Nakamura, M.; Satou, A.; Shimada, K.; Nakamura, S. Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma in the Gastrointestinal Tract in the Modern Era. Cancers 2022, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’angelis, G.L. Helicobacter pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Biomed. 2018, 89, 72–76. [Google Scholar]
- Duan, M.; Li, Y.; Liu, J.; Zhang, W.; Dong, Y.; Han, Z.; Wan, M.; Lin, M.; Lin, B.; Kong, Q.; et al. Transmission Routes and Patterns of Helicobacter pylori. Helicobacter 2023, 28, e12945. [Google Scholar] [CrossRef]
- Hikaru, H.; Engevik, K.A.; Matthis, A.L.; Ottemann, K.M.; Montrose, M.H.; Aihara, E. Helicobacter pylori Uses the TlpB Receptor To Sense Sites of Gastric Injury. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. Biomed. Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the Human Stomach: Persistence Strategies of the Bacterial Pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N.; Fischer, W. Composition, Structure and Function of the Helicobacter pylori Cag Pathogenicity Island Encoded Type IV Secretion System. Future Microbiol. 2015, 10, 955–965. [Google Scholar] [CrossRef]
- HATAKEYAMA, M. Structure and Function of Helicobacter pylori CagA, the First-Identified Bacterial Protein Involved in Human Cancer. Proc. Jpn. Acad. Ser. B 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Yong, X.; Tang, B.; Li, B.-S.; Xie, R.; Hu, C.-J.; Luo, G.; Qin, Y.; Dong, H.; Yang, S.-M. Helicobacter pylori Virulence Factor CagA Promotes Tumorigenesis of Gastric Cancer via Multiple Signaling Pathways. Cell Commun. Signal. 2015, 13, 30. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Maeda, S.; Mentis, A.F. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2007, 12, 10–14. [Google Scholar] [CrossRef]
- Messina, B.; Lo Sardo, F.; Scalera, S.; Memeo, L.; Colarossi, C.; Mare, M.; Blandino, G.; Ciliberto, G.; Maugeri-Saccà, M.; Bon, G. Hippo Pathway Dysregulation in Gastric Cancer: From Helicobacter pylori Infection to Tumor Promotion and Progression. Cell Death Dis. 2023, 14, 21. [Google Scholar] [CrossRef]
- Cover, T.L.; Blanke, S.R. Helicobacter pylori VacA, a Paradigm for Toxin Multifunctionality. Nat. Rev. Microbiol. 2005, 3, 320–332. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef]
- Chauhan, N.; Tay, A.C.Y.; Marshall, B.J.; Jain, U. Helicobacter pylori VacA, a Distinct Toxin Exerts Diverse Functionalities in Numerous Cells: An Overview. Helicobacter 2019, 24, e12544. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Belayneh, Y.M. Helicobacter pylori and Duodenal Ulcer: Systematic Review of Controversies in Causation. Clin. Exp. Gastroenterol. 2019, 12, 441–447. [Google Scholar] [CrossRef]
- Dincă, A.L.; Meliț, L.E.; Mărginean, C.O. Old and New Aspects of H. pylori-Associated Inflammation and Gastric Cancer. Children 2022, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediat. Inflamm. 2022, 2022, 2944156. [Google Scholar] [CrossRef]
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA Promotes CagA Accumulation in Gastric Epithelial Cells during Helicobacter pylori Infection. Sci. Rep. 2019, 9, 38. [Google Scholar] [CrossRef]
- Venerito, M.; Krieger, T.; Ecker, T.; Leandro, G.; Malfertheiner, P. Meta-Analysis of Bismuth Quadruple Therapy versus Clarithromycin Triple Therapy for Empiric Primary Treatment of Helicobacter pylori Infection. Digestion 2013, 88, 33–45. [Google Scholar] [CrossRef]
- Yamaoka, Y. Mechanisms of Disease: Helicobacter pylori Virulence Factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef]
- Ha, N.-C.; Oh, S.-T.; Sung, J.Y.; Cha, K.A.; Lee, M.H.; Oh, B.-H. Supramolecular Assembly and Acid Resistance of Helicobacter pylori Urease. Nat. Struct. Biol. 2001, 8, 505–509. [Google Scholar] [CrossRef]
- Mobley, H.L.; Mendz, G.L.; Hazell, S.L. Helicobacter pylori: Physiology and Genetics; ASM Press: Washington, DC, USA, 2001; ISBN 1555812139. [Google Scholar]
- Ansari, S.; Yamaoka, Y. Survival of Helicobacter pylori in Gastric Acidic Territory. Helicobacter 2017, 22, e12386. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Idowu, S.; Bertrand, P.P.; Walduck, A.K. Gastric Organoids: Advancing the Study of H. pylori Pathogenesis and Inflammation. Helicobacter 2022, 27, e12891. [Google Scholar] [CrossRef]
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular Basis for Colonization and Survival in Gastric Environment and Resistance to Antibiotics. A Short Review. Infect. Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef]
- Agarwal, N.; Jaiswal, N.; Gulati, K.; Gangele, K.; Nagar, N.; Kumar, D.; Poluri, K.M. Molecular Insights into Conformational Heterogeneity and Enhanced Structural Integrity of Helicobacter pylori DNA Binding Protein Hup at Low PH. Biochemistry 2021, 60, 3236–3252. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Goers Sweeney, E.; Guillemin, K.; Amieva, M.R. Multiple Acid Sensors Control Helicobacter pylori Colonization of the Stomach. PLoS Pathog. 2017, 13, e1006118. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori Infection: An Overview of Bacterial Virulence Factors and Pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- White, J.R.; Winter, J.A.; Robinson, K. Differential Inflammatory Response to Helicobacter pylori Infection: Etiology and Clinical Outcomes. J. Inflamm. Res. 2015, 8, 137–147. [Google Scholar]
- Zeyaullah, M.d.; AlShahrani, A.M.; Ahmad, I. Association of Helicobacter pylori Infection and Host Cytokine Gene Polymorphism with Gastric Cancer. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8810620. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Amedei, A.; Cappon, A.; Del Prete, G.; de Bernard, M. The Neutrophil-Activating Protein of Helicobacter pylori (HP-NAP) as an Immune Modulating Agent. FEMS Immunol. Med. Microbiol. 2007, 50, 157–164. [Google Scholar] [CrossRef]
- Su, B.; Ceponis, P.J.M.; Lebel, S.; Huynh, H.; Sherman, P.M. Helicobacter pylori Activates Toll-Like Receptor 4 Expression in Gastrointestinal Epithelial Cells. Infect. Immun. 2003, 71, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J. Immunol. Res. 2015, 2015, 981328. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.P.; D’Elios, M.M. Cytotoxic T Cells in H. pylori-Related Gastric Autoimmunity and Gastric Lymphoma. J. Biomed. Biotechnol. 2010, 2010, 104918. [Google Scholar] [CrossRef] [PubMed]
- Reyes Victor, E.; Peniche, A.G. Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion. In Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 229–265. ISBN 978-3-030-15138-6. [Google Scholar]
- Kumar, S.; Patel, G.K.; Ghoshal, U.C. Helicobacter pylori-Induced Inflammation: Possible Factors Modulating the Risk of Gastric Cancer. Pathogens 2021, 10, 1099. [Google Scholar] [CrossRef]
- Caron, T.J.; Scott, K.E.; Fox, J.G.; Hagen, S.J. Tight Junction Disruption: Helicobacter pylori and Dysregulation of the Gastric Mucosal Barrier. World J. Gastroenterol. 2015, 21, 11411–11427. [Google Scholar] [PubMed]
- Graham, D.Y.; Miftahussurur, M.; Yamaoka, Y. Helicobacter pylori as an Oncogenic Pathogen, Revisited. Expert Rev. Mol. Med. 2017, 19, e4. [Google Scholar] [CrossRef]
- Díaz, P.; Valenzuela Valderrama, M.; Bravo, J.; Quest, A.F.G. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front. Microbiol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Skoog, E. Helicobacter spp. Interactions with Mucins: Adhesion and Mucin Regulation of Pathogen Proliferation and Gene Expression; Department of Medical Biochemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy at University of Gothenburg: Göteborg, Sweden, 2014; ISBN 9789162888718. [Google Scholar]
- Nazanin, N.; Johansson, M.E.; Raghavan, S.; Lindén, S.K. Helicobacter pylori Infection Impairs the Mucin Production Rate and Turnover in the Murine Gastric Mucosa. Infect. Immun. 2013, 81, 829–837. [Google Scholar] [CrossRef]
- Padra, M.; Benktander, J.; Robinson, K.; Lindén, S.K. Carbohydrate-Dependent and Antimicrobial Peptide Defence Mechanisms Against Helicobacter pylori Infections. In Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 179–207. ISBN 978-3-030-15138-6. [Google Scholar]
- Fock, K.M. The Epidemiology and Prevention of Gastric Cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef]
- Duan, C.; Cao, H.; Zhang, L.-H.; Xu, Z. Harnessing the CRISPR-Cas Systems to Combat Antimicrobial Resistance. Front. Microbiol. 2021, 12, 716064. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori Infection. Helicobacter 2014, 19, 1–5. [Google Scholar] [CrossRef]
- Moss, S.F. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, P.; Hyvärinen, H. Role of Helicobacter pylori in the Pathogenesis of Gastritis, Peptic Ulcer and Gastric Cancer. Scand. J. Gastroenterol. 1993, 28, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; De Francesco, V.; Repici, A.; Manta, R.; Tomao, S.; Annibale, B.; Vaira, D. Helicobacter pylori and Functional Dyspepsia: An Unsolved Issue? World J. Gastroenterol. 2014, 20, 8957–8963. [Google Scholar] [PubMed]
- Moayyedi, P.; Soo, S.; Deeks, J.; Delaney, B.; Harris, A.; Innes, M.; Oakes, R.; Wilson, S.; Roalfe, A.; Bennett, C.; et al. Eradication of Helicobacter pylori for Non-ulcer Dyspepsia. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Majumdar, D.; Bebb, J. Helicobacter pylori Infection and Peptic Ulcers. Medicine 2019, 47, 292–300. [Google Scholar] [CrossRef]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori Infection. Mo. Med. 2018, 115, 219–224. [Google Scholar]
- Ferro, A.; Peleteiro, B.; Malvezzi, M.; Bosetti, C.; Bertuccio, P.; Levi, F.; Negri, E.; La Vecchia, C.; Lunet, N. Worldwide Trends in Gastric Cancer Mortality (1980–2011), with Predictions to 2015, and Incidence by Subtype. Eur. J. Cancer 2014, 50, 1330–1344. [Google Scholar] [CrossRef]
- Khatoon, J.; Rai, R.P.; Prasad, K.N. Role of Helicobacter pylori in Gastric Cancer: Updates. World J. Gastrointest. Oncol. 2016, 8, 147–158. [Google Scholar] [CrossRef]
- Mégraud, F.; Bessède, E.; Varon, C. Helicobacter pylori Infection and Gastric Carcinoma. Clin. Microbiol. Infect. 2015, 21, 984–990. [Google Scholar] [CrossRef]
- Smith, M.G. Cellular and Molecular Aspects of Gastric Cancer. World J. Gastroenterol. 2006, 12, 2979. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M. Helicobacter pylori: Gastric Cancer and Beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, P.Y.; Aboul-Soud, M.A.M. From Inflammation to Gastric Cancer: Role of Helicobacter pylori. Oncol. Lett. 2017, 13, 543–548. [Google Scholar] [CrossRef]
- Kalali, B.; Formichella, L.; Gerhard, M. Diagnosis of Helicobacter pylori: Changes towards the Future. Diseases 2015, 3, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.M.; Retnakumar, R.J.; Chouhan, D.; Devi, T.N.B.; Dharmaseelan, S.; Devadas, K.; Thapa, N.; Tamang, J.P.; Lamtha, S.C.; Chattopadhyay, S. Helicobacter pylori in Human Stomach: The Inconsistencies in Clinical Outcomes and the Probable Causes. Front. Microbiol. 2021, 12, 713955. [Google Scholar]
- Mladenova, I. Clinical Relevance of Helicobacter pylori Infection. J. Clin. Med. 2021, 10, 3473. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Off. J. Am. Coll. Gastroenterol.|ACG 2017, 112, 212–239. [Google Scholar]
- Shatila, M.; Thomas, A.S. Current and Future Perspectives in the Diagnosis and Management of Helicobacter pylori Infection. J. Clin. Med. 2022, 11, 5086. [Google Scholar] [CrossRef]
- Dore, M.P.; Pes, G.M. What Is New in Helicobacter pylori Diagnosis. An Overview. J. Clin. Med. 2021, 10, 2091. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Yamaoka, Y. Diagnostic Methods of Helicobacter pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. Biomed. Res. Int. 2016, 2016, 4819423. [Google Scholar] [CrossRef]
- Patel, S.K.; Pratap, C.B.; Jain, A.K.; Gulati, A.K.; Nath, G. Diagnosis of Helicobacter pylori: What Should Be the Gold Standard? World J. Gastroenterol. 2014, 20, 12847–12859. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, T.; Ganji, L. The Diagnostic Tests for Detection of Helicobacter pylori Infection. Monoclon. Antib. Immunodiagn. Immunother. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Rojas-Rengifo, D.F.; Mendoza, B.; Jaramillo, C.; Rodríguez-Urrego, P.A.; Vera-Chamorro, J.F.; Alvarez, J.; Delgado, M.d.P.; Jimenez-Soto, L.F. Helicobacter pylori Culture as a Key Tool for Diagnosis in Colombia. J. Infect. Dev. Ctries. 2019, 13, 720–726. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, X.; Li, P.; Zhu, Y. Effect of Helicobacter pylori Eradication on Gastric Precancerous Lesions: A Systematic Review and Meta-Analysis. Helicobacter 2023, 28, e13013. [Google Scholar] [CrossRef] [PubMed]
- Herbrink, P.; van Doorn, L.J. Serological Methods for Diagnosis of Helicobacter pylori Infection and Monitoring of Eradication Therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Siavoshi, F.; Saniee, P.; Khalili-Samani, S.; Hosseini, F.; Malakutikhah, F.; Mamivand, M.; Shahreza, S.; Sharifi, A.H. Evaluation of Methods for H. pylori Detection in PPI Consumption Using Culture, Rapid Urease Test and Smear Examination. Ann. Transl. Med. 2015, 3, 11. [Google Scholar] [CrossRef]
- Skrebinska, S.; Mégraud, F.; Bessède, E. Diagnosis of Helicobacter pylori Infection. Helicobacter 2018, 23, e12515. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Moosavi, L. Urea breath test. [Updated 2022 Aug 8]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542286/ (accessed on 13 December 2023).
- Ferwana, M.; Abdulmajeed, I.; Alhajiahmed, A.; Madani, W.; Firwana, B.; Hasan, R.; Altayar, O.; Limburg, P.J.; Murad, M.H.; Knawy, B. Accuracy of Urea Breath Test in Helicobacter pylori Infection: Meta-Analysis. World J. Gastroenterol. 2015, 21, 1305–1314. [Google Scholar] [CrossRef]
- Sadowski, D.C.; van Zanten, S.V. Dyspepsia. Can. Med. Assoc. J. 2015, 187, 276. [Google Scholar] [CrossRef][Green Version]
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Shimoyama, T. Stool Antigen Tests for the Management of Helicobacter pylori Infection. World J. Gastroenterol. 2013, 19, 8188–8191. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of Monoclonal Stool Antigen Test for the Diagnosis of H. pylori Infection: A Systematic Review and Meta-Analysis. Off. J. Am. Coll. Gastroenterol.|ACG 2006, 101, 1921–1930. [Google Scholar] [CrossRef]
- Ricci, C.; Holton, J.; Vaira, D. Diagnosis of Helicobacter pylori: Invasive and Non-Invasive Tests. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Best, L.M.J.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-Invasive Diagnostic Tests for Helicobacter pylori Infection. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Lee, H.S. Histopathologic Diagnosis of H. pylori Infection and Associated Gastric Diseases. In Helicobacter pylori; Kim, N., Ed.; Springer: Singapore, 2016; pp. 119–127. ISBN 978-981-287-706-2. [Google Scholar]
- Yadav, R.; Sagar, M. Comparison of Different Histological Staining Methods for Detection of Helicobacter pylori Infection in Gastric Biopsy. Cureus 2022, 14, e27316. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P.; the Participants in the International Workshop on the Histopathology of Gastritis, H. 1994 Classification and Grading of Gastritis: The Updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Meliț, L.E.; Săsăran, M.O. Traditional and Modern Diagnostic Approaches in Diagnosing Pediatric Helicobacter pylori Infection. Children 2022, 9, 994. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Lorenzetti, R.; Winn, S.; Morini, S. A Clinical Practice Viewpoint: To Culture or Not to Culture Helicobacter pylori? Dig. Liver Dis. 2003, 35, 357–361. [Google Scholar] [CrossRef]
- Hirschl, A.M.; Makristathis, A. Methods to Detect Helicobacter pylori: From Culture to Molecular Biology. Helicobacter 2007, 12, 6–11. [Google Scholar] [CrossRef]
- Nurgalieva, Z.Z.; Conner, M.E.; Opekun, A.R.; Zheng, C.Q.; Elliott, S.N.; Ernst, P.B.; Osato, M.; Estes, M.K.; Graham, D.Y. B-Cell and T-Cell Immune Responses to Experimental Helicobacter pylori Infection in Humans. Infect. Immun. 2005, 73, 2999–3006. [Google Scholar] [CrossRef]
- Macin, S.; Alp, A.; Sener, B.; Sokmensuer, C.; Orhan, D.; Ozen, H.; Kav, T.; Akyon, Y. Comparison of Culture, Real- Time-PCR, ELISA, and Histopathological Examination Methods for Identification of Helicobacter pylori. Istanb. Med. J. 2018, 19, 138–142. [Google Scholar] [CrossRef]
- Bénéjat, L.; Ducournau, A.; Lehours, P.; Mégraud, F. Real-Time PCR for Helicobacter pylori Diagnosis. The Best Tools Available. Helicobacter 2018, 23, e12512. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El Serag, H.B.; Saruuljavkhlan, B.; Azzaya, D.; Matsumoto, T.; Uchida, T.; Oyuntsetseg, K.; Oyunbileg, N.; Davaadorj, D.; Yamaoka, Y. Advantage of 16S RRNA Amplicon Sequencing in Helicobacter pylori Diagnosis. Helicobacter 2021, 26, e12790. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Cho, I.K.; Lee, C.H.; Song, G.G.; Lim, J.H. Clinical Outcomes of Standard Triple Therapy Plus Probiotics or Concomitant Therapy for Helicobacter pylori Infection. Gut Liver 2018, 12, 165–172. [Google Scholar] [CrossRef]
- Rokkas, T.; Gisbert, J.P.; Malfertheiner, P.; Niv, Y.; Gasbarrini, A.; Leja, M.; Megraud, F.; O’Morain, C.; Graham, D.Y. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-Analysis. Gastroenterology 2021, 161, 495–507.e4. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Sabourian, R.; Foroumadi, A. Treatment of Helicobacter pylori Infection: Current and Future Insights. World J. Clin. Cases 2016, 4, 5. [Google Scholar] [CrossRef]
- Smith, S.I.; Yamaoka, Y. Antibiotic Resistance and Therapy for Helicobacter pylori Infection. Antibiotics 2023, 12, 1669. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, S.J.; Lee, Y.C.; Yang, H.J.; Park, S.Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.H.; Nam, S.Y.; et al. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver 2021, 15, 168–195. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Helicobacter pylori in the Post-Antibiotics Era: From Virulence Factors to New Drug Targets and Therapeutic Agents. Arch. Microbiol. 2023, 205, 301. [Google Scholar] [CrossRef]
- Luther, J.; Higgins, P.D.R.; Schoenfeld, P.S.; Moayyedi, P.; Vakil, N.; Chey, W.D. Empiric Quadruple vs. Triple Therapy for Primary Treatment of Helicobacter pylori Infection: Systematic Review and Meta-Analysis of Efficacy and Tolerability. Off. J. Am. Coll. Gastroenterol.|ACG 2010, 105, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.F.; Hsu, P.I. Second-Line Rescue Treatment of Helicobacter pylori Infection: Where Are We Now? World J. Gastroenterol. 2018, 24, 4548–4553. [Google Scholar] [PubMed]
- Essa, A.S.; Kramer, J.R.; Graham, D.Y.; Treiber, G. Meta-Analysis: Four-Drug, Three-Antibiotic, Non-Bismuth-Containing “Concomitant Therapy” Versus Triple Therapy for Helicobacter pylori Eradication. Helicobacter 2009, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Shin, J.M.; Howden, C.W. Review Article: The Clinical Pharmacology of Proton Pump Inhibitors. Aliment. Pharmacol. Ther. 2006, 23, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Cartee, N.M.P.; Wang, M.M. Binding of Omeprazole to Protein Targets Identified by Monoclonal Antibodies. PLoS ONE 2020, 15, e0239464. [Google Scholar] [CrossRef]
- Shin, J.M.; Sachs, G. Pharmacology of Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Kandulski, A.; Venerito, M. Proton-Pump Inhibitors: Understanding the Complications and Risks. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 697–710. [Google Scholar] [CrossRef]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, X.; Liu, X.; Song, Y.; Song, C.; Wu, S.; An, Y.; Yuan, R.; Wang, Y.; Xie, Y. The Effect of Antibiotic Resistance on Helicobacter pylori Eradication Efficacy: A Systematic Review and Meta-Analysis. Helicobacter 2020, 25, e12714. [Google Scholar] [CrossRef]
- Zanotti Giuseppe and Cendron, L. Structural Aspects of Helicobacter pylori Antibiotic Resistance. In Helicobacter pylori in Human Diseases: Advances in Microbiology, Infectious Diseases and Public Health Volume 11; Shigeru, K., Backert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 227–241. ISBN 978-3-030-21916-1. [Google Scholar]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-Analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Prevalence of Antibiotic Heteroresistance Associated with Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Microb. Pathog. 2022, 170, 105720. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Vukovic, J.; Modun, D.; Sundov, Z.; Tonkic, A. Historical Overview of the Maastricht Consensus Reports for the Management of Helicobacter pylori Infection. Where Are We Today? Austin J. Infect. Dis. 2022, 9. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Update on Non-Bismuth Quadruple (Concomitant) Therapy for Eradication of Helicobacter pylori. Clin. Exp. Gastroenterol. 2012, 5, 23–34. [Google Scholar]
- Cheng, H.-C.; Chang, W.-L.; Chen, W.-Y.; Yang, H.-B.; Wu, J.-J.; Sheu, B.-S. Levofloxacin-Containing Triple Therapy to Eradicate the Persistent H. pylori after a Failed Conventional Triple Therapy. Helicobacter 2007, 12, 359–363. [Google Scholar] [CrossRef]
- Hsu, P.-I.; Tsai, F.-W.; Kao, S.-S.; Hsu, W.-H.; Cheng, J.-S.; Peng, N.-J.; Tsai, K.-W.; Hu, H.-M.; Wang, Y.-K.; Chuah, S.-K.; et al. Ten-Day Quadruple Therapy Comprising Proton Pump Inhibitor, Bismuth, Tetracycline, and Levofloxacin Is More Effective than Standard Levofloxacin Triple Therapy in the Second-Line Treatment of Helicobacter pylori Infection: A Randomized Controlled Trial. Off. J. Am. Coll. Gastroenterol.|ACG 2017, 112, 1374–1381. [Google Scholar]
- Hsu, P.-I.; Tsay, F.-W.; Kao, J.Y.; Peng, N.-J.; Chen, Y.-H.; Tang, S.-Y.; Kuo, C.-H.; Kao, S.-S.; Wang, H.-M.; Wu, I.-T.; et al. Tetracycline-Levofloxacin versus Amoxicillin-Levofloxacin Quadruple Therapies in the Second-Line Treatment of Helicobacter pylori Infection. Helicobacter 2021, 26, e12840. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Calvet, X.; O’Connor, A.; Mégraud, F.; O’Morain, C.A. Sequential Therapy for Helicobacter pylori Eradication: A Critical Review. J. Clin. Gastroenterol. 2010, 44, 313–325. [Google Scholar]
- O’Connor, H.J. Forty Years of Helicobacter pylori Infection and Changes in Findings at Esophagogastroduodenoscopy. Helicobacter 2023, 28, e13026. [Google Scholar] [CrossRef]
- Kim, S.Y. Antibiotic Treatment for Helicobacter pylori: Is the End Coming? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 183. [Google Scholar] [CrossRef]
- Su, Y.-L.; Huang, H.-L.; Huang, B.-S.; Chen, P.-C.; Chen, C.-S.; Wang, H.-L.; Lin, P.-H.; Chieh, M.-S.; Wu, J.-J.; Yang, J.-C.; et al. Combination of OipA, BabA, and SabA as Candidate Biomarkers for Predicting Helicobacter pylori-Related Gastric Cancer. Sci. Rep. 2016, 6, 36442. [Google Scholar] [CrossRef] [PubMed]
- Safarov, T.; Kiran, B.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S. An Overview of Nanotechnology-Based Treatment Approaches against Helicobacter pylori. Expert Rev. Anti Infect. Ther. 2019, 17, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Lao, Y.-H.; Wang, H.; Zhang, J.; Yi, K.; Chen, Z.; Han, J.; Song, W.; Tao, Y.; Li, M. Combatting Helicobacter pylori with Oral Nanomedicines. J. Mater. Chem. B 2021, 9, 9826–9838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, W.; Zhang, J.; Xia, X. Eradication of Helicobacter pylori: The Power of Nanosized Formulations. Nanomedicine 2020, 15, 527–542. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Mestre, A.; Sathiya Narayanan, R.; Rivas, D.; John, J.; Abdulqader, M.A.; Khanna, T.; Chakinala, R.C.; Gupta, S. Role of Probiotics in the Management of Helicobacter pylori. Cureus 2022, 14, e26463. [Google Scholar] [CrossRef]
- Mestrovic, A.; Perkovic, N.; Tonkic, A.; Sundov, Z.; Kumric, M.; Bozic, J. Personalized Approach in Eradication of Helicobacter Pylori Infection. Antibiotics 2023, 12, 7. [Google Scholar] [CrossRef]
- Graham, D.Y. Transitioning of Helicobacter pylori Therapy from Trial and Error to Antimicrobial Stewardship. Antibiotics 2020, 9, 671. [Google Scholar] [CrossRef]
- Addissouky, T.A.; Wang, Y.; El Sayed, I.E.T.; Baz, A.E.; Ali, M.M.A.; Khalil, A.A. Recent Trends in Helicobacter pylori Management: Harnessing the Power of AI and Other Advanced Approaches. Beni Suef Univ. J. Basic. Appl. Sci. 2023, 12, 80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. https://doi.org/10.3390/microorganisms12010222
Ali A, AlHussaini KI. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms. 2024; 12(1):222. https://doi.org/10.3390/microorganisms12010222
Chicago/Turabian StyleAli, Asghar, and Khalid I. AlHussaini. 2024. "Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies" Microorganisms 12, no. 1: 222. https://doi.org/10.3390/microorganisms12010222
APA StyleAli, A., & AlHussaini, K. I. (2024). Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms, 12(1), 222. https://doi.org/10.3390/microorganisms12010222







