The Effects of Local Weed Species on Arbuscular Mycorrhizal Fungal Communities in an Organic Winter Wheat (Triticum durum L.) Field in Lebanon
Abstract
:1. Introduction
2. Material and Methods
2.1. Site Description
2.2. Experimental Approach
2.3. Soil Chemical Properties
2.4. The Determination of Weed Species Richness
2.5. The Determination of the Density and Viability of AMF Propagules in Soils
2.6. Target Plant Growth Experiments in Greenhouse
2.7. The Level of Mycorrhizal Root Colonization
2.8. DNA Extraction, PCR Amplification and Illumina-Based Sequencing
2.9. Bioinformatic Analysis
2.10. Statistical Analysis
2.11. Data Availability
3. Results
3.1. The AMF Colonization and Alpha Diversity of Root AM Fungal Community
3.2. AM Fungal Community Distribution
3.3. AM Fungal Community Composition
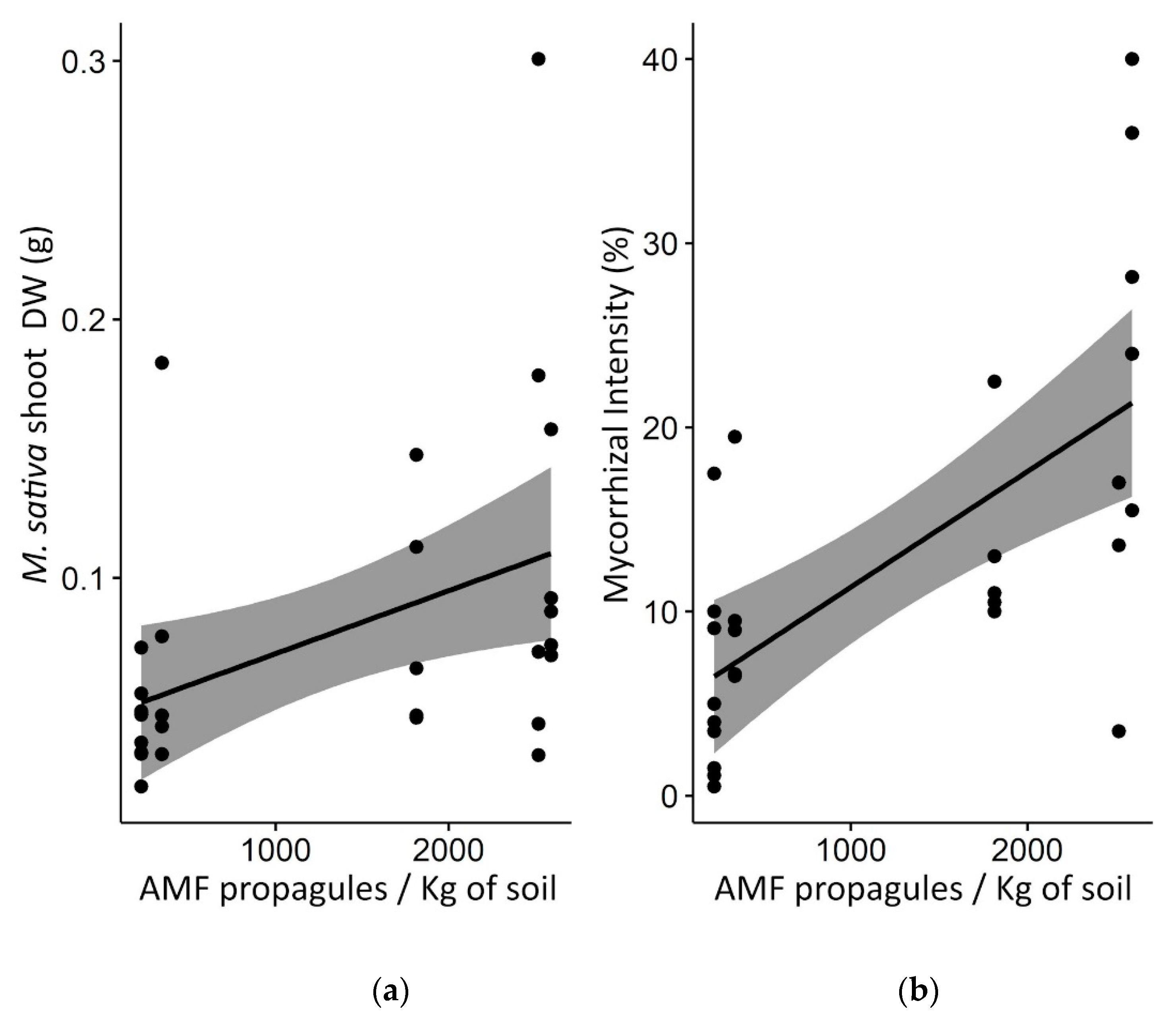
3.4. AMF Propagules
3.5. The Indirect Effect of Weeds on M. sativa Trap Plant Productivity and Mycorrhization in Greenhouse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 24. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Cambecedes, J.; Vela, E. Is the Mesopotamian region a main source of Western European segetal plants? Bot. Lett. 2020, 167, 290–299. [Google Scholar] [CrossRef]
- Coulon, F.; André, J. Analyse des Pratiques Agricoles Favorables aux Plantes Messicoles en Midi-Pyrénées Conservatoire Botanique des Pyrénées et de Midi-Pyrénées; Solagro: Toulouse, France, 2010. [Google Scholar]
- Willcox, G. Searching for the origins of arable weeds in the Near East. Veg. Hist. Archaeobot. 2012, 21, 163–167. [Google Scholar] [CrossRef]
- Korres, N.E. Agronomic weed control: A trustworthy approach for sustainable weed management. In Non-Chemical Weed Control, 1st ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2018; pp. 97–114. [Google Scholar] [CrossRef]
- Richner, N.; Holderegger, R.; Linder, H.P.; Walter, T. Reviewing change in the arable flora of Europe: A meta-analysis. Weed Res. 2015, 55, 1–13. [Google Scholar] [CrossRef]
- Barbercheck, M.E.; Wallace, J. Weed–insect interactions in annual cropping systems. Ann. Entomol. Soc. Am. 2021, 114, 276–291. [Google Scholar] [CrossRef]
- Gaba, S.; Perronne, R.; Fried, G.; Gardarin, A.; Bretagnolle, F.; Biju-Duval, L.; Colbach, N.; Cordeau, S.; Fernandez-Aparicio, M.; Gauvrit, C.; et al. Response and effect traits of arable weeds in agro-ecosystems: A review of current knowledge. Weed Res. 2017, 57, 123–147. [Google Scholar] [CrossRef]
- Trinchera, A.; Testani, E.; Roccuzzo, G.; Campanelli, G.; Ciaccia, C. Agroecological service crops drive plant mycorrhization in organic horticultural systems. Microorganisms 2021, 9, 410. [Google Scholar] [CrossRef]
- Albrecht, H.; Cambecèdes, J.; Lang, M.; Wagner, M. Management options for the conservation of rare arable plants in Europe. Bot. Lett. 2016, 163, 389–415. [Google Scholar] [CrossRef]
- Wortman, S.E. Weedy fallow as an alternative strategy for reducing nitrogen loss from annual cropping systems. Agron. Sustain. Dev. 2016, 36, 61. [Google Scholar] [CrossRef]
- Diehl, E.; Wolters, V.; Birkhofer, K. Arable weeds in organically managed wheat fields foster carabid beetles by resource-and structure-mediated effects. Arthropod Plant Interact. 2012, 6, 75–82. [Google Scholar] [CrossRef]
- Blaix, C.; Moonen, A.C.; Dostatny, D.F.; Izquierdo, J.; Le Corff, J.; Morrison, J.; von Redwitz, C.; Schumacher, M.; Westerman, P.R. R. Quantification of regulating ecosystem services provided by weeds in annual cropping systems using a systematic map approach. Weed Res. 2018, 58, 151–164. [Google Scholar] [CrossRef]
- Thomas, S.R.; Goulson, D.; Holland, J.M. Resource provision for farmland gamebirds: The value of beetle banks. Ann. Appl. Biol. 2021, 139, 111–118. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What good is weed diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef]
- El Omari, B.; El Ghachtouli, N. Arbuscular mycorrhizal fungi-weeds interaction in cropping and unmanaged ecosystems: A review. Symbiosis 2021, 83, 279–292. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Binet, M.N.; Marchal, C.; Lipuma, J.; Geremia, R.A.; Bagarri, O.; Candaele, B.; Fraty, D.; David, B.; Perigon, S.; Barbreau, V.; et al. Plant health status effects on arbuscular mycorrhizal fungi associated with Lavandula angustifolia and Lavandula intermedia infected by Phytoplasma in France. Sci. Rep. 2020, 10, 20305. [Google Scholar] [CrossRef]
- Mouhamadou, B.; Puissant, J.; Personeni, E.; Desclos-Theveniau, M.; Kastl, E.M.; Schloter, M.; Zinger, L.; Roy, J.; Geremia, R.A.; Lavorel, S. Effects of two grass species on the composition of soil fungal communities. Biol. Fertil. Soils 2013, 49, 1131–1139. [Google Scholar] [CrossRef]
- Legay, N.; Lavorel, S.; Baxendale, C.; Krainer, U.; Bahn, M.; Binet, M.N.; Cantarel, A.M.; Colace, M.P.; Foulquier, A.; Kastl, E.M.; et al. Influence of plant traits, soil microbial properties, and abiotic parameters on nitrogen turnover of grassland ecosystems. Ecosphere 2016, 7, e01448. [Google Scholar] [CrossRef]
- Kubota, H.; Quideau, S.A.; Hucl, P.J.; Spaner, D.M. The effect of weeds on soil arbuscular mycorrhizal fungi and agronomic traits in spring wheat (Triticum aestivum L.) under organic management in Canada. Can. J. Plant Sci. 2015, 95, 615–627. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Goss, M.J. Soil and weed management for enhancing arbuscular mycorrhiza colonization of wheat. Soil Use Manag. 2013, 29, 540–546. [Google Scholar] [CrossRef]
- Trinchera, A.; Warren Raffa, D. Weeds: An Insidious Enemy or a Tool to Boost Mycorrhization in Cropping Systems? Microorganisms 2023, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Ichter, J.; Poncet, L.; Touroult, J. Catalogues des Méthodes et des Protocoles. Phase 1: Étude de Définition et Proposition d’une Démarche; Service du Patrimoine Naturel, Muséum National d’Histoire Naturelle: Paris, France, 2014; 30p. [Google Scholar]
- Porter, W.M. The ‘most probable number’ method for enumerating infective propagules of vesicular arbuscular mycorrhizal fungi in soil. Aust. J. Soil Res. 1979, 17, 515–519. [Google Scholar] [CrossRef]
- Binet, M.N.; Sage, L.; Malan, C.; Clément, J.C.; Redecker, D.; Wipf, D.; Geremia, R.A.; Lavorel, S.; Mouhamadou, B. Effects of mowing on fungal endophytes and arbuscular mycorrhizal fungi in subalpine grasslands. Fungal Ecol. 2013, 6, 248–255. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition, 2nd ed.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1966. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedure for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–163. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Gollotte, A.; van Tuinen, D.; Atkinson, D. Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 2004, 14, 111–117. [Google Scholar] [CrossRef]
- Boyer, F.; Mercier, C.; Bonin, A.; Le Bras, Y.; Taberlet, P.; Coissac, E. Obitools: A unix- inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 2016, 16, 176–182. [Google Scholar] [CrossRef]
- Van Dongen, S. Graph clustering via a discrete uncoupling process. SIAM J. Matrix Anal. Appl. 2008, 30, 121–141. [Google Scholar] [CrossRef]
- Zinger, L.; Lionnet, C.; Benoiston, A.S.; Donald, J.; Mercier, C.; Boyer, F. metabaR: An R package for the evaluation and improvement of DNA metabarcoding data quality. Methods Ecol. Evol. 2021, 12, 586–592. [Google Scholar] [CrossRef]
- Solimos, P.; Stevens, M.H.H.; Wagner, H.H. Vegan: Community Ecology Package. R Package Version 1.15-4; Ordination methods, diversity analysis and other functions for community and vegetation ecologists; The R Project for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Guzman, A.; Montes, M.; Hutchins, L.; DeLaCerda, G.; Yang, P.; Kakouridis, A.; Dahlquist-Willard, R.M.; Firestone, M.K.; Bowles, T.; Kremen, C. Crop diversity enriches arbuscular mycorrhizal fungal communities in an intensive agricultural landscape. New Phytol. 2021, 231, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Garcia de Leon, D.; Zobel, M.; Moora, M.; Bueno, C.G.; Barceló, M.; Gertz, M.; Léon, D.; Meng, Y.; Pillar, V.D.; et al. Plant functional groups associate with distinct arbuscular mycorrhizal fungal communities. New Phytol. 2020, 226, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, N.T.; Hawkes, C.V. Plant neighborhood control of arbuscular mycorrhizal community composition. New Phytol. 2009, 183, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Binet, M.N.; Van Tuinen, D.; Deprêtre, N.; Koszela, N.; Chambon, C.; Gianinazzi, S. Arbuscular mycorrhizal fungi associated with Artemisia umbelliformis Lam, an endangered aromatic species in Southern French Alps, influence plant P and essential oil contents. Mycorrhiza 2011, 21, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Alaux, P.L.; Mison, C.; Senés-Guerrero, C.; Moreau, V.; Manssens, G.; Foucart, G.; Manssens, G.; Foucart, G.; Cranenbrouck, S.; Declerck, S. Diversity and species composition of arbuscular mycorrhizal fungi across maize fields in the southern part of Belgium. Mycorrhiza 2021, 31, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ricono, C.; Fournier, P.; Mondy, S.; Vandenkoornhuyse, P.; Mony, C. Neighbourhood effect of weeds on wheat root endospheric mycobiota. J. Ecol. 2023, 111, 994–1008. [Google Scholar] [CrossRef]
- Lyu, D.; Smith, D.L. The root signals in rhizospheric inter-organismal communications. Front. Plant Sci. 2022, 13, 5328. [Google Scholar] [CrossRef]
- Varela-Cervero, S.; Vasar, M.; Davison, J.; Barea, J.M.; Öpik, M.; Azcón-Aguilar, C. The composition of arbuscular mycorrhizal fungal communities differs among the roots, spores and extraradical mycelia associated with five Mediterranean plant species. Environ. Microbiol. 2015, 17, 2882–2895. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Valiente-Banuet, A.; Verdú, M. Mycorrhizal symbiosis increases the benefits of plant facilitative interactions. Ecography 2019, 42, 447–455. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fung. Biol. 2022, 3, 723892. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]


| Df | Sum of Squares | R2 | F | Pr (>F) | |
|---|---|---|---|---|---|
| Weed species richness | 1 | 0.20320 | 0.19164 | 4.5835 | 0.001 |
| Total soil N (%) | 1 | 0.11813 | 0.11141 | 2.6646 | 0.021 |
| Total soil C (%) | 1 | 0.04023 | 0.03794 | 0.9074 | 0.428 |
| Soil pH | 1 | 0.03374 | 0.03182 | 0.7610 | 0.623 |
| Residuals | 15 | 0.66500 | 0.62718 | ||
| Total | 19 | 1.06029 | 1.000 |
| OTUs | Number of OTUs | Total Relative Abundance (%) | |||
|---|---|---|---|---|---|
| Td | Tdw | W | WTd | ||
| OTUs shared between all conditions | 146 | 94.43 | 92 | 94.23 | 92.78 |
| OTUs shared between Tdw, W and WTd | 22 | 0.84 | 1.02 | 1.66 | |
| OTUs shared between Td, W and WTd | 15 | 0.62 | 0.72 | 0.83 | |
| Td | Tdw | Pr (>F) | |
|---|---|---|---|
| Funneliformis sp5 | 0.020 (0.019) | 0.076 (0.047) | 0.040 |
| Funneliformis sp7 | 0.020 (0.006) | 0.028 (0.005) | 0.041 |
| Funneliformis sp8 | 0.001 (0.003) | 0.008 (0.005) | 0.039 |
| Funneliformis sp12 | 0.001 (0.003) | 0.007 (0.004) | 0.045 |
| Funneliformis sp14 | 0.015 (0.011) | 0.044 (0.021) | 0.030 |
| Glomus sp2 | 0.011 (0.007) | 0.002 (0.004) | 0.037 |
| Rhizophagus sp1 | 0.030 (0.010) | 0.048 (0.013) | 0.041 |
| Rhizophagus sp2 | 0.025 (0.090) | 0.036 (0.004) | 0.040 |
| Rhizophagus sp3 | 0.005 (0.003) | 0.011 (0.003) | 0.013 |
| Rhizophagus sp4 | 0.018 (0.003) | 0.024 (0.003) | 0.016 |
| Rhizophagus sp14 | 0.016 (0.008) | 0.003 (0.004) | 0.011 |
| Glomeromycotina 1 | 0.006 (0.004) | 0.012 (0.003) | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayek, S.; Marchal, C.; Huc, S.; Lapébie, L.; Abdulhak, S.; Van Es, J.; Barbreau, V.; Mouhamadou, B.; Binet, M.-N. The Effects of Local Weed Species on Arbuscular Mycorrhizal Fungal Communities in an Organic Winter Wheat (Triticum durum L.) Field in Lebanon. Microorganisms 2024, 12, 75. https://doi.org/10.3390/microorganisms12010075
Hayek S, Marchal C, Huc S, Lapébie L, Abdulhak S, Van Es J, Barbreau V, Mouhamadou B, Binet M-N. The Effects of Local Weed Species on Arbuscular Mycorrhizal Fungal Communities in an Organic Winter Wheat (Triticum durum L.) Field in Lebanon. Microorganisms. 2024; 12(1):75. https://doi.org/10.3390/microorganisms12010075
Chicago/Turabian StyleHayek, Soukayna, Camille Marchal, Stéphanie Huc, Ludivine Lapébie, Sylvain Abdulhak, Jérémie Van Es, Viviane Barbreau, Bello Mouhamadou, and Marie-Noëlle Binet. 2024. "The Effects of Local Weed Species on Arbuscular Mycorrhizal Fungal Communities in an Organic Winter Wheat (Triticum durum L.) Field in Lebanon" Microorganisms 12, no. 1: 75. https://doi.org/10.3390/microorganisms12010075
APA StyleHayek, S., Marchal, C., Huc, S., Lapébie, L., Abdulhak, S., Van Es, J., Barbreau, V., Mouhamadou, B., & Binet, M.-N. (2024). The Effects of Local Weed Species on Arbuscular Mycorrhizal Fungal Communities in an Organic Winter Wheat (Triticum durum L.) Field in Lebanon. Microorganisms, 12(1), 75. https://doi.org/10.3390/microorganisms12010075






