The Cultivation of Halophilic Microalgae Shapes the Structure of Their Prokaryotic Assemblages
Abstract
:1. Introduction
- How do the diversity and composition of the prokaryotic communities change upon isolation of halophilic algae in laboratory cultures?
- Does the taxonomic affiliation of halophilic microalgae determine the composition of their prokaryotic assemblages?
- Are the prokaryotic assemblages of halophilic microalgae composed randomly, or are there specific prokaryotic taxa featuring microalgal species?
2. Materials and Methods
2.1. Sampling, Alga Cultures, and Growth Conditions
2.2. DNA Extraction
2.3. Preparation of DNA Libraries and Sequencing
2.4. Bioinformatic Pipeline
2.5. Statistical Analysis and Visualization
3. Results
3.1. Characteristics of the Isolated Monoalgal Cultures
3.2. General Characteristics of the 16S rDNA Metabarcoding Data
3.3. Alpha-Diversity and Taxonomic Composition of the Natural Prokaryotic Communities from the Hypersaline Sites
3.4. Alpha-Diversity and Taxonomic Composition of the Control Prokaryotic Communities
3.5. Alpha-Diversity of Prokaryotic Assemblages Associated with the Monoalgal Cultures
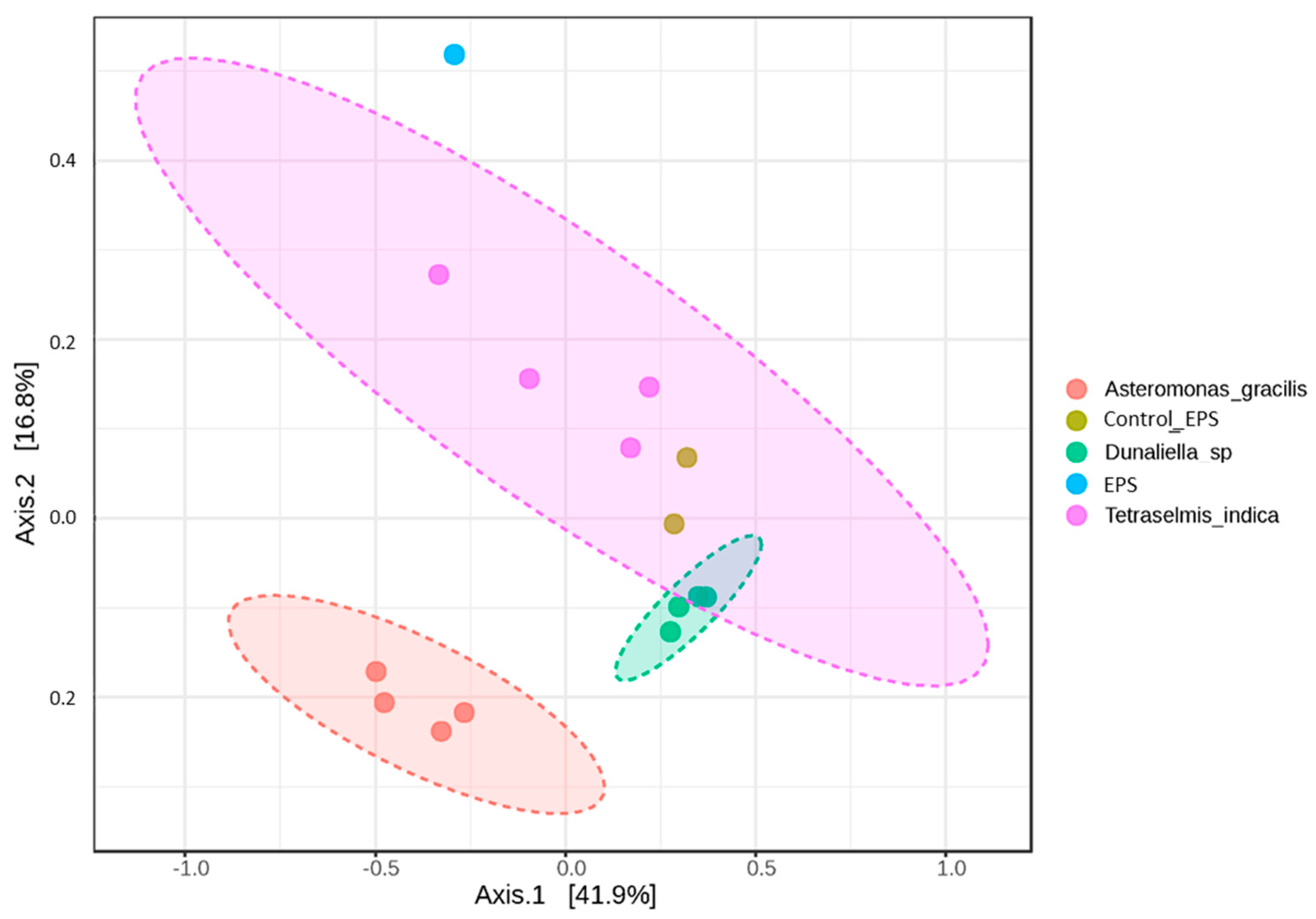
3.6. Beta-Diversity of Prokaryotic Assemblages Associated with the Monoalgal Cultures
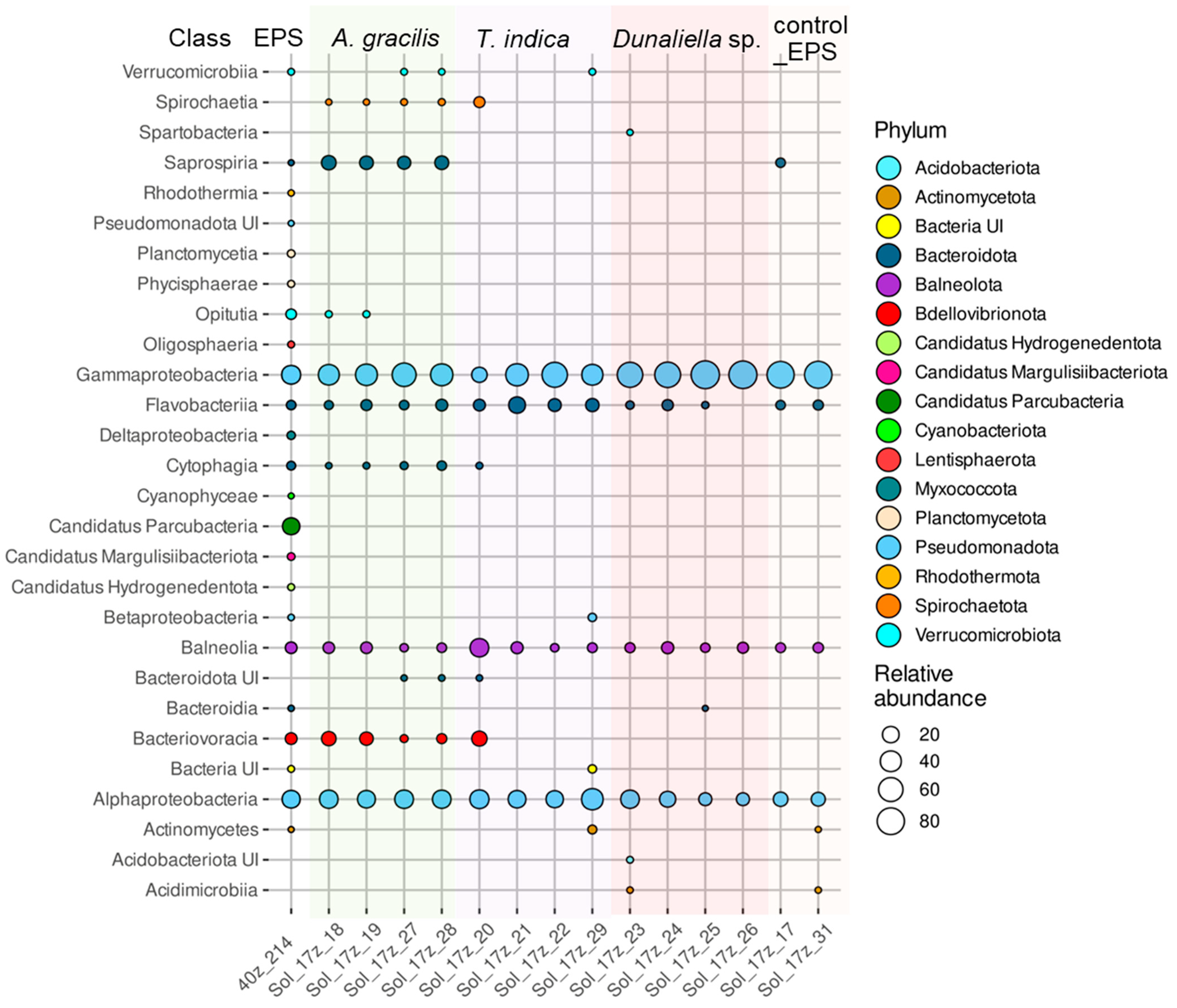
3.7. Taxonomic Composition of the Prokaryotic Assemblages Associated with the Monoalgal Cultures
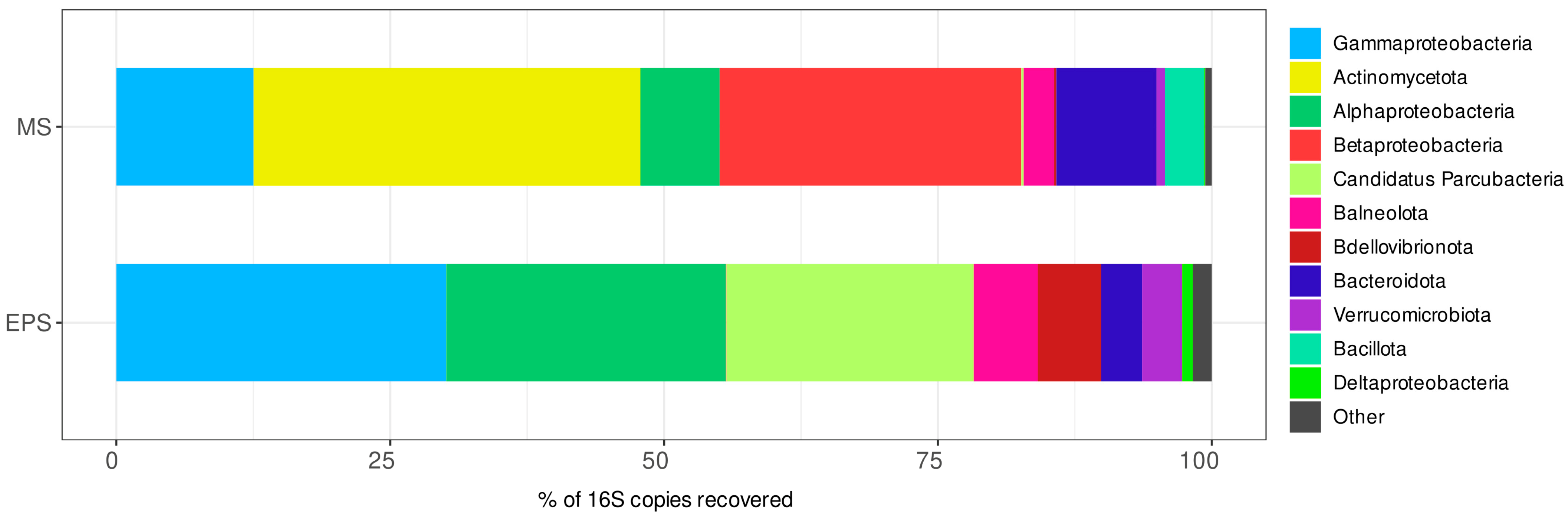
3.7.1. Taxonomic Composition of the Prokaryotic Assemblages Associated with the Monoalgal Cultures from the Ephemeral Pond near Solyanka River (EPS)
3.7.2. Taxonomic Composition of the Prokaryotic Assemblages Associated with the Monoalgal Cultures from Malaya Smorogda River (MS)
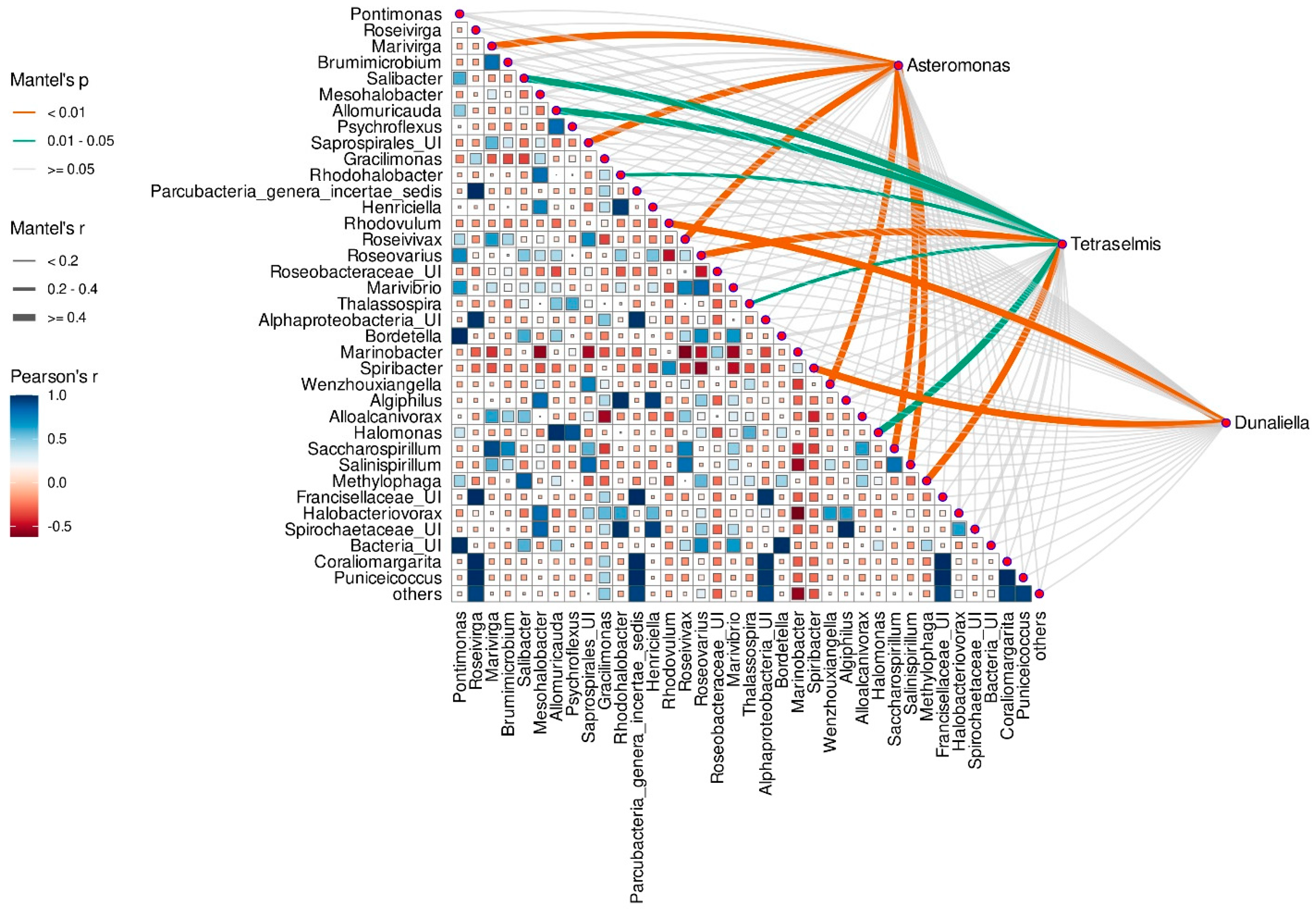
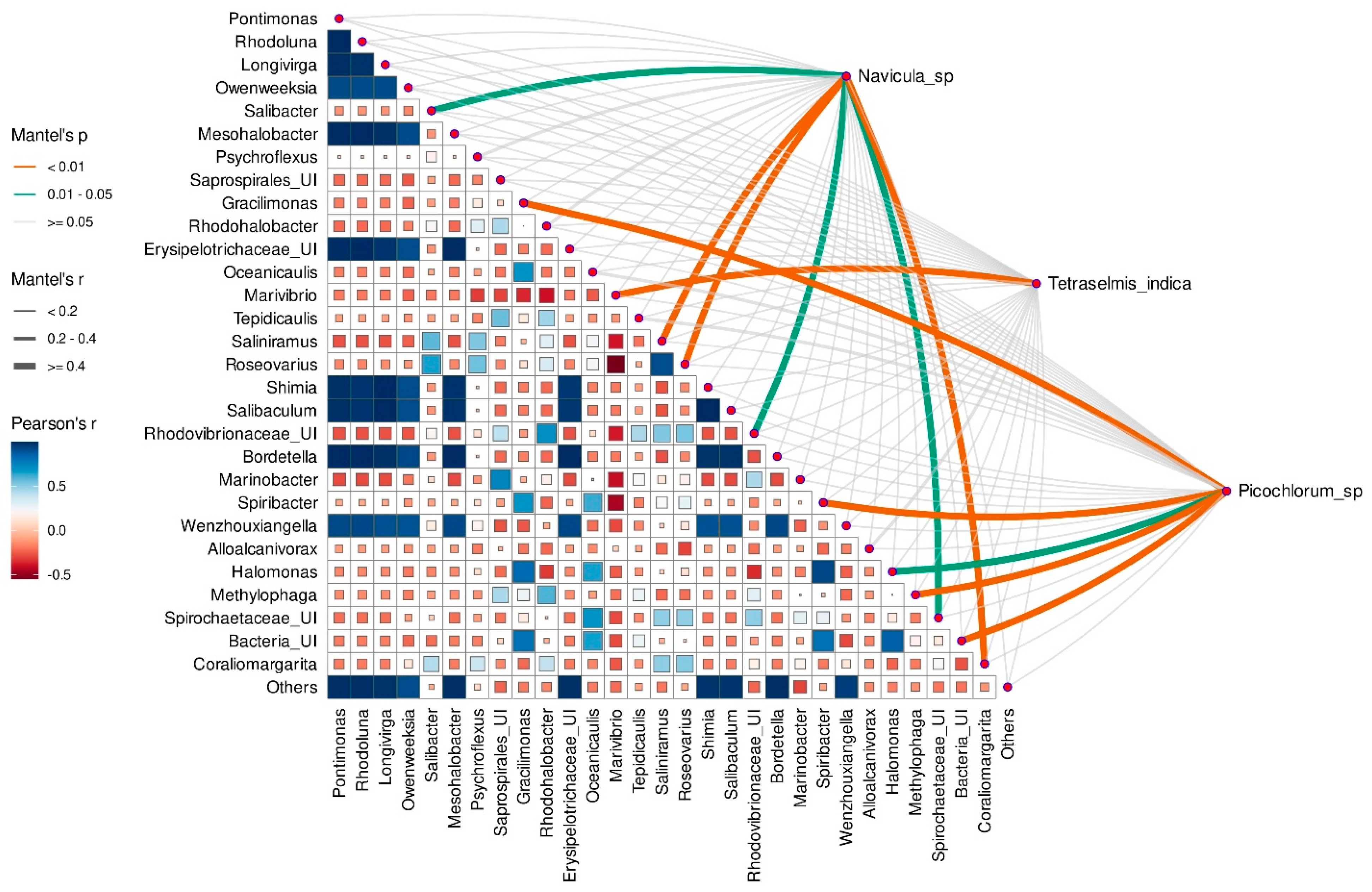
3.8. Correlation between Microalgae Species and Taxonomic Composition of Prokaryotic Assembleges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tiwari, T.; Kaur, G.A.; Singh, P.K.; Balayan, S.; Mishra, A.; Tiwari, A. Emerging Bio-Capture Strategies for Greenhouse Gas Reduction: Navigating Challenges towards Carbon Neutrality. Sci. Total Environ. 2024, 929, 172433. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.M.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of Cyanobacteria/Microalgae and Bacteria: Biotechnological Potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and Signalling between a Cosmopolitan Phytoplankton and Associated Bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic Carbon and Sulfur Cycling between Surface Ocean Plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the Phycosphere: The Ecological Interface for Phytoplankton–Bacteria Relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Shibl, A.A.; Isaac, A.; Ochsenkühn, M.A.; Cárdenas, A.; Fei, C.; Behringer, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom Modulation of Select Bacteria through Use of Two Unique Secondary Metabolites. Proc. Natl. Acad. Sci. USA 2020, 117, 27445–27455. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Shibl, A.A.; Amin, S.A. The Diatom Microbiome: New Perspectives for Diatom-Bacteria Symbioses. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 679–712. ISBN 978-3-030-92499-7. [Google Scholar]
- Krug, L.; Erlacher, A.; Markut, K.; Berg, G.; Cernava, T. The Microbiome of Alpine Snow Algae Shows a Specific Inter-Kingdom Connectivity and Algae-Bacteria Interactions with Supportive Capacities. ISME J. 2020, 14, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Bruto, M.; Oger, P.M.; Got, P.; Bernard, C.; Melayah, D.; Cloarec, L.A.; Duval, C.; Escalas, A.; Duperron, S.; Guigard, L.; et al. Phytoplanktonic Species in the Haloalkaline Lake Dziani Dzaha Select Their Archaeal Microbiome. Mol. Ecol. 2023, 32, 6824–6838. [Google Scholar] [CrossRef]
- Park, Y.; Je, K.-W.; Lee, K.; Jung, S.-E.; Choi, T.-J. Growth Promotion of Chlorella ellipsoidea by Co-Inoculation with Brevundimonas sp. Isolated from the Microalga. Hydrobiologia 2008, 598, 219–228. [Google Scholar] [CrossRef]
- Grossart, H.; Simon, M. Interactions of Planktonic Algae and Bacteria: Effects on Algal Growth and Organic Matter Dynamics. Aquat. Microb. Ecol. 2007, 47, 163–176. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased Growth of the Microalga Chlorella vulgaris When Coimmobilized and Cocultured in Alginate Beads with the Plant-Growth-Promoting Bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef]
- Mayali, X.; Doucette, G.J. Microbial Community Interactions and Population Dynamics of an Algicidal Bacterium Active against Karenia brevis (Dinophyceae). Harmful Algae 2002, 1, 277–293. [Google Scholar] [CrossRef]
- Joint, I.; Henriksen, P.; Fonnes, G.A.; Bourne, D.; Thingstad, T.F.; Riemann, B. Competition for Inorganic Nutrients between Phytoplankton and Bacterioplankton in Nutrient Manipulated Mesocosms. Aquat. Microb. Ecol. 2002, 29, 145–159. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Fukami, K.; Nishijima, T.; Ishida, Y. Stimulative and Inhibitory Effects of Bacteria on the Growth of Microalgae. In Live Food in Aquaculture; Hagiwara, A., Snell, T.W., Lubzens, E., Tamaru, C.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 185–191. [Google Scholar]
- Hernández Acevedo, H.E.; Flores Ramos, L.; Villamón Cifuentes, F.; Ruiz Soto, A.; Aguilar Samanamud, C.P. Characterization and Production Potential of Carotenes in Peruvian Strains of Dunaliella salina Teodoresco. J. World Aquac. Soc. 2022, 53, 765–780. [Google Scholar] [CrossRef]
- Avron, M.; Benamotz, A. Dunaliella: Physiology, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Hotos, G.N. A Short Review on the Halotolerant Green Microalga Asteromonas gracilis Artari with Emphasis on Its Uses. Asian J. Fish. Aquat. Res. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Fawzy, M.A. Fatty Acid Characterization and Biodiesel Production by the Marine Microalga Asteromonas gracilis: Statistical Optimization of Medium for Biomass and Lipid Enhancement. Mar. Biotechnol. 2017, 19, 219–231. [Google Scholar] [CrossRef]
- Kirkwood, A.E.; Henley, W.J. Algal community dynamics and halotolerance in a terrestrial, hypersaline environment. J. Phycol. 2006, 42, 537–547. [Google Scholar] [CrossRef]
- Ignatenko, M.E.; Selivanova, E.; Khlopko, Y.A.; Yatsenko-Stepanova, T.N. Algal and Cyanobacterial Diversity in Saline Rivers of the Elton Lake Basin (Russia) Studied via Light Microscopy and next-Generation Sequencing. Biosyst. Divers. 2021, 29, 59–66. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.-W.; Richards, T.A. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kobert, J.K.; Flouri, T.; Stamatakis, A. PEAR—A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using Microbiome Analyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. linkET: Everything Is Linkable, R Package Version 0.0.3. 2021. Available online: https://github.com/Hy4m/linkET (accessed on 1 July 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kublanovskaya, A.; Solovchenko, A.; Fedorenko, T.; Chekanov, K.; Lobakova, E. Natural Communities of Carotenogenic Chlorophyte Haematococcus lacustris and Bacteria from the White Sea Coastal Rock Ponds. Microb. Ecol. 2020, 79, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Vacant, S.; Benites, L.F.; Salmeron, C.; Intertaglia, L.; Norest, M.; Cadoudal, A.; Sanchez, F.; Caceres, C.; Piganeau, G. Long-Term Stability of Bacterial Associations in a Microcosm of Ostreococcus tauri (Chlorophyta, Mamiellophyceae). Front. Plant Sci. 2022, 13, 814386. [Google Scholar] [CrossRef] [PubMed]
- Bardavid, R.E.; Khristo, P.; Oren, A. Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles 2008, 12, 5–14. [Google Scholar] [CrossRef]
- Williams, T.J.; Allen, M.; Tschitschko, B.; Cavicchioli, R. Glycerol metabolism of haloarchaea. Environ. Microbiol. 2017, 19, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. A Hundred Years of Dunaliella Research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef]
- Arora, M.; Anil, A.C.; Leliaert, F.; Delany, J.; Mesbahi, E. Tetraselmis indica (Chlorodendrophyceae, Chlorophyta), a New Species Isolated from Salt Pans in Goa, India. Eur. J. Phycol. 2013, 48, 61–78. [Google Scholar] [CrossRef]
- Fayó, R.; Pan, J.; Espinosa, M.A. Microbial Mat and Surface Sediment Communities from a Shallow Oxbow Lake in the Colorado River Floodplain, Argentina. Geomicrobiol. J. 2020, 37, 937–949. [Google Scholar] [CrossRef]
- Henley, W.J.; Hironaka, J.L.; Guillou, L.; Buchheim, M.A.; Buchheim, J.A.; Fawley, M.W.; Fawley, K.P. Phylogenetic analysis of the ‘Nannochloris-like’ algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Phycologia 2004, 43, 641–652. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Selivanova, E.A.; Chekanov, K.A.; Sidorov, R.A.; Nemtseva, N.V.; Lobakova, E.S. Induction of secondary carotenogenesis in new halophile microalgae from the genus Dunaliella (Chlorophyceae). Biochemistry 2015, 80, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Kazeroni, N.; Baboli, M. Fatty Acid Composition of the Marine Micro Alga Tetraselmis chuii Butcher in Response to Culture Conditions. J. Algal. Biomass Utln 2015, 6, 49–55. [Google Scholar]
- Pugkaew, W.; Meetam, M.; Yokthongwattana, K.; Leeratsuwan, N.; Pokethitiyook, P. Effects of Salinity Changes on Growth, Photosynthetic Activity, Biochemical Composition, and Lipid Productivity of Marine Microalga Tetraselmis suecica. J. Appl. Phycol. 2019, 31, 969–979. [Google Scholar] [CrossRef]
- Etesami, E.; Saba, F.; Noroozi, M.; Amoozegar, M.A.; Khaniki, G.B.; Fazeli, S.A.S. Caspian Sea’s Navicula Salinicola Hustedt 1939 and Effect of the Prolonged Culture on Its Fatty Acid Profile. Int. J. Aquat. Biol. 2017, 5, 268–274. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, J.-Y.; Sang, J.; Mu, D.; Du, Z. Mesohalobacter halotolerans Gen. Nov., Sp. Nov., Isolated from a Marine Solar Saltern. Int. J. Syst. Evol. Microbiol. 2020, 70, 3588–3596. [Google Scholar] [CrossRef]
- Munoz, R.; Rosselló-Móra, R.; Amann, R. Revised Phylogeny of Bacteroidetes and Proposal of Sixteen New Taxa and Two New Combinations Including Rhodothermaeota Phyl. Nov. Syst. Appl. Microbiol. 2016, 39, 281–296. [Google Scholar] [CrossRef]
- Wang, H.; Hill, R.T.; Zheng, T.; Hu, X.; Wang, B. Effects of Bacterial Communities on Biofuel-Producing Microalgae: Stimulation, Inhibition and Harvesting. Crit. Rev. Biotechnol. 2016, 36, 341–352. [Google Scholar] [CrossRef]
- Fulbright, S.P.; Robbins-Pianka, A.; Berg-Lyons, D.; Knight, R.; Reardon, K.F.; Chisholm, S.T. Bacterial Community Changes in an Industrial Algae Production System. Algal Res. 2018, 31, 147–156. [Google Scholar] [CrossRef]
- Abell, G.C.J.; Bowman, J.P. Colonization and Community Dynamics of Class Flavobacteria on Diatom Detritus in Experimental Mesocosms Based on Southern Ocean Seawater. FEMS Microbiol. Ecol. 2005, 53, 379–391. [Google Scholar] [CrossRef]
- Krüger, K.; Chafee, M.; Ben Francis, T.; Glavina del Rio, T.; Becher, D.; Schweder, T.; Amann, R.I.; Teeling, H. In Marine Bacteroidetes the Bulk of Glycan Degradation during Algae Blooms Is Mediated by Few Clades Using a Restricted Set of Genes. ISME J. 2019, 13, 2800–2816. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Muntyan, M.S.; Toshchakov, S.V.; Korzhenkov, A.A.; Kublanov, I.V. Phenotypic and Genomic Properties of a Novel Deep-Lineage Haloalkaliphilic Member of the Phylum Balneolaeota from Soda Lakes Possessing Na+-Translocating Proteorhodopsin. Front. Microbiol. 2018, 9, 2672. [Google Scholar] [CrossRef]
- Kimbrel, J.A.; Samo, T.J.; Ward, C.; Nilson, D.; Thelen, M.P.; Siccardi, A.; Zimba, P.; Lane, T.W.; Mayali, X. Host Selection and Stochastic Effects Influence Bacterial Community Assembly on the Microalgal Phycosphere. Algal Res. 2019, 40, 101489. [Google Scholar] [CrossRef]
- Orellana, L.H.; Francis, T.B.; Ferraro, M.; Hehemann, J.-H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota Are Specialist Consumers of Sulfated Methyl Pentoses during Diatom Blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Powell, S.M.; Wilson, R.; Bowman, J.P. Extensive Gene Acquisition in the Extremely Psychrophilic Bacterial Species Psychroflexus torquis and the Link to Sea-Ice Ecosystem Specialism. Genome Biol. Evol. 2014, 6, 133–148. [Google Scholar] [CrossRef]
- Pinhassi, J.; Sala, M.M.; Havskum, H.; Peters, F.; Guadayol, Ò.; Malits, A.; Marrasé, C. Changes in Bacterioplankton Composition under Different Phytoplankton Regimens. Appl. Environ. Microbiol. 2004, 70, 6753–6766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Su, J.; Lai, Q.; Li, X.; Yang, X.; Dong, P.; Zheng, T. Phaeocystidibacter luteus Gen. Nov., Sp. Nov., a Member of the Family Cryomorphaceae Isolated from the Marine Alga Phaeocystis globosa, and Emended Description of Owenweeksia hongkongensis. Int. J. Syst. Evol. Microbiol. 2013, 63, 1143–1148. [Google Scholar] [CrossRef]
- DeLong, E.F.; Franks, D.G.; Alldredge, A.L. Phylogenetic Diversity of Aggregate-attached vs. Free-living Marine Bacterial Assemblages. Limnol. Oceanogr. 1993, 38, 924–934. [Google Scholar] [CrossRef]
- Burke, C.; Thomas, T.; Lewis, M.; Steinberg, P.; Kjelleberg, S. Composition, Uniqueness and Variability of the Epiphytic Bacterial Community of the Green Alga Ulva australis. ISME J. 2011, 5, 590–600. [Google Scholar] [CrossRef]
- Furusawa, G.; Yoshikawa, T.; Yasuda, A.; Sakata, T. Algicidal Activity and Gliding Motility of Saprospira sp. SS98-5. Can. J. Microbiol. 2003, 49, 92–100. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Nielsen, P.H. The Family Saprospiraceae. In The Prokaryotes: Other Major Lineages of Bacteria and The Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 863–889. ISBN 978-3-642-38954-2. [Google Scholar]
- Choi, D.H.; Zhang, G.I.; Noh, J.H.; Kim, W.-S.; Cho, B.C. Gracilimonas tropica Gen. Nov., Sp. Nov., Isolated from a Synechococcus Culture. Int. J. Syst. Evol. Microbiol. 2009, 59, 1167–1172. [Google Scholar] [CrossRef]
- Lee, Y.; Weerawongwiwat, V.; Kim, J.-H.; Yoon, J.-H.; Lee, J.-S.; Sukhoom, A.; Kim, W. Gracilimonas sediminicola Sp. Nov., a Moderately Halotolerant Bacterium Isolated from Seaweed Sediment Collected in the East Sea, Republic of Korea. Int. J. Syst. Evol. Microbiol. 2023, 73, 005912. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-J.; Liu, Z.-W.; Feng, X.; Zou, Q.-H.; Du, Z.-J. Rhodohalobacter mucosus Sp. Nov., Isolated from a Marine Solar Saltern. Arch. Microbiol. 2021, 203, 2419–2424. [Google Scholar] [CrossRef]
- Zecher, K.; Hayes, K.R.; Philipp, B. Evidence of Interdomain Ammonium Cross-Feeding from Methylamine- and Glycine Betaine-Degrading Rhodobacteraceae to Diatoms as a Widespread Interaction in the Marine Phycosphere. Front. Microbiol. 2020, 11, 533894. [Google Scholar] [CrossRef] [PubMed]
- Wienhausen, G.; Noriega-Ortega, B.E.; Niggemann, J.; Dittmar, T.; Simon, M. The Exometabolome of Two Model Strains of the Roseobacter Group: A Marketplace of Microbial Metabolites. Front. Microbiol. 2017, 8, 1985. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, Y.; Cao, J.; Xu, J.; Kong, Z.; Zhang, L.; Liao, K.; Zhou, C.; Yan, X. Analysis of Bacterial Community Diversity within Seven Bait-Microalgae. Algal Res. 2020, 51, 102033. [Google Scholar] [CrossRef]
- Kessler, R.W.; Weiss, A.; Kuegler, S.; Hermes, C.; Wichard, T. Macroalgal-Bacterial Interactions: Role of Dimethylsulfoniopropionate in Microbial Gardening by Ulva (Chlorophyta). Mol. Ecol. 2018, 27, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Zheng, C.; Ke, L.-X. Marivibrio halodurans Gen. Nov., Sp. Nov., a Marine Bacterium in the Family Rhodospirillaceae Isolated from Underground Rock Salt. Int. J. Syst. Evol. Microbiol. 2017, 67, 4266–4271. [Google Scholar] [CrossRef]
- Arora, M.; AC, A.; Delany, J.; Rajarajan, N.; Emami, K.; Mesbahi, E. Carbohydrate-Degrading Bacteria Closely Associated with Tetraselmis indica: Influence on Algal Growth. Aquat. Biol. 2012, 15, 61–71. [Google Scholar] [CrossRef]
- Kaeppel, E.C.; Gärdes, A.; Seebah, S.; Grossart, H.-P.; Ullrich, M.S. Marinobacter adhaerens Sp. Nov., Isolated from Marine Aggregates Formed with the Diatom Thalassiosira weissflogii. Int. J. Syst. Evol. Microbiol. 2012, 62, 124–128. [Google Scholar] [CrossRef]
- Sonnenschein, E.C.; Syit, D.A.; Grossart, H.-P.; Ullrich, M.S. Chemotaxis of Marinobacter adhaerens and Its Impact on Attachment to the Diatom Thalassiosira weissflogii. Appl. Environ. Microbiol. 2012, 78, 6900–6907. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of Iron-Siderophore Chelates Promotes Bacterial-Algal Mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, L.; Du, Z.-Z.; Zheng, W.-S.; Lu, D.-C.; Du, Z.-J. Spiribacter halobius Sp. Nov., a Novel Halophilic Gammaproteobacterium with a Relatively Large Genome. Front. Mar. Sci. 2022, 9, 1028967. [Google Scholar] [CrossRef]
- López-Pérez, M.; Ghai, R.; Leon, M.J.; Rodríguez-Olmos, Á.; Copa-Patiño, J.L.; Soliveri, J.; Sanchez-Porro, C.; Ventosa, A.; Rodriguez-Valera, F. Genomes of “Spiribacter”, a streamlined, successful halophilic bacterium. BMC Genom. 2013, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Li, T.D.; Doronina, N.V.; Ivanova, E.G.; Trotsenko, I.A. Vitamin B12-independent Strains of Methylophaga marina Isolated from Red Sea Algae. Mikrobiologiia 2007, 76, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.M.; Kimbrel, J.A.; Geng, H.; Tran-Gyamfi, M.B.; Yu, E.T.; Sale, K.L.; Lane, T.W.; Mayali, X. Bacterial Community Assembly, Succession, and Metabolic Function during Outdoor Cultivation of Microchloropsis salina. mSphere 2022, 7, e0023122. [Google Scholar] [CrossRef]
- Chernikova, T.N.; Bargiela, R.; Toshchakov, S.V.; Shivaraman, V.; Lunev, E.A.; Yakimov, M.M.; Thomas, D.N.; Golyshin, P.N. Hydrocarbon-Degrading Bacteria Alcanivorax and Marinobacter Associated With Microalgae Pavlova lutheri and Nannochloropsis oculata. Front. Microbiol. 2020, 11, 572931. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Gong, H.; Ma, M.; Li, S.; Yang, H.; Zhang, H.; Liu, J. Identification and Characterization of a Novel High-Activity Amylosucrase from Salinispirillum sp. LH10-3-1. Appl. Microbiol. Biotechnol. 2023, 107, 1725–1736. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Z.; Zhou, X.; Xie, Z.; Wang, Y.; Wang, D.; Feng, L.; Yang, G.; Ge, Y.; Zhang, X. Saccharospirillum alexandrii Sp. Nov., Isolated from the Toxigenic Marine Dinoflagellate Alexandrium catenella LZT09. Int. J. Syst. Evol. Microbiol. 2020, 70, 820–826. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Jankowiak, J.; Koch, F.; Gobler, C.J. Prokaryotic and Eukaryotic Microbiomes Associated with Blooms of the Ichthyotoxic Dinoflagellate Cochlodinium (Margalefidinium) polykrikoides in New York, USA, Estuaries. PLoS ONE 2019, 14, e0223067. [Google Scholar] [CrossRef]



| OTU (NCBI Accession No.) | Closest Homologue (NCBI Accession No.) | Query Cover (%) | Similarity (%) | Identified as (Number of Cultures) | Taxonomy (Phylum, Class) | Sampling Site |
|---|---|---|---|---|---|---|
| 19Z-93_132-05-7 (OR037277) | Dunaliella sp. (MN907401) | 100 | 99.76 | Dunaliella sp. (4) | Chlorophyta, Chlorophyceae | EPS |
| 19Z-93_132-05-9 (OR037278) | Navicula salinicola (MT012298) | 100 | 99.28 | Navicula sp. 1 (2) | Bacillariophyta, Bacillariophyceae | MS |
| 19Z-93_132-05-24 (OR037279) | Navicula salinicola (MT012298) | 100 | 98.89 | Navicula sp. 2 (3) | Bacillariophyta, Bacillariophyceae | MS |
| 19Z-93_132-05-1 (OR037280) | Tetraselmis indica (HQ651184) | 100 | 99.76 | Tetraselmis indica (4) | Chlorophyta, Chlorodendrophyceae | EPS |
| 19Z-93_132-05-1 (OR037280) | Tetraselmis indica (HQ651184) | 100 | 99.76 | Tetraselmis indica (4) | Chlorophyta, Chlorodendrophyceae | MS |
| 19Z-93_132-05-3 (OR037281) | Picochlorum sp. (MK973100) | 100 | 99.76 | Picochlorum sp. (5) | Chlorophyta, Trebouxiophyceae | MS |
| 19Z-93_132-05-2 (OR037282) | Asteromonas gracilis (JN033244) | 100 | 99.76% | Asteromonas gracilis (4) | Chlorophyta, Chlorophyceae | EPS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selivanova, E.A.; Yakimov, M.M.; Kataev, V.Y.; Khlopko, Y.A.; Balkin, A.S.; Plotnikov, A.O. The Cultivation of Halophilic Microalgae Shapes the Structure of Their Prokaryotic Assemblages. Microorganisms 2024, 12, 1947. https://doi.org/10.3390/microorganisms12101947
Selivanova EA, Yakimov MM, Kataev VY, Khlopko YA, Balkin AS, Plotnikov AO. The Cultivation of Halophilic Microalgae Shapes the Structure of Their Prokaryotic Assemblages. Microorganisms. 2024; 12(10):1947. https://doi.org/10.3390/microorganisms12101947
Chicago/Turabian StyleSelivanova, Elena A., Michail M. Yakimov, Vladimir Y. Kataev, Yuri A. Khlopko, Alexander S. Balkin, and Andrey O. Plotnikov. 2024. "The Cultivation of Halophilic Microalgae Shapes the Structure of Their Prokaryotic Assemblages" Microorganisms 12, no. 10: 1947. https://doi.org/10.3390/microorganisms12101947








