Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions
Abstract
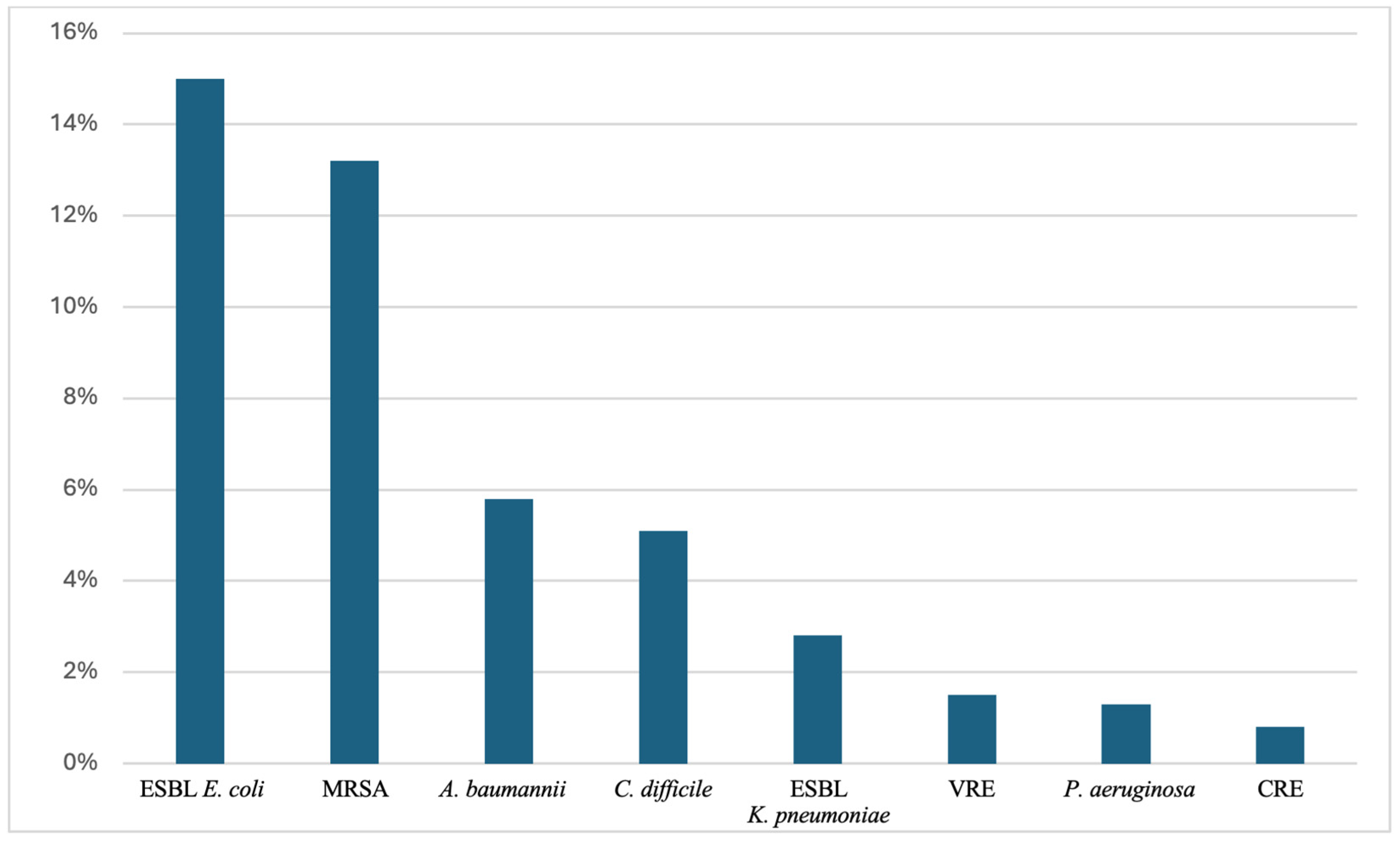
:1. Introduction
2. Key MDROs in the Elderly
2.1. MRSA
2.2. VRE
2.3. S. pneumoniae
2.4. Enterobacteriaceae
2.5. C. difficile
2.6. Mycobacterium tuberculosis
2.7. P. aeruginosa
2.8. A. baumannii
3. Risk Factors for Infections from MDROs in the Elderly
3.1. Immunosenescence
3.2. Polypharmacy
3.3. Sarcopenia and Malnutrition
3.4. Frailty and Decreased Mobility
3.5. Cognitive Impairment
3.6. Multimorbidity
3.7. Frequent Hospitalizations and Long-Term Care Facility Residency
4. Strategies to Combat Antibiotic Resistance in the Elderly
4.1. Novel Antibiotics
4.2. Bacteriophage Therapy
4.3. Antivirulence Therapies
4.4. Probiotics and Fecal Microbiota Transplantation
4.5. Vaccine Development
4.5.1. Multi-Epitope Vaccines Using Immuno-informatics and mRNA Technologies
4.5.2. Nanoparticle-Based Vaccines
4.5.3. Reverse Vaccinology and Antigen Discovery
4.5.4. Lipopeptide Adjuvants
4.5.5. Other Strategies to Enhance Immunity Following Vaccination
4.6. Antimicrobial Stewardship Programs
4.7. Advanced Diagnostic Techniques
5. The Role of ML in Combating Antibiotic Resistance in the Elderly
5.1. ML in Diagnosis
5.2. Predictive Models for Antibiotic Resistance and Antibiotic Stewardship
5.3. ML in Vaccine Development
5.4. ML in Antibiotic Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 5 September 2024).
- Cristina, M.L.; Spagnolo, A.M.; Giribone, L.; Demartini, A.; Sartini, M. Epidemiology and Prevention of Healthcare-Associated Infections in Geriatric Patients: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5333. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Theodorakis, N.; Feretzakis, G.; Vamvakou, G.; Hitas, C.; Kalantzi, S.; Spyridaki, A.; Apostolos, A.; Verykios, V.S.; Toutouzas, K. Nationwide mortality trends from 2001 to 2020 in Greece: Health policy implications under the scope of aging societies. Hellenic J. Cardiol. 2024, in press. [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Cassone, M.; Mody, L. Colonization with Multi-Drug Resistant Organisms in Nursing Homes: Scope, Importance, and Management. Curr. Geriatr. Rep. 2015, 4, 87–95. [Google Scholar] [CrossRef]
- Madrazo, M.; Esparcia, A.; López-Cruz, I.; Alberola, J.; Piles, L.; Viana, A.; Eiros, J.M.; Artero, A. Clinical impact of multidrug-resistant bacteria in older hospitalized patients with community-acquired urinary tract infection. BMC Infect. Dis. 2021, 21, 1232. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M. Multi-drug resistant gram-negative bacterial pneumonia: Etiology, risk factors, and drug resistance patterns. Pneumonia 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Henig, O.; Kaye, K.S. Bacterial Pneumonia in Older Adults. Infect Dis. Clin. N. Am. 2017, 31, 689–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef]
- González-Vázquez, R.; Córdova-Espinoza, M.G.; Escamilla-Gutiérrez, A.; Herrera-Cuevas, M.d.R.; González-Vázquez, R.; Esquivel-Campos, A.L.; López-Pelcastre, L.; Torres-Cubillas, W.; Mayorga-Reyes, L.; Mendoza-Pérez, F.; et al. Detection of mecA Genes in Hospital-Acquired MRSA and SOSA Strains Associated with Biofilm Formation. Pathogens 2024, 13, 212. [Google Scholar] [CrossRef]
- Dashtbani-Roozbehani, A.; Brown, M.H. Efflux Pump Mediated Antimicrobial Resistance by Staphylococci in Health-Related Environments: Challenges and the Quest for Inhibition. Antibiotics 2021, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Loeffler, A.; Witney, A.A.; Gould, K.A.; Lloyd, D.H.; Lindsay, J.A. Extensive horizontal gene transfer during Staphylococcus aureus co-colonization in vivo. Genome Biol. Evol. 2014, 6, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Resistance in Vancomycin-Resistant Enterococci. Infect Dis. Clin. N. Am. 2020, 34, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Mobarez, A.M.; Najar-Peerayeh, S.; Mirzaee, M. Prevalence of biofilm formation and vancomycin-resistant genes among Enterococcus faecium isolated from clinical and environmental specimens in Lorestan hospitals. Iran. J. Microbiol. 2018, 10, 74–81. [Google Scholar]
- Lehmkuhl, J.; Schneider, J.S.; Werth, K.L.V.; Scherff, N.; Mellmann, A.; Kampmeier, S. Role of membrane vesicles in the transmission of vancomycin resistance in Enterococcus faecium. Sci. Rep. 2024, 14, 1895. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance Among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Springer: Berlin/Heidelberg, Germany, 2018; pp. 13–38. [Google Scholar]
- Schroeder, M.R.; Stephens, D.S. Macrolide Resistance in Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2016, 6, 98. [Google Scholar] [CrossRef]
- Chao, Y.; Marks, L.R.; Pettigrew, M.M.; Hakansson, A.P. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front. Cell. Infect. Microbiol. 2015, 4, 194. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Tsai, Y.K.; Fung, C.P.; Lin, J.C.; Chen, J.H.; Chang, F.Y.; Chen, T.L.; Siu, L.K. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2011, 55, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cross, T.S.; Dörr, T. Analysis of AcrB in Klebsiella pneumoniae reveals natural variants promoting enhanced multidrug resistance. Res. Microbiol. 2022, 173, 103901. [Google Scholar] [CrossRef]
- Hawkey, J.; Wyres, K.L.; Judd, L.M.; Harshegyi, T.; Blakeway, L.; Wick, R.R.; Jenney, A.W.J.; Holt, K.E. ESBL plasmids in Klebsiella pneumoniae: Diversity, transmission and contribution to infection burden in the hospital setting. Genome Med. 2022, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Blau, K.; Gallert, C. Prevalence, Antimicrobial Resistance and Toxin-Encoding Genes of Clostridioides difficile from Environmental Sources Contaminated by Feces. Antibiotics 2023, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Lagos-Moraga, S.; Calderón-Romero, P.; Pizarro-Guajardo, M.; Paredes-Sabja, D. Updates on Clostridium difficile spore biology. Anaerobe 2017, 45, 3–9. [Google Scholar] [CrossRef]
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D’Ambrosio, L.; van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB—Latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022, 124 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef]
- Takawira, F.T.; Mandishora, R.S.D.; Dhlamini, Z.; Munemo, E.; Stray-Pedersen, B. Mutations in rpoB and katG genes of multidrug resistant mycobacterium tuberculosis undetectable using genotyping diagnostic methods. Pan Afr. Med. J. 2017, 27, 145. [Google Scholar] [CrossRef]
- Giovagnorio, F.; De Vito, A.; Madeddu, G.; Parisi, S.G.; Geremia, N. Resistance in Pseudomonas aeruginosa: A Narrative Review of Antibiogram Interpretation and Emerging Treatments. Antibiotics 2023, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Dela Ahator, S.; Liu, Y.; Wang, J.; Zhang, L.H. The virulence factor regulator and quorum sensing regulate the type I-F CRISPR-Cas mediated horizontal gene transfer in Pseudomonas aeruginosa. Front. Microbiol. 2022, 13, 987656. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Colquhoun, J.M.; Farokhyfar, M.; Hutcheson, A.R.; Anderson, A.; Bethel, C.R.; Bonomo, R.A.; Clarke, A.J.; Rather, P.N. OXA-23 β-Lactamase Overexpression in Acinetobacter baumannii Drives Physiological Changes Resulting in New Genetic Vulnerabilities. mBio 2021, 12, e0313721. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Insights on the Horizontal Gene Transfer of Carbapenemase Determinants in the Opportunistic Pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct Target Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Lee, K.A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Tawam, D.; Baladi, M.; Jungsuwadee, P.; Earl, G.; Han, J. The Positive Association between Proton Pump Inhibitors and Clostridium Difficile Infection. Innov. Pharm. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Gemikonakli, G.; Mach, J.; Hilmer, S.N. Interactions Between the Aging Gut Microbiome and Common Geriatric Giants: Polypharmacy, Frailty, and Dementia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1019–1028. [Google Scholar] [CrossRef]
- Sachdev, C.; Anjankar, A.; Agrawal, J. Self-Medication With Antibiotics: An Element Increasing Resistance. Cureus 2022, 14, e30844. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Vamvakou, G.; Verykios, V.S.; Polymeris, A.; Nikolaou, M. Testosterone therapy for functional hypogonadism in middle-aged and elderly males: Current evidence and future perspectives. Hormones 2024, preprint. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Sinha, P.; Guerrant, R.L. The Costly Vicious Cycle of Infections and Malnutrition. J. Infect. Dis. 2024, 229, 1611–1613. [Google Scholar] [CrossRef]
- Theodorakis, N.; Nikolaou, M.; Hitas, C.; Anagnostou, D.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Triantafylli, G.; Metheniti, P.; Papaconstantinou, I. Comprehensive Peri-Operative Risk Assessment and Management of Geriatric Patients. Diagnostics 2024, 14, 2153. [Google Scholar] [CrossRef]
- Kumar, N.R.; Balraj, T.A.; Shivashankar, K.K.; Jayaram, T.C.; Prashant, A. Inflammaging in Multidrug-Resistant Sepsis of Geriatric ICU Patients and Healthcare Challenges. Geriatrics 2024, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Binsuwaidan, R.; Khan, M.A.; Alzahrani, R.H.; Aldusaymani, A.M.; Almallouhi, N.M.; Alsabti, A.S.; Ali, S.; Khan, O.S.; Youssef, A.M.; Alnajjar, L.I. Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital. Antibiotics 2023, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, T.; Li, Y.; Luo, L.; Li, Z.; Zhao, Q. The pulmonary infection risk factors in long-term bedridden patients: A meta-analysis. Am. J. Transl. Res. 2021, 13, 11014–11025. [Google Scholar] [PubMed]
- Zhu, C.; Liu, H.; Wang, Y.; Jiao, J.; Li, Z.; Cao, J.; Song, B.; Jin, J.; Liu, Y.; Wen, X.; et al. Prevalence, incidence, and risk factors of urinary tract infection among immobile inpatients in China: A prospective, multi-centre study. J. Hosp. Infect. 2020, 104, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Shaffer, M.L.; Loeb, M.B.; Givens, J.L.; Habtemariam, D.; Kiely, D.K.; D’Agata, E. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern. Med. 2014, 174, 1660–1667. [Google Scholar] [CrossRef]
- Hofman, M.R.; van den Hanenberg, F.; Sierevelt, I.N.; Tulner, C.R. Elderly patients with an atypical presentation of illness in the emergency department. Neth. J. Med. 2017, 75, 241–246. [Google Scholar]
- Falcone, M.; Meier, J.J.; Marini, M.G.; Caccialanza, R.; Aguado, J.M.; Del Prato, S.; Menichetti, F. Diabetes and acute bacterial skin and skin structure infections. Diabetes Res. Clin. Pract. 2021, 174, 108732. [Google Scholar] [CrossRef]
- Su, G.; Xu, H.; Riggi, E.; He, Z.; Lu, L.; Lindholm, B.; Marrone, G.; Wen, Z.; Liu, X.; Johnson, D.W.; et al. Association of Kidney Function with Infections by Multidrug-Resistant Organisms: An Electronic Medical Record Analysis. Sci. Rep. 2018, 8, 13372. [Google Scholar] [CrossRef]
- Pailhoriès, H.; Herrmann, J.L.; Velo-Suarez, L.; Lamoureux, C.; Beauruelle, C.; Burgel, P.R.; Héry-Arnaud, G. Antibiotic resistance in chronic respiratory diseases: From susceptibility testing to the resistome. Eur. Respir. Rev. 2022, 31, 210259. [Google Scholar] [CrossRef]
- Mesquita, E.T. Infections in Heart Failure—Impact on Mortality. Arq. Bras. Cardiol. 2018, 110, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hong, Y.; Hong, J.Y. Risk for multidrug-resistant tuberculosis in patients treated with anti-tumor necrosis factor agents. Front. Med. 2023, 10, 1108119. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Ye, W. Prevention and control of hospital-acquired infections with multidrug-resistant organism: A review. Medicine 2024, 103, e37018. [Google Scholar] [CrossRef] [PubMed]
- Spigaglia, P.; Mastrantonio, P.; Barbanti, F. Antibiotic Resistances of Clostridium difficile. Adv. Exp. Med. Biol. 2018, 1050, 137–159. [Google Scholar] [PubMed]
- Piddock, L.J. Teixobactin, the first of a new class of antibiotics discovered by iChip technology? J. Antimicrob. Chemother. 2015, 70, 2679–2680. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, N.; Wei, W.; Jiang, C. Outcomes and Nephrotoxicity Associated with Vancomycin Treatment in Patients 80 Years and Older. Clin. Interv. Aging 2021, 16, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Sollima, A.; Rossini, F.; Lanza, P.; Pallotto, C.; Meschiari, M.; Gentile, I.; Stellini, R.; Lenzi, A.; Mulé, A.; Castagna, F.; et al. Role of Cefiderocol in Multidrug-Resistant Gram-Negative Central Nervous System Infections: Real Life Experience and State-of-the-Art. Antibiotics 2024, 13, 453. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef]
- Gu, H.; Liu, D.; Zeng, X.; Peng, L.S.; Yuan, Y.; Chen, Z.F.; Zou, Q.M.; Shi, Y. Aging exacerbates mortality of Acinetobacter baumannii pneumonia and reduces the efficacies of antibiotics and vaccine. Aging 2018, 10, 1597–1608. [Google Scholar] [CrossRef]
- Zampaloni, C.; Mattei, P.; Bleicher, K.; Winther, L.; Thäte, C.; Bucher, C.; Adam, J.M.; Alanine, A.; Amrein, K.E.; Baidin, V.; et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 2024, 625, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Topola, E. Meropenem/Vaborbactam: β-Lactam/β-Lactamase Inhibitor Combination, the Future in Eradicating Multidrug Resistance. Antibiotics 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jean, S.S.; Lee, Y.L.; Lu, M.C.; Ko, W.C.; Liu, P.Y.; Hsueh, P.R. Carbapenem-Resistant Enterobacterales in Long-Term Care Facilities: A Global and Narrative Review. Front. Cell. Infect. Microbiol. 2021, 11, 601968. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Burgess, D.S. Plazomicin: A new aminoglycoside in the fight against antimicrobial resistance. Ther. Adv. Infect. Dis. 2020, 7, 2049936120952604. [Google Scholar] [CrossRef] [PubMed]
- Lizza, B.D.; Betthauser, K.D.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. New Perspectives on Antimicrobial Agents: Ceftolozane-Tazobactam. Antimicrob. Agents Chemother. 2021, 65, e0231820. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef]
- Ku, N.S.; Kim, Y.C.; Kim, M.H.; Song, J.E.; Oh, D.H.; Ahn, J.Y.; Kim, S.B.; Kim, H.W.; Jeong, S.J.; Han, S.H.; et al. Risk factors for 28-day mortality in elderly patients with extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae bacteremia. Arch. Gerontol. Geriatr. 2014, 58, 105–109. [Google Scholar] [CrossRef]
- Soraci, L.; Cherubini, A.; Paoletti, L.; Filippelli, G.; Luciani, F.; Laganà, P.; Gambuzza, M.E.; Filicetti, E.; Corsonello, A.; Lattanzio, F. Safety and Tolerability of Antimicrobial Agents in the Older Patient. Drugs Aging 2023, 40, 499–526. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.E.; Nicolau, D.P. Clostridium difficile infection in the elderly: An update on management. Clin. Interv. Aging 2017, 12, 1799–1809. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum sensing inhibitors as Therapeutics: Bacterial biofilm inhibition. Bioorg. Chem. 2023, 136, 106551. [Google Scholar] [CrossRef]
- Wijit, K.; Sonthisombat, P.; Diewsurin, J. A score to predict Pseudomonas aeruginosa infection in older patients with community-acquired pneumonia. BMC Infect. Dis. 2023, 23, 700. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Komiya, K.; Umeki, K.; Hiramatsu, K.; Kadota, J.I. Impact of Pseudomonas aeruginosa coverage on the prognosis of elderly patients with community-acquired pneumonia. J. Infect. Chemother. 2023, 29, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Jump, R.L. Clostridium difficile infection in older adults. Aging Health 2013, 9, 403–414. [Google Scholar] [CrossRef] [PubMed]
- François, B.; Mercier, E.; Gonzalez, C.; Asehnoune, K.; Nseir, S.; Fiancette, M.; Desachy, A.; Plantefève, G.; Meziani, F.; de Lame, P.A.; et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus. Intensive Care Med. 2018, 44, 1787–1796. [Google Scholar] [CrossRef]
- Reig, S.; Le Gouellec, A.; Bleves, S. What Is New in the Anti-Pseudomonas aeruginosa Clinical Development Pipeline Since the 2017 WHO Alert? Front. Cell. Infect. Microbiol. 2022, 12, 909731. [Google Scholar] [CrossRef]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2018, 63, e01110–e01118. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Feretzakis, G.; Tzelves, L.; Paxinou, E.; Hitas, C.; Vamvakou, G.; Verykios, V.S.; Nikolaou, M. Integrating Machine Learning with Multi-Omics Technologies in Geroscience: Towards Personalized Medicine. J. Pers. Med. 2024, 14, 931. [Google Scholar] [CrossRef]
- McFarland, L.V. Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics 2015, 4, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.L.L.; Albano, M.O.; Martins, C.D.S.; Warren, C.A.; Brito, G.A.C. Role of probiotics in preventing Clostridioides difficile infection in older adults: An integrative review. Front. Med. 2023, 10, 1219225. [Google Scholar] [CrossRef]
- Carstensen, J.W.; Chehri, M.; Schønning, K.; Rasmussen, S.C.; Anhøj, J.; Godtfredsen, N.S.; Andersen, C.Ø.; Petersen, A.M. Use of prophylactic Saccharomyces boulardii to prevent Clostridium difficile infection in hospitalized patients: A controlled prospective intervention study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Wombwell, E.; Patterson, M.E.; Bransteitter, B.; Gillen, L.R. The Effect of Saccharomyces boulardii Primary Prevention on Risk of Hospital-onset Clostridioides difficile Infection in Hospitalized Patients Administered Antibiotics Frequently Associated with C. difficile Infection. Clin. Infect. Dis. 2021, 73, e2512–e2518. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.K.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. eClinicalMedicine 2020, 29, 100642. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. npj Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Dey, J.; Mahapatra, S.R.; Singh, P.K.; Prabhuswamimath, S.C.; Misra, N.; Suar, M. Designing of multi-epitope peptide vaccine against Acinetobacter baumannii through combined immunoinformatics and protein interaction-based approaches. Immunol. Res. 2023, 71, 639–662. [Google Scholar] [CrossRef]
- Asadinezhad, M.; Khoshnood, S.; Asadollahi, P.; Ghafourian, S.; Sadeghifard, N.; Pakzad, I.; Zeinivand, Y.; Omidi, N.; Hematian, A.; Kalani, B.S. Development of innovative multi-epitope mRNA vaccine against Pseudomonas aeruginosa using in silico approaches. Brief. Bioinform. 2023, 25, bbad502. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.; Molaei, A.; Allahyari Fard, N. Multi-epitope vaccines, from design to expression; an in silico approach. Hum. Immunol. 2024, 85, 110804. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Pati, S.; Ray, R.R. Nanoparticles based antibacterial vaccines: Novel strategy to combat antimicrobial resistance. Process Biochem. 2022, 119, 82–89. [Google Scholar] [CrossRef]
- Manju, K.; Raj, S.N.; Ranjini, H.K.; Nayaka, S.C.; Ashwini, P.; Satish, S.; Prasad, M.N.N.; Chouhan, R.S.; Baker, S. Nanovaccines to combat drug resistance: The next-generation immunisation. Future J. Pharm. Sci. 2023, 9, 64. [Google Scholar] [CrossRef]
- Khanifar, J.; Hosseini, R.H.; Kazemi, R.; Ramandi, M.F.; Amani, J.; Salmanian, A.H. Prevention of EHEC infection by chitosan nano-structure coupled with synthetic recombinant antigen. J. Microbiol. Methods 2019, 157, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Galgani, I.; Vadivelu, V.K.; Phogat, S. Reverse development of vaccines against antimicrobial-resistant pathogens. npj Vaccines 2024, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Poh, C.L. The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria. Vaccines 2023, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Sebilleau, C.O.; Sucheck, S.J. Lipopeptide adjuvants for antibiotics and vaccines: The future step in the fight against multidrug-resistant and extensively drug-resistant pathogens. Explor. Drug Sci. 2024, 2, 203–233. [Google Scholar] [CrossRef]
- Alves, J.; Prendki, V.; Chedid, M.; Yahav, D.; Bosetti, D.; Rello, J.; ESCMID Study group of infections in the elderly (ESGIE). Challenges of antimicrobial stewardship among older adults. Eur. J. Intern. Med. 2024, 124, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.S.; Keller, S.C.; Crnich, C.J.; Jump, R.L.P.; Yoshikawa, T.T. Antibiotic Stewardship for Older Adults in Ambulatory Care Settings: Addressing an Unmet Challenge. J. Am. Geriatr. Soc. 2020, 68, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Mauro, J.; Kannangara, S.; Peterson, J.; Livert, D.; Tuma, R.A. Rigorous antibiotic stewardship in the hospitalized elderly population: Saving lives and decreasing cost of inpatient care. JAC Antimicrob. Resist. 2021, 3, dlab118. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Chen, H.; Li, N.; Wang, Y.; Li, Y.; Liang, W. Rapid Detection of blaKPC, blaNDM, blaOXA-48-like and blaIMP Carbapenemases in Enterobacterales Using Recombinase Polymerase Amplification Combined with Lateral Flow Strip. Front. Cell. Infect. Microbiol. 2021, 11, 772966. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Yeh, E.; Perry, S.; Luo, R.F.; Pinsky, B.A.; Lee, B.P.; Sisodiya, D.; Baron, E.J.; Banaei, N. Real-time PCR testing for mecA reduces vancomycin usage and length of hospitalization for patients infected with methicillin-sensitive staphylococci. J. Clin. Microbiol. 2010, 48, 785–790. [Google Scholar] [CrossRef]
- Schwab, T.C.; Perrig, L.; Göller, P.C.; Guebely De la Hoz, F.F.; Lahousse, A.P.; Minder, B.; Günther, G.; Efthimiou, O.; Omar, S.V.; Egger, M.; et al. Targeted next-generation sequencing to diagnose drug-resistant tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2024, 24, 1162–1176. [Google Scholar] [CrossRef]
- Chen, X.F.; Hou, X.; Xiao, M.; Zhang, L.; Cheng, J.W.; Zhou, M.L.; Huang, J.J.; Zhang, J.J.; Xu, Y.C.; Hsueh, P.R. Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) Analysis for the Identification of Pathogenic Microorganisms: A Review. Microorganisms 2021, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.C.; de Aguiar, R.S.; Jardim, W.M.; Melo, L.H.; Mendonça, T.; Cavalcanti, A.B.; de França, P.H.C. Cohorting to prevent acquisition of multidrug-resistant bacteria: An interrupted time series study. Am. J. Infect. Control 2019, 47, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hedhoud, Y.; Mekhaznia, T.; Amroune, M. An improvement of the CNN-XGboost model for pneumonia disease classification. Pol. J. Radiol. 2023, 88, e483–e493. [Google Scholar] [CrossRef] [PubMed]
- Al Meslamani, A.Z.; Sobrino, I.; de la Fuente, J. Machine learning in infectious diseases: Potential applications and limitations. Ann. Med. 2024, 56, 2362869. [Google Scholar] [CrossRef] [PubMed]
- Pinto-de-Sá, R.; Sousa-Pinto, B.; Costa-de-Oliveira, S. Brave New World of Artificial Intelligence: Its Use in Antimicrobial Stewardship—A Systematic Review. Antibiotics 2024, 13, 307. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Marelli, C.; Guastavino, S.; Mora, S.; Rosso, N.; Signori, A.; Campi, C.; Giacomini, M.; Bassetti, M. Explainable and Interpretable Machine Learning for Antimicrobial Stewardship: Opportunities and Challenges. Clin. Ther. 2024, 46, 474–480. [Google Scholar] [CrossRef]
- Sakagianni, A.; Koufopoulou, C.; Feretzakis, G.; Kalles, D.; Verykios, V.S.; Myrianthefs, P.; Fildisis, G. Using Machine Learning to Predict Antimicrobial Resistance—A Literature Review. Antibiotics 2023, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; Kalles, D.; Martsoukou, M.; Lada, M.; Skarmoutsou, N.; Christopoulos, C.; Valakis, K.; Velentza, A.; et al. Using Machine Learning Techniques to Aid Empirical Antibiotic Therapy Decisions in the Intensive Care Unit of a General Hospital in Greece. Antibiotics 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Feretzakis, G.; Sakagianni, A.; Loupelis, E.; Kalles, D.; Skarmoutsou, N.; Martsoukou, M.; Christopoulos, C.; Lada, M.; Petropoulou, S.; Velentza, A.; et al. Machine Learning for Antibiotic Resistance Prediction: A Prototype Using Off-the-Shelf Techniques and Entry-Level Data to Guide Empiric Antimicrobial Therapy. Healthc. Inform. Res. 2021, 27, 214–221. [Google Scholar] [CrossRef]
- Olawade, D.B.; Teke, J.; Fapohunda, O.; Weerasinghe, K.; Usman, S.O.; Ige, A.O.; Clement David-Olawade, A. Leveraging artificial intelligence in vaccine development: A narrative review. J. Microbiol. Methods 2024, 224, 106998. [Google Scholar] [CrossRef]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a structural class of antibiotics with explainable deep learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef] [PubMed]
| MDR Bacteria | Major Mechanisms of Resistance | Common Infections | References |
|---|---|---|---|
| MRSA |
| Skin, respiratory, and bloodstream infections, infective endocarditis. Particularly in hospital/long-term care settings. | [11,12,13] |
| VRE |
| Urinary tract, bloodstream and wound infections, infective endocarditis. Particularly in hospital/long-term care settings. | [14,15,16] |
| S. pneumoniae |
| Pneumonia and meningitis. | [17,18,19] |
| Enterobacteriaceae |
| Urinary tract, respiratory, bloodstream and wound infections. Particularly in hospital/long-term care settings. | [20,21,22,23] |
| C. difficile |
| Healthcare-associated diarrhea/pseudomembranous colitis. Often following prolonged antibiotic use. | [24,25] |
| MDR/XDR M. tuberculosis |
| Tuberculosis. Particularly in immunocompromised or elderly patients. | [26,27] |
| P. aeruginosa |
| Respiratory (e.g., ventilator-associated pneumonia), urinary tract, bloodstream, and wound infections. Particularly in hospital/long-term care settings. | [28,29,30] |
| A. baumannii |
| Respiratory (e.g., ventilator-associated pneumonia), urinary tract, bloodstream, and wound infections. Particularly in the intensive care unit. | [31,32,33,34] |
| Risk Factors | Description | References |
|---|---|---|
| Immunosenescence |
| [35,36] |
| Polypharmacy |
| [37,38,39] |
| Sarcopenia and Malnutrition |
| [40,41,42] |
| Frailty and Decreased Mobility |
| [43,44,45,46,47] |
| Cognitive Impairment |
| [48,49] |
| Multimorbidity |
| [50,51,52,53,54] |
| Frequent Hospitalizations and LTCF Residency |
| [55,56] |
| Antibiotic | Key MDRO Targets | Limitations | References |
|---|---|---|---|
| Teixobactin |
|
| [57,58] |
| Cefiderocol |
|
| [59,60] |
| Plazomicin |
|
| [65] |
| Ceftolozane/Tazobactam |
|
| [66] |
| Ceftazidime/Avibactam |
|
| [67] |
| Meropenem/Vaborbactam |
|
| [63] |
| Murepavadin |
|
| [62] |
| Strategies | Description | References |
|---|---|---|
| Novel antibiotics |
| [57,58,59,60,61,62,63,64,65,66,67,68] |
| Bacteriophage therapy |
| [69,70,71] |
| Antivirulence therapies |
| [72,73,74,75,76,77,78] |
| Probiotics and fecal microbiota transplantation |
| [79,80,81,82,83,84] |
| Vaccine development |
| [85,86,87,88,89,90,91,92,93,94,95,96] |
| Antimicrobial stewardship programs |
| [86,97,98] |
| Advanced diagnostic techniques |
| [99,100,101,102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Boufeas, I.Z.; Sakagianni, A.; Paxinou, E.; Verykios, V.S.; et al. Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms 2024, 12, 1978. https://doi.org/10.3390/microorganisms12101978
Theodorakis N, Feretzakis G, Hitas C, Kreouzi M, Kalantzi S, Spyridaki A, Boufeas IZ, Sakagianni A, Paxinou E, Verykios VS, et al. Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms. 2024; 12(10):1978. https://doi.org/10.3390/microorganisms12101978
Chicago/Turabian StyleTheodorakis, Nikolaos, Georgios Feretzakis, Christos Hitas, Magdalini Kreouzi, Sofia Kalantzi, Aikaterini Spyridaki, Iris Zoe Boufeas, Aikaterini Sakagianni, Evgenia Paxinou, Vassilios S. Verykios, and et al. 2024. "Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions" Microorganisms 12, no. 10: 1978. https://doi.org/10.3390/microorganisms12101978
APA StyleTheodorakis, N., Feretzakis, G., Hitas, C., Kreouzi, M., Kalantzi, S., Spyridaki, A., Boufeas, I. Z., Sakagianni, A., Paxinou, E., Verykios, V. S., & Nikolaou, M. (2024). Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms, 12(10), 1978. https://doi.org/10.3390/microorganisms12101978









