Cyanobacterial Blooms in City Parks: A Case Study Using Zebrafish Embryos for Toxicity Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
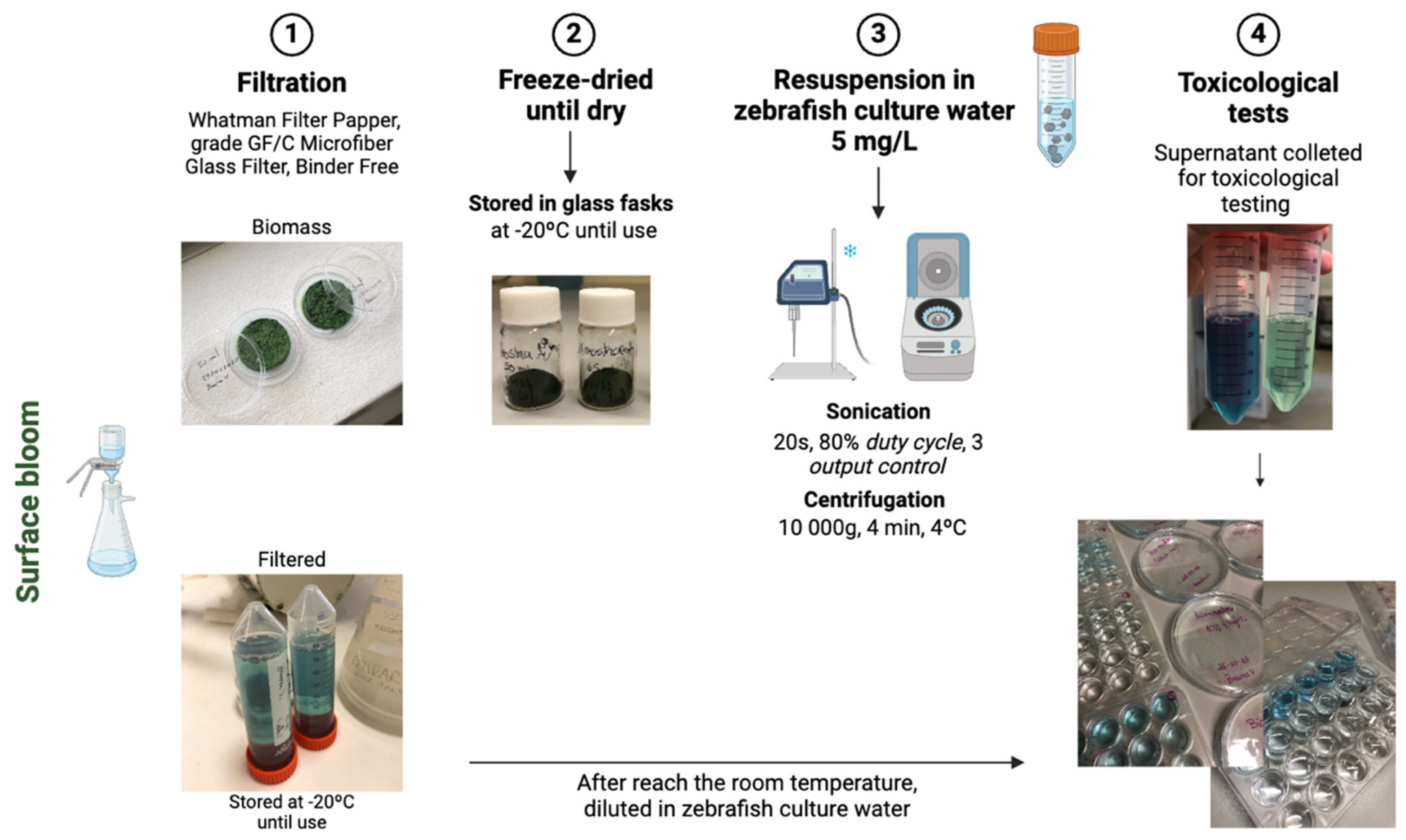
2.2. Preparation of the Extracts
2.3. Quantification
2.4. Test Organisms
2.5. Ecotoxicity Tests
2.6. Biochemical Analysis
2.7. Data Analyses
3. Results
3.1. Cyanobacteria Extract—Site 1 and Site 2
3.1.1. Mortality and Hatching
3.1.2. Development Delay
3.1.3. Locomotor Behavior
3.1.4. Biochemical Determinations
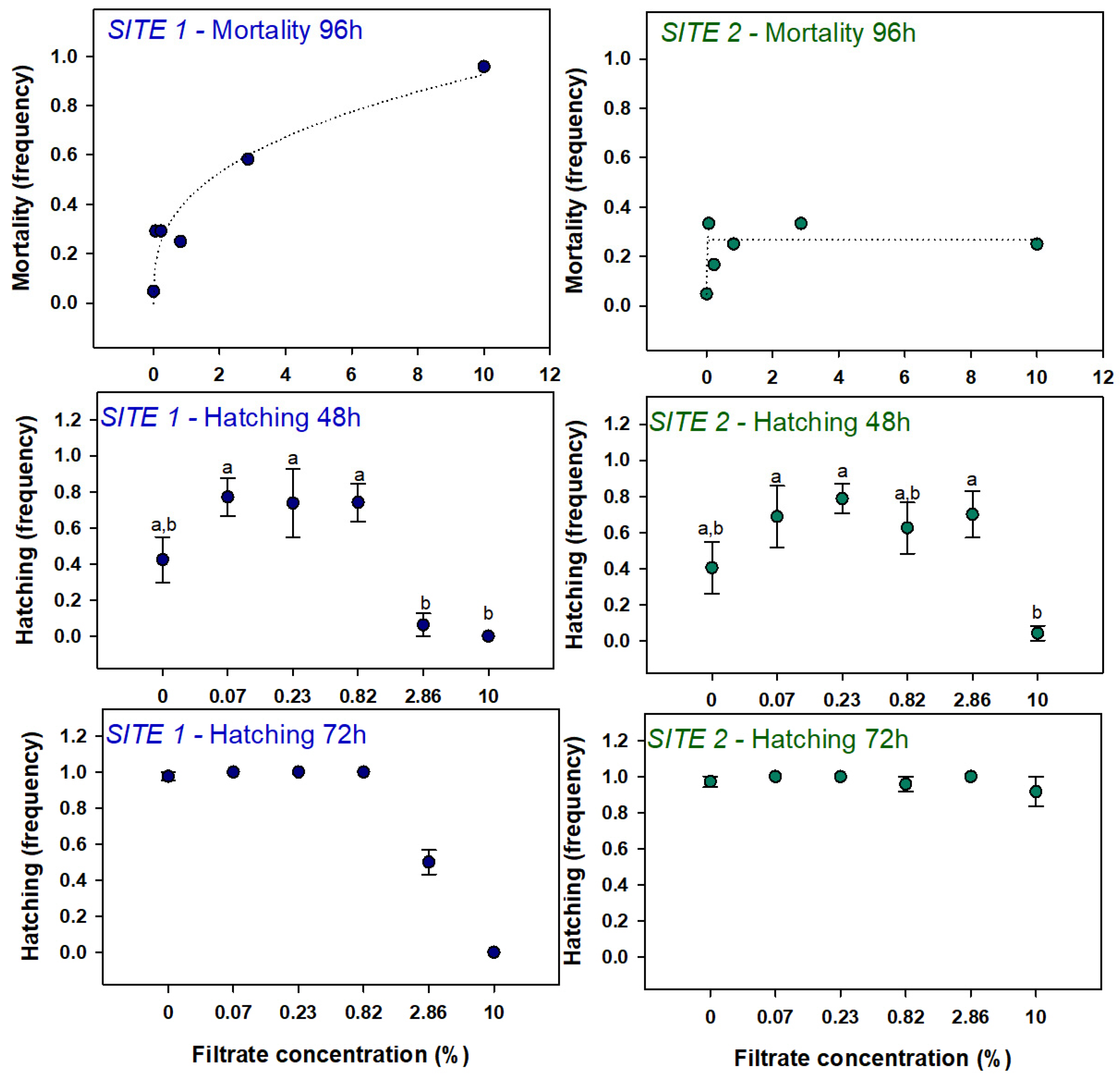
3.2. Filtrates—Site 1 and Site 2
3.2.1. Mortality and Hatching
3.2.2. Locomotor Behavior
3.2.3. Biochemical Determinations
3.3. Water Column—Site 1 and Site 2
4. Discussion
4.1. Toxicity of Cyanobacteria Extract
4.2. Filtrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Res. Found. 2015, 6, 1254. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P.; Villafañe, V.E.; Helbling, E.W. Productivity of aquatic primary producers under global climate change. R. Soc. Chem. 2014, 13, 1370–1392. [Google Scholar] [CrossRef] [PubMed]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- El-Shehawy, R.; Gorokhova, E.; Fernández-Piñas, F.; del Campo, F.F. Global warming and hepatotoxin production by cyanobacteria: What can we learn from experiments? Water Res. 2012, 46, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Kanoshina, I.; Lips, U.; Leppänen, J.M. The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2003, 2, 29–41. [Google Scholar] [CrossRef]
- Mowe, M.A.D.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2015, 74, 205–224. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. Long-term monitoring reveals carbon-nitrogen metabolism key to microcystin production in eutrophic lakes. Front. Microbiol. 2015, 6, 456. [Google Scholar] [CrossRef]
- Davidson, K.; Gowen, R.J.; Harrison, P.J.; Fleming, L.E.; Hoagland, P.; Moschonas, G. Anthropogenic nutrients and harmful algae in coastal waters. J. Environ. Manag. 2014, 146, 206–216. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef]
- Smith, V.H.; Joye, S.B.; Howarth, R.W. Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 2006, 51 Pt 2, 351–355. [Google Scholar] [CrossRef]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.M.; Parsons, M.L.; Rensel, J.E.J.; Townsend, D.W.; et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Trevino-Garrison, I.; DeMent, J.; Ahmed, F.S.; Haines-Lieber, P.; Langer, T.; Ménager, H.; Neff, J.; Van der Merwe, D.; Carney, E. Human illnesses and animal deaths associated with freshwater harmful algal blooms—Kansas. Toxins 2015, 7, 353–366. [Google Scholar] [CrossRef]
- Pham, T.L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Waajen, G.W.A.M.; Faassen, E.J.; Lürling, M. Eutrophic urban ponds suffer from cyanobacterial blooms: Dutch examples. Environ. Sci. Pollut. Res. 2014, 21, 9983–9994. [Google Scholar] [CrossRef]
- Qin, X.; Xia, W.; Hu, X.; Shao, Z. Dynamic variations of cyanobacterial blooms and their response to urban development and climate change in Lake Chaohu based on Landsat observations. Environ. Sci. Pollut. Res. 2022, 29, 33152–33166. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. Brookes. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Leal Filho, W.; Barbir, J.; Sima, M.; Kalbus, A.; Nagy, G.J.; Paletta, A.; Villamizar, A.; Martinez, R.; Azeiteiro, U.M.; Pereira, M.J.; et al. Reviewing the role of ecosystems services in the sustainability of the urban environment: A multi-country analysis. J. Clean. Prod. 2020, 262, 121338. [Google Scholar] [CrossRef]
- Yan, H.; Wu, F.; Dong, L. Influence of a large urban park on the local urban thermal environment. Sci. Total Environ. 2018, 622–623, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.V.; Handley, P.; Morison, J.I.; Doick, K.J. The Role of Urban Trees and Greenspaces in Reducing Urban Air Temperatures. 2019. Available online: https://www.researchgate.net/publication/332902468 (accessed on 20 February 2024).
- Wan, C.; Shen, G.Q.; Choi, S. Effects of physical and psychological factors on users’ attitudes, use patterns, and perceived benefits toward urban parks. Urban. For. Urban. Green. 2020, 51, 126691. [Google Scholar] [CrossRef]
- Lucini, C.; D’angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, brain, and regeneration: Insights from zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef]
- Lau, B.Y.B.; Mathur, P.; Gould, G.G.; Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 2581–2586. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef]
- Norton, W. Towards developmental models of psychiatric disorders in zebrafish. Front. Neural Circuits 2013, 7, 79. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokariota—1. Teil Choococcales. In Süßwasserflora von Mitteleuropa. Begründet von A. Pascher. Band 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Spektrum, Akademischer Verlag: Heidelberg/Berlin, Germnay, 1999. [Google Scholar]
- Acs, A.; Kovacs, A.W.; Csepregi, J.Z.; Törő, N.; Kiss, G.; Győri, J.; Vehovszky, Á.; Kováts, N.; Farkas, A. The ecotoxicological evaluation of Cylindrospermopsis raciborskii from Lake Balaton (Hungary) employing a battery of bioassays and chemical screening. Toxicon 2013, 70, 98–106. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2013. [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.M.; de Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A. Methods of Enzymatic Analysis, Enzymes: Oxirreductases Transferases; Verlag Chemie: Basel, Switzerland, 1983; Volume III. [Google Scholar]
- Clairborne, A. CRC Handbook of Methods in Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and zebrafish: Old assays to address a current issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Quintaneiro, C.; Gagliardi, M.; Capone, L.; Domingues, I. Des embrionario. Captar 2022, 11. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Gantar, M.; Mayer, G.D.; Gibbs, P.D.L.; Berry, J.P. Effects of Cyanobacterial Lipopolysaccharides from Microcystis on Glutathione-Based Detoxification Pathways in the Zebrafish (Danio rerio) Embryo. Toxins 2012, 4, 390–404. [Google Scholar] [CrossRef]
- Tzima, E.; Serifi, I.; Tsikari, I.; Alzualde, A.; Leonardos, I.; Papamarcaki, T. Transcriptional and behavioral responses of zebrafish larvae to microcystin-LR exposure. Int. J. Mol. Sci. 2017, 18, 365. [Google Scholar] [CrossRef]
- Wang, P.J.; Chien, M.S.; Wu, F.J.; Chou, H.N.; Lee, S.J. Inhibition of embryonic development by microcystin-LR in zebrafish, Danio rerio. Toxicon 2005, 45, 303–308. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Wu, Y.; Shao, D.; Ye, B.; Zhang, Y.; Liu, J.; Wang, J.; Jia, X. Mitochondrial and endoplasmic reticulum pathways involved in microcystin-LR-induced apoptosis of the testes of male frog (Rana nigromaculata) in vivo. J. Hazard. Mater. 2013, 252–253, 382–389. [Google Scholar] [CrossRef]
- Kist, L.W.; Piato, A.L.; da Rosa, J.G.; Koakoski, G.; Barcellos, L.J.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Acute exposure to microcystin-producing cyanobacterium microcystis aeruginosa alters adult zebrafish (Danio rerio) swimming performance parameters. J. Toxicol. 2011, 2011, 280304. [Google Scholar] [CrossRef]
- Wei, H.; Wang, S.; Xu, E.G.; Liu, J.; Li, X.; Wang, Z. Synergistic toxicity of microcystin-LR and Cu to zebrafish (Danio rerio). Sci. Total Environ. 2020, 713, 136393. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.; Xie, P.; Jiang, Y.; Wu, L.; Zhang, X. Protein expression profiling in the zebrafish (Danio rerio) embryos exposed to the microcystin-LR. Proteomics 2011, 11, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Oberemm, A.; Fastner, J.; Steinberg, C.E.W. Research Note Effects Of Microcystin-Lr And Cyanobacterial Crude Extracts On Embryo-Larval Development Of Zebrafish (Danio Rerio). Water Res. 1997, 31, 2918–2921. [Google Scholar] [CrossRef]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. 1999, 14, 77–88. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Gong, Z.; Balasubramanian, R. Toxicological implications of microcystins for zebrafish embryos in the presence of other environmental pollutants. Environ. Toxicol. Chem. 2013, 32, 1574–1581. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, W.; Gu, Y.; Xie, L.; Jiang, W.; Gao, Y.; Yang, L. Synergetic enhancement toxicity of copper, cadmium and microcystin-LR to the Ceratophyllum demersum L. Toxicon 2020, 186, 151–159. [Google Scholar] [CrossRef]
- Jacquet, C.; Thermes, V.; de Luze, A.; Puiseux-Dao, S.; Bernard, C.; Joly, J.S.; Bourrat, F.; Edery, M. Effects of microcystin-LR on development of medaka fish embryos (Oryzias latipes). Toxicon 2004, 43, 141–147. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, W.; Liu, C.; Li, L.; Yu, L.; Zhao, S.; Li, G. Microcystin-LR exposure induces developmental neurotoxicity in zebrafish embryo. Environ. Pollut. 2016, 213, 793–800. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Phenotypic Plasticity and the Origins of Diversity. Annu. Rev. Ecol. Syst. 1989, 20, 249–278. [Google Scholar] [CrossRef]
- Honkanen, R.E.; Zwiller JE, M.R.; Moore, R.E.; Daily, S.L.; Khatra, B.S.; Dukelow, M.; Boynton, A.L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J. Biol. Chem. 1990, 265, 19401–19404. [Google Scholar] [CrossRef]
- Rao, J.; Begum, G.; Pallela, R.; Usman, P.; Rao, R. Changes in Behavior and Brain Acetylcholinesterase Activity in Mosquito Fish, Gambusia affinis in Response to the Sub-Lethal Exposure to Chlorpyrifos. Int. J. Environ. Res. Public. Health 2005, 2, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Anras, M.-L.B.; Lagardère, J.P. Measuring cultured fish swimming behaviour: First results on rainbow trout using acoustic telemetry in tanks. Aquaculture 2004, 240, 175–186. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. 2016, 23, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Kist, L.W.; Rosemberg, D.B.; Pereira TC, B.; De Azevedo, M.B.; Richetti, S.K.; de Castro Leão, J.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Microcystin-LR acute exposure increases AChE activity via transcriptional ache activation in zebrafish (Danio rerio) brain. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2012, 155, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Emran, F.; Rihel, J.; Dowling, J.E. A behavioral assay to measure responsiveness of Zebrafish to changes in light intensities. J. Vis. Exp. 2008, 20, e923. [Google Scholar] [CrossRef]
- Ahmad, F.; Richardson, M.K.; Noldus, L.P.J.J.; Tegelenbosch, R.A.J. Zebrafish embryos and larvae in behavioural assays. Behaviour 2012, 149, 1241–1281. [Google Scholar]
- Qian, H.; Liu, G.; Lu, T.; Sun, L. Developmental neurotoxicity of Microcystis aeruginosa in the early life stages of zebrafish. Ecotoxicol. Environ. Saf. 2018, 151, 35–41. [Google Scholar] [CrossRef]
- Lema, S.C.; Schultz, I.R.; Scholz, N.L.; Incardona, J.P.; Swanson, P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE 47). Aquat. Toxicol. 2007, 82, 296–307. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Huang, C.; Hu, B.; Hu, C.; Zhou, B. Acute exposure to DE-71 causes alterations in visual behavior in zebrafish larvae. Environ. Toxicol. Chem. 2013, 32, 1370–1375. [Google Scholar] [CrossRef]
- Pípal, M.; Legradi, J.; Smutná, M.; Kočí, T.; Priebojová, J.; Bláhová, L.; Krauss, M.; Hilscherová, K. Neurobehavioral effects of cyanobacterial biomass field extracts on zebrafish embryos and potential role of retinoids. Aquat. Toxicol. 2020, 228, 105613. [Google Scholar] [CrossRef]
- Paskerová, H.; Hilscherová, K.; Bláha, L. Oxidative stress and detoxification biomarker responses in aquatic freshwater vertebrates exposed to microcystins and cyanobacterial biomass. Environ. Sci. Pollut. Res. 2012, 19, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, S.-F.; Cao, Y.-F. Oxidative stress response in zebrafish (Danio rerio) gill experimentally exposed to subchronic microcystin-LR. Environ. Monit. Assess. 2012, 184, 6775–6787. [Google Scholar] [CrossRef]
- Prieto, A.; Jos, A.; Pichardo, S.; Moreno, I.; Camean, A. Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat. Toxicol. 2006, 77, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Almeida, A.R.; Tacão, M.; Machado, A.L.; Golovko, O.; Zlabek, V.; Domingues, I.; Henriques, I. Long-term effects of oxytetracycline exposure in zebrafish: A multi-level perspective. Chemosphere 2019, 222, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.C.; Printes, L.B.; Rocha, O. Use of cholinesterase activity as an ecotoxicological marker to assess anatoxin-a(s) exposure: Responses of two cladoceran species belonging to contrasting geographical regions. Harmful Algae 2016, 55, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. 2006, 25, 72–86. [Google Scholar] [CrossRef]
- Vajková, V.; Navrátil, S.; Palıkóva, M. The Effect of Intraperitoneall Y Applied Pure Microcystin LR on Haematological, Biochemical and Morphological Indices of Silver Carp (Hypophthalmichthys molitrix Val.). Acta Vet. Brno 1998, 67, 281–287. [Google Scholar] [CrossRef]
- Sigee, D.C.; Selwyn, A.; Gallois, P.; Dean, A.P. Patterns of cell death in freshwater colonial cyanobacteria during the late summer bloom. Phycologia 2007, 46, 284–292. [Google Scholar] [CrossRef]
- Rashidan, K.K.; Bird, D.F. Role of predatory bacteria in the termination of a cyanobacterial bloom. Microb. Ecol. 2001, 41, 97–105. [Google Scholar] [CrossRef]
- Suttle, C.A. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 1994, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, B.; Amaral, J.; Pereira, M.J.; Domingues, I. Cyanobacterial Blooms in City Parks: A Case Study Using Zebrafish Embryos for Toxicity Characterization. Microorganisms 2024, 12, 2003. https://doi.org/10.3390/microorganisms12102003
Vieira B, Amaral J, Pereira MJ, Domingues I. Cyanobacterial Blooms in City Parks: A Case Study Using Zebrafish Embryos for Toxicity Characterization. Microorganisms. 2024; 12(10):2003. https://doi.org/10.3390/microorganisms12102003
Chicago/Turabian StyleVieira, Bruna, João Amaral, Mário Jorge Pereira, and Inês Domingues. 2024. "Cyanobacterial Blooms in City Parks: A Case Study Using Zebrafish Embryos for Toxicity Characterization" Microorganisms 12, no. 10: 2003. https://doi.org/10.3390/microorganisms12102003
APA StyleVieira, B., Amaral, J., Pereira, M. J., & Domingues, I. (2024). Cyanobacterial Blooms in City Parks: A Case Study Using Zebrafish Embryos for Toxicity Characterization. Microorganisms, 12(10), 2003. https://doi.org/10.3390/microorganisms12102003







