Abstract
This study is a continuation of research on sustainable food packaging materials made from locally available feedstock and industrial by-products within the Baltic Sea region. Its main focus is the impact of wheat bran filler and Saccharomyces cerevisiae additive, which was used to develop a novel bio-coating for paper composite packaging, on the biodegradation efficiency of paper composites under aerobic and anaerobic conditions. In this study, we analyzed the effect of 15% and 40% concentrations of wheat bran filler and Saccharomyces cerevisiae biomass on the biodegradation efficiency of paper composites. This research was conducted under controlled environmental conditions, with aerobic biodegradation tested at 46 °C in a compost-based mesophilic–thermophilic environment and anaerobic biodegradation tested at 55 °C in an active inoculum thermophilic environment. The results show that the presence of wheat bran filler significantly improves biodegradation efficiency compared to microcrystalline cellulose reference material. Under aerobic conditions, the biodegradation efficiency for the 40% wheat bran and yeast sample was 6.34%, compared to only 0.71% for the cellulose reference material. In anaerobic conditions, the 15% wheat bran and yeast sample showed a biodegradation efficiency of 96.62%, compared to 82.32% for the cellulose reference material.
1. Introduction
This study builds upon prior research aimed at developing fully green food packaging material sourced from locally available feedstock and industrial by-products within the Baltic Sea region. Previous stages of research have identified the benefits of bio-based and biodegradable fast food packaging for the circular economy [1], analyzed locally available sustainable feedstock materials [2], and tested material samples for physical–mechanical- and barrier-related properties [3]. The findings from previous studies highlight several key points, as follows:
- (a)
- Biodegradable food packaging offers significant benefits to the circular economy by purifying waste streams, diverting food waste from landfills, and providing sustainable alternatives for non-recyclable packaging [4]. This approach facilitates the return of biomaterials to the soil, thereby enhancing biological recycling benefits [5,6,7,8].
- (b)
- The utilization of plant-based feedstocks, particularly those sourced from industrial waste or by-products, highlights the importance of renewable resources with lower environmental impacts [2].
- (c)
- The application of paper (both wood and non-wood fiber) in fully green food packaging remains limited due to stringent barrier property requirements. The natural components of yeast, such as proteins and glucans, have unique characteristics that could enhance fully green food packaging barrier properties.
Several studies have shown that Saccharomyces cerevisiae biomass can benefit uncoated paper food packaging due to its potential for biofilm formation [9,10,11,12]. Additionally, it can benefit coated paper packaging by impacting plastic degradation [13,14] and microplastic removal [15]. Seeking to improve the barrier properties of the paper composite created in a previous experimental stage of research [3], Saccharomyces cerevisiae was added as a potential biofilm-forming additive. The observed results indicated that the addition of yeast affected barrier properties variably: it increased air permeability while enhancing surface hydrophobicity. The material samples that demonstrated the best hydrophobicity properties were selected for further research.
This study aims to investigate the impact of wheat bran filler and added Saccharomyces cerevisiae on material biodegradation efficiency. Key factors influencing material biodegradation may include the specific composition of biodegradable packaging materials (concentration of wheat bran filler and Saccharomyces cerevisiae biomass), the environmental conditions under which biodegradation is tested, and the interaction between the material and microbial communities in the biodegradation environment. Maintaining consistent environmental conditions is essential for both aerobic and anaerobic digestion to achieve their full potential. In both environments, optimizing key factors such as temperature, pH levels, substrate composition, and nutrient availability is critical for enhancing microbial activity [16,17,18,19,20,21,22,23,24,25,26]. Therefore, this study covers the analysis of the impact of wheat bran filler concentration and Saccharomyces cerevisiae biomass on material biodegradation under different temperatures and inoculum conditions: aerobic biodegradation at 46 °C in a compost-based environment, and anaerobic biodegradation at 55 °C in an active inoculum environment.
The key impact of this research is the identification of optimal conditions and wheat bran filler compositions for enhancing the biodegradation efficiency of paper composites, as well as providing insights into the biodegradation potential of yeast biomass. The findings demonstrate that the inclusion of Saccharomyces cerevisiae and higher concentrations of wheat bran significantly improve the biodegradation process, offering valuable insights for the development of sustainable and efficient biodegradable packaging materials. However, the validity of the aerobic test is compromised due to the insufficient degradation of the reference material (0.71% in 30 days), indicating the need for further testing under improved conditions to ensure accurate and reliable results.
2. Materials and Methods
2.1. Test Samples
Paper composite samples (see Table 1) that demonstrated the best hydrophobic properties in a previous research stage [3] were selected for aerobic and anaerobic biodegradation efficiency testing. Different filler compositions were chosen to compare the effect of bran on material biodegradation efficiency. For analyzing the effect of yeast, samples containing the same concentration of bran, with and without yeast, were selected. The detailed materials description, chemical composition, and production procedure of the paper composite samples are provided in our previous publication [3].

Table 1.
Materials selected for aerobic and anaerobic tests.
2.2. Inoculum
Compost used for the aerobic test was taken from a composting plant north of Milan treating selected biowaste and green waste. The anaerobic inoculum was taken from a thermophilic digestor operating at 50 °C in Carimate WWTP (Como, Italy).
2.3. Aerobic Biodegradation Tests
Tests were performed in the LIA lab of DICA Politecnico di Milano. Laboratory equipment used in the study included the OxiTop® respirometric system (WTW, Xylem Analytics, Weilheim, Germany) for measuring biological oxygen demand (BOD) under aerobic conditions. Well-aerated compost from a properly operating aerobic composting plant was prepared and used as inoculum following ISO 14855-2 [27].
The inoculum was further diluted with an inorganic test medium prepared following ISO 14851:2019 [28]. Three types of dry test substrates were cut into 10 × 10 mm squares and then added to the diluted inoculum. Microcrystalline cellulose was used as a reference material. Detailed substrate and inoculum characterization are provided in Table 2.

Table 2.
Materials characterization and mean values of aerobic degradation test.
For testing, 1110 mL volume glass vessels were used, and sodium hydroxide was present in the top cap for CO2 absorption. To ensure even mixing and temperature homogeneity during BOD testing, vessels were placed on the IS 12 inductive stirring system. Test vessels were incubated in a temperature-controlled oven, with gentle continuous mixing using a magnetic stirrer. The temperature was set at 46 °C throughout the entire 30-day incubation period. To prevent oxygen deficiency, air was supplied to the test vessels via an aerator for 5 min every 5–6 days.
The selected temperature is in the range of optimal composting temperatures [29], where both mesophilic and thermophilic bacteria are active [30], and is optimal for microbial activity and pathogen elimination [31,32].
Substrate, blank assays, and positive controls were carried out in triplicates.
The aerobic biodegradability achieved for each test specimen at the end of the test was calculated as follows—the oxygen pressure values generated from the substrate (hPa/bott) were obtained from the respirometric measuring system controller data. Every 14 days, 360 measurements were generated, resulting in a total of 772 measurements during the entire incubation period. The data were transferred to a PC and analyzed afterward. The measured pressure obtained with respirometric methods was converted into the BOD value using the following equation:
where
BOD = (M(O2)/(R * Tm)) * ((Vtot − Vl)/VI) * α * (Tm/T0) * Δp(O2)
- M(O2) = Molecular weight of oxygen (32,000 mg/mol)
- R = Gas constant (83.144 L·hPa/(mol·K))
- T0 = Temperature (273.15 K)
- Tm = Measuring temperature (319.15 K for performed BOD)
- Vtot = Bottle volume [mL]
- V1 = Sample volume [mL]
- α = Bunsen absorption coefficient (0.03103)
- Δp(O2) = Difference in the partial oxygen pressure [hPa]
The test results represent the maximum level of biodegradation determined from the plateau stage of the biodegradation curve [28].
The specific biological oxygen demand of each tested material (BODtotal) was calculated as the difference between oxygen consumption in the test flasks and the blanks.
Given that the COD of the sample added to each bottle (CODsample) represents the total oxygen demand for the chemical oxidation of the organic matter inserted, the oxygen demand obtained in long-duration aerobic biodegradation tests like the ones here performed (BODtotal), is a close proxy of the biodegradable organic substrate in the bottle (CODsample bio) and the ratio of BODtotal/CODsamples gives a close proxy of the fraction CODsample bio/CODsample and of the biodegradability of the organic compound present in the bottle.
A more accurate calculation of the CODbio according to the COD mass balance principles, however, should have considered that a part of the CODsample bio is converted to bacterial cells by growth (without oxygen consumption), and a fraction of this remains at the end of the test as unbiodegradable cell residuals from bacterial decay. Finally, BODtotal values always slightly underestimate the quantity of biodegradable organics in the sample (CODbio). Formula to calculate CODbio from BODtotal are well described in the literature [33] and have been here applied, assuming that 10% of the initial CODbio remains as unbiodegradable organics residues, as follows:
2.4. Anaerobic Biodegradation
Tests were performed in the Fab-e lab of DICA Politecnico di Milano. Laboratory equipment used in the study included Gas Endeavour III (GE III, BPC Instruments, Lund, Sweden) for measuring methane production under anaerobic conditions.
The anaerobic inoculum was left without feeding for 7 days (degassing). Afterward, according to Italian standard UNI/TS 11703:2018 [34], nutrient media were added, and the inoculum was further diluted. Italian standard UNI/TS11703:2018 was used along with the best BMA test practices [35].
Three types of dry test substrates, cut into 10 × 10 mm squares, were then added to the diluted inoculum. Microcrystalline cellulose was used as a reference material. The final concentration ratio of total solids in the vessel and the pH at the start of the test were adjusted to 7, as described in ISO 14853 [36]. The mass of the substrate to be dosed was calculated by dividing the mass of the substrate to be dosed vs. by the volatile solids to total solids ratio (VS/TS-S) and was precisely scaled. Detailed substrate and inoculum characterization, allowing for a maximum error of 1%, is provided in Table 3.

Table 3.
Materials characterization and mean values of anaerobic degradation test.
Biomethane potential (BMP) tests were carried out using a Gas Endeavour III (GE III, BPC Instrument, Sweden) consisting of 18 units of 500 mL volume reactors and the same number of gas flow meters (flow cells) attached to a detection unit for automatic data acquisition. BMP tests were conducted under thermophilic conditions at 55 °C [37,38,39]. Test vessels were incubated in a temperature-controlled water bath, with gentle and continuous (1 min running, 2 min stop) mixing applied to the vessels throughout the entire 30-day incubation period [40,41].
Substrate, blank assays, and positive controls were carried out in triplicates.
The anaerobic biodegradability achieved for each test specimen was calculated as follows—from the total cumulative methane values generated from each test (NmLCH4/bott), the net methane values were calculated by deducting the blank vessel value. Then, the net values were normalized to the COD of the substrate added (NmLCH4/mgCOD) (CH4 final). Then, the biodegradability achieved during the test was calculated by dividing the normalized production by the equivalence factor α: 0.32 mL CH4produced/mg CODbiodegraded considering 10% of the substrate COD is consumed for biomass growth and is not giving any methane production [42].
Biodegradation efficiency is then calculated based on the amount of methane produced compared to the substrate COD added at the beginning of the test according to the following formula:
Biodegradation efficiency (%) = (CH4Final/Theoretical CH4) * 100
2.5. Analytical Determination
Total solids (TS) and volatile solids (VS) were determined following ISO 14855-2 [27].
Chemical Oxygen Demand (COD) was determined using the Dichromate Method [43]. The COD values obtained were used as the initial COD in the anaerobic material biodegradation data analysis.
3. Results and Discussion
3.1. Aerobic Biodegradation Results
The data in Table 4 present the summary of measurements related to the aerobic biodegradation process of different samples involving bran filler and yeast additive. The key metrics include the initial theoretical Chemical Oxygen Demand (COD) determined using the Dichromate Method (see Section 2.5), the COD (mg O2/bottle) generated during the incubation period, and the final COD with deducted new microorganism growth and repair activity, and calculated biodegradation efficiency, allowing for a maximum error of 1%.

Table 4.
The aerobic test data.
The reference sample shows minimal change, indicating a low biodegradation of cellulose (0.71%).
Wheat bran concentration appears to have a notable impact on biodegradation. Wheat bran is rich in non-starch polysaccharides, such as arabinoxylans and β-glucans, which provide a good substrate for microbial activity. These components enhance the growth and activity of microorganisms involved in biodegradation, leading to increased breakdown of organic material. Additionally, wheat bran contains various essential nutrients that support microbial metabolism, making it a valuable additive in biodegradation processes [44].
The B15Y sample shows a modest increase in BOD, suggesting a lower biodegradation rate compared to samples with higher wheat bran concentrations. The biodegradation efficiency for this sample is 2.24%. The sample B40Y demonstrates a higher BOD increase compared to B15Y, indicating a higher biodegradation rate. The biodegradation efficiency for this sample is 4.07%. Increasing the amount of wheat bran in the biodegradation process provides more substrate for microorganisms, which can lead to more efficient degradation of organic matter. Higher concentrations of wheat bran also mean more available nutrients and structural polysaccharides [45,46], which can stimulate microbial activity and enhance the overall biodegradation rate.
Yeast addition seems to enhance biodegradation. The 40% wheat bran sample without yeast shows a biodegradation efficiency of 4.07%, while the 40% wheat bran sample with yeast exhibits the highest BOD increase among the samples, suggesting that yeast enhances biodegradation. The biodegradation efficiency for this sample is 6.34%. Saccharomyces cerevisiae biomass contains a variety of nutrients such as proteins, vitamins, and minerals, which can be released into the environment and used by other microorganisms. These nutrients can enhance the growth and activity of bacteria and other microbes that are active in the biodegradation process, leading to improved efficiency [47]. Yeast cell biomass can also provide structural support and serve as a physical matrix for microbial colonization [48]. The physical presence of yeast cells can thus contribute to a more effective breakdown of substrates by supporting microbial communities.
In general, the data indicate that higher concentrations of wheat bran result in higher biodegradation efficiencies. Additionally, the presence of yeast enhances the biodegradation process, as evidenced by the highest efficiency observed in the 40% wheat bran with yeast sample. This sample demonstrated a 35% higher biodegradation rate compared to the sample without yeast.
Aerobic biodegradation test validity: the validity of the aerobic test is questionable due to the exceptionally low biodegradation efficiency of the reference material (cellulose), which degraded by only 0.71% over 30 days. According to ISO 14855-2 [27], the test is considered valid only if the degree of biodegradation of the reference material exceeds 70% after 45 days. Given that the biodegradation rate falls significantly below this threshold, the low biodegradation rates suggest that the test environment, inoculum, or equipment [49] may not have been optimal for supporting aerobic microbial activity. Consequently, the aerobic biodegradation efficiency of other materials tested in this environment may also be underestimated.
3.2. Anaerobic Biodegradation Results
The data in Table 5 present the summary of measurements related to the BMP anaerobic biodegradation process of different samples involving bran filler and yeast additive. The key metrics include Chemical Oxygen Demand (COD), theoretical and final methane (CH4) production, and biodegradation efficiency.

Table 5.
The anaerobic test data.
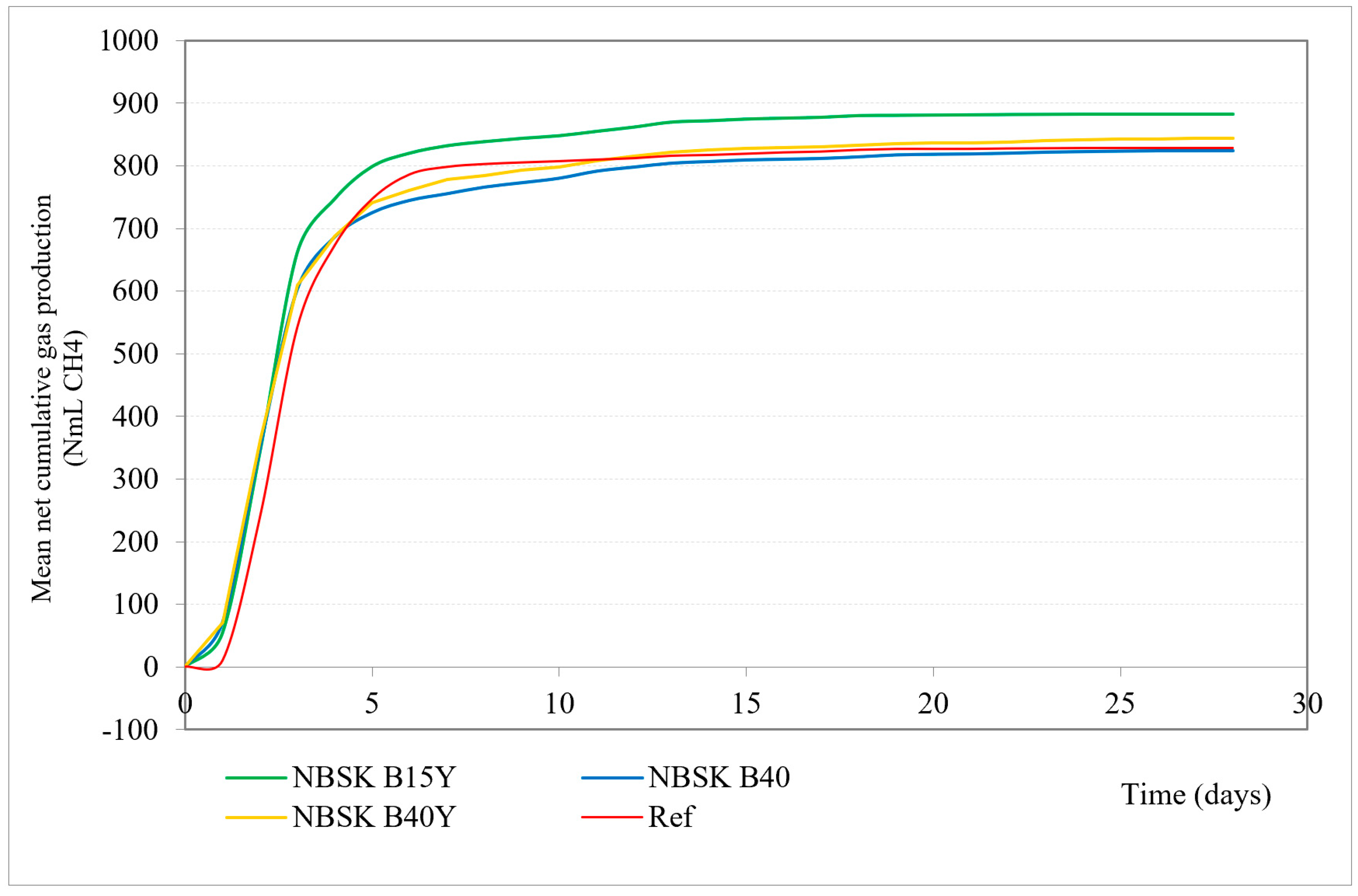
Figure 1 and Table 6 present performed materials anaerobic biodegradation test mean net cumulative gas production (NmL CH4) over time.
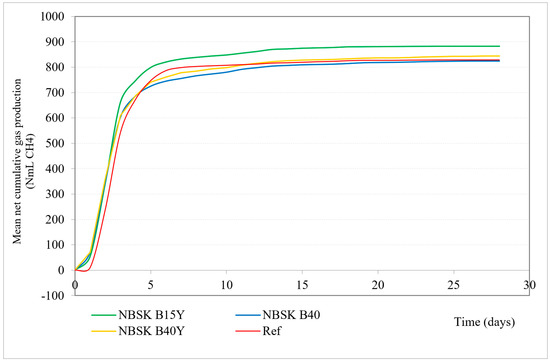
Figure 1.
Mean net cumulative gas production (NmL CH4) in 30 days. Green line: 15% bran filler, yeast additive; yellow line: 40% bran filler, yeast additive; red line: reference/cellulose; blue line: 40% bran filler, no yeast additive.

Table 6.
Mean net cumulative gas production (NmL CH4) day-by-day test data.
All material sample treatments show a rapid increase in gas production within the first 5 days, followed by a plateau phase.
The sample with 15% bran filler and yeast additive shows the highest gas production, reaching around 883.60 NmL CH4, maintaining the highest level throughout the period. The sample with 40% bran filler and yeast additive shows slightly lower gas production, indicating that increasing the bran content reduces material biodegradation. The observation that lower bran filler content (15%) with yeast additive is more efficient for biodegradation and methane production compared to higher bran filler content (40%) can be influenced by different lignin content and other factors. Lignin is known to be resistant to microbial degradation, particularly in the absence of oxygen, due to its complex structure [50,51,52,53]. Therefore, higher lignin content can inhibit the biodegradation process [54], and lignin concentration in the tested paper composites correlates negatively with biodegradation efficiency [55].
Another factor might be microbial synergy. The interaction between different microbial communities (bacteria, archaea, and fungi) is crucial for efficient biodegradation [56,57,58]. A balanced substrate composition (like 15% bran filler) may support a more synergistic microbial environment compared to a higher bran content (40%).
Even though increasing bran filler content from 15% to 40% slightly reduces the gas production (when yeast additive is present), both bran filler contents show significantly higher gas production compared to the cellulose reference, indicating that cellulose alone is less effective for methane production compared to bran-based composites.
The B40 sample with 40% bran filler and no yeast additive shows slightly lower (826.30 NmL CH4) gas production compared to B40Y, which is the same bran content with yeast additive (847.40 NmL CH4), indicating a non-significant positive effect of the yeast additive on gas production. On the other hand, B40Y biodegradation is 88.14%, slightly lower compared to B40’s 88.50%. This could be explained by initial rapid degradation in the beginning and incomplete degradation. The presence of yeast additive could enhance the initial microbial activity, rapidly breaking down easily accessible organic matter and producing a significant amount of gas early in the process. Once the easily degradable components are consumed, the remaining substrate may not be as readily accessible or degradable by the microbial community, resulting in lower overall biodegradation.
4. Conclusions
The comparison of aerobic and anaerobic degradation: The performed tests reveal distinct differences in efficiency and outcomes. Under aerobic conditions, the biodegradation efficiency is significantly lower compared to anaerobic biodegradation, with values ranging from 0.71% to 6.34%. B40Y exhibits the highest aerobic efficiency at 6.34%, followed by B40 (4.07%) and B15Y (2.24%). Cellulose shows the lowest efficiency at 0.71%.
In contrast, anaerobic biodegradation shows much higher efficiencies, with biodegradation percentages reaching up to 96.62% for B15Y, while B40 and B40Y demonstrate slightly lower values of 88.50% and 88.14%, respectively. However, these values are still significantly higher compared to the control (cellulose, 82.32%).
This stark difference can be attributed to the metabolic capabilities of anaerobic microbes, which efficiently convert organic matter into biogas, particularly methane (CH4).
Aerobic biodegradation (46 °C, Mesophiles and Thermophiles): Higher concentrations of wheat bran lead to higher biodegradation efficiencies due to the rich non-starch polysaccharides and essential nutrients present in wheat bran, which support and stimulate both mesophilic and thermophilic microbial activity.
Anaerobic biodegradation (55 °C, Thermophiles): Thermophiles at 55 °C have fewer metabolic pathways available for degrading organic matter without oxygen. The breakdown of organic matter is slower, and the presence of lignin in wheat bran further complicates the process due to its resistance to microbial degradation.
The addition of yeast: Even in an inactive form yeast additive provides additional nutrients that might stimulate microbial growth and activity, enhancing biodegradation efficiency under aerobic conditions and having an insignificant positive impact in anaerobic conditions.
Future research: Due to the observed exceptionally low aerobic biodegradation efficiency, future research will focus on optimizing test conditions, and testing materials at a temperature range of 50–60 °C, using compost with a 40–60% moisture content as the inoculum. Advanced testing equipment with an automatic oxygen supply will be utilized.
Author Contributions
All authors contributed significantly to this manuscript. Z.M. and V.V. were responsible for the original idea and the theoretical aspects of the paper; Z.M., A.G., G.M. and F.M. prepared the methodology design; Z.M. drafted the manuscript; Z.M., A.G. and G.M. were responsible for testing and result analysis; F.M. supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Research was conducted as part of the execution of Project “Mission-driven Implementation of Science and Innovation Programmes” (No. 02-002-P-0001), funded by the Economic Revitalization and Resilience Enhancement Plan “New Generation Lithuania”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Data Availability Statement
The authors declare that the data supporting the indings of this study are available within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Markevičiūtė, Z.; Varžinskas, V. Smart Material Choice: The Importance of Circular Design Strategy Applications for Bio-Based Food Packaging Preproduction and End-of-Life Life Cycle Stages. Sustainability 2022, 14, 6366. [Google Scholar] [CrossRef]
- Markevičiūtė, Z.; Varžinskas, V. Plant-Origin Feedstock Applications in Fully Green Food Packaging: The Potential for Tree-Free Paper and Plant-Origin Bio-Plastics in the Baltic Sea Region. Sustainability 2022, 14, 7393. [Google Scholar] [CrossRef]
- Markevičiūtė, Z.; Lyytikäinen, J.; Leminen, V.; Varžinskas, V. Grain by-products and Saccharomyces cerevisiae application in paper packaging material: Impact on physical–mechanical and barrier properties. Discov. Sustain. 2024, 5, 82. [Google Scholar] [CrossRef]
- Kachook, O.; Cramer, K.; Gendell, A. Understanding the Role of Compostable Packaging in North America, Sustainable Packaging Coalition. 2021. Available online: https://sustainablepackaging.org/wp-content/uploads/2023/07/UnderstandingCompostablePackagingGuide.pdf (accessed on 27 September 2024).
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Odegard, I.; Nusselder, S.; Lindgreen, E.R.; Bergsma, G.; Graaff, L. Biobased Plastics in a Circular Economy—Policy Suggestions for Biobased and Biobased Biodegradable Plastics; CE Delft: Delft, The Netherlands, 2017. [Google Scholar]
- Spierling, S.; Knüpffer, E.; Behnsena, H.; Mudersbacha, M.; Kriegb, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endresa, H.-J. Bio-based plastics—A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Delgado, J.F.; Sceni, P.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Development of innovative biodegradable films based on biomass of Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Water vapour transport in biopolymeric materials: Effects of thickness and water vapour pressure gradient on yeast biomass-based films. J. Polym. Environ. 2022, 30, 2976–2989. [Google Scholar] [CrossRef]
- Lago, A.; Delgado, J.F.; Rezzani, G.D.; Cottet, C.; Ramírez Tapias, Y.A.; Peltzer, M.A.; Salvay, A.G. Multi-Component Biodegradable Materials Based on Water Kefir Grains and Yeast Biomasses: Effect of the Mixing Ratio on the Properties of the Films. Polymers 2023, 15, 2594. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.C.; Abeywickrama, T.D.; Rahman, F.A. Role of genetically engineered yeast in plastic degradation. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Daverey, A., Dutta, K., Joshi, S., Gea, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 567–584. [Google Scholar]
- Zahmatkesh Anbarani, M.; Esmaeili Nasrabadi, A.; Bonyadi, Z. Use of Saccharomyces cerevisiae as new technique to remove polystyrene from aqueous medium: Modeling, optimization, and performance. Appl. Water Sci. 2023, 13, 166. [Google Scholar] [CrossRef]
- Rozzi, A.; Remigi, E. Methods of assessing microbial activity and inhibition under anaerobic conditions: A literature review. Rev. Environ. Sci. Bio/Technol. 2004, 3, 93–115. [Google Scholar] [CrossRef]
- Zavala, M.; Funamizu, N.; Takakuwa, T. Temperature effect on aerobic biodegradation of feces using sawdust as a matrix. Water 2004, 38, 2406–2416. [Google Scholar] [CrossRef]
- Lim, B.-R.; Huang, X.; Hu, H.-Y.; Goto, N.; Fujie, K. Effects of temperature on biodegradation characteristics of organic pollutants and microbial community in a solid phase aerobic bioreactor treating high strength organic wastewater. Water Sci. Technol. 2001, 43, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, T.; Li, T.; Lü, F.; He, P. Comparison of sludge digestion under aerobic and anaerobic conditions with a focus on the degradation of proteins at mesophilic temperature. Bioresour. Technol. 2013, 140, 131–137. [Google Scholar] [CrossRef]
- Ma, A.; As, A.; Ma, C.; Zhang, M.A.; Xu, Z.A.; As, A.; Mf, A.; Wei, W.A.; Ly, B. Ultra-high temperature aerobic fermentation pretreatment composting: Parameters optimization, mechanisms and compost quality assessment. J. Environ. Chem. Eng. 2021, 9, 105453. [Google Scholar]
- Xu, H.; Li, Y.; Hua, D.; Zhao, Y.; Chen, L.; Zhou, L.; Chen, G. Effect of microaerobic microbial pretreatment on anaerobic digestion of a lignocellulosic substrate under controlled pH conditions. Bioresour. Technol. 2021, 328, 124852. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Taherzadeh, M.J. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Subirats, J.; Sharpe, H.; Topp, E. Fate of Clostridia and other spore-forming Firmicute bacteria during feedstock anaerobic digestion and aerobic composting. J. Environ. Manag. 2022, 309, 114643. [Google Scholar] [CrossRef]
- Liu, R.; Gong, H.; Xu, Y.; Cai, C.; Hua, Y.; Li, L.; Dai, L.; Dai, X. The transition temperature (42 °C) from mesophilic to thermophilic microorganisms enhances biomethane potential of corn stover. Sci. Total Environ. 2021, 759, 143549. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Taherzadeh, M.J. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Microbial community changes in methanogenic granules during the transition from mesophilic to thermophilic conditions. Appl. Microbiol. Biotechnol. 2016, 101, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 14851:2019; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2019.
- Rich, N.; Bharti, A. Assessment of different types of in-vessel composters and its effect on stabilization of MSW compost. Int. Res. J. Eng. Techno. 2015, 2, 37–42. [Google Scholar]
- Steiniger, B.; Hupfauf, S.; Insam, H.; Schaum, C. Exploring Anaerobic Digestion from Mesophilic to Thermophilic Temperatures—Operational and Microbial Aspects. Fermentation 2023, 9, 798. [Google Scholar] [CrossRef]
- Wang, P.; Han, S.; Lin, Y. Chapter 8—Role of microbes and microbial dynamics during composting. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–220. [Google Scholar]
- Tannouri, A.; Rizk, Z.; Daccache, M.; Ghanem, C.; Azzi, V.; Maroun, R.G.; Hobaika, Z.; Salameh, D. Study of Raw Material Pretreatment and the Microbiota Selection Effect on the Composting Process Efficiency. Agronomy 2023, 13, 2048. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Metcalf & Eddy. In Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Higher Education: New York, NY, USA, 2014. [Google Scholar]
- The new Italian standard UNI/TS 11703:2018 on the BMP assay. In Method for the Measurement of the Potential Production of Methane from Wet Anaerobic Digestion—Matrices in Feed; Comitato Termotecnico Italiano (CTI): Milan, Italy, 2018.
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- ISO 14853:2016; Plastics—Determination of the Ultimate Anaerobic Biodegradation of Plastic Materials in an Aqueous System—Method by Measurement of Biogas Production. International Organization for Standardization: Geneva, Switzerland, 2016.
- Sameena, B.; Sudharshan, J.; Gangagni, R.A.; Nicky, E. Comparison of mesophilic and thermophilic methane production potential of acids rich and high-strength landfill leachate at different initial organic loadings and food to inoculum ratios. Sci. Total Environ. 2020, 715, 136658. [Google Scholar]
- Kang, J.-H.; Kang, S.-W.; Kim, W.-J.; Kim, D.-H.; Im, S.-W. Anaerobic Co-Digestion of Bioplastics and Food Waste under Mesophilic and Thermophilic Conditions: Synergistic Effect and Biodegradation. Fermentation 2022, 8, 638. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic Digestion of Solid Organic Substrates in Batch Mode: An Overview Relating to Methane Yields and Experimental Procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- ASTM D1252-06(2020); Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. ASTM International: West Conshohocken, PA, USA, 2020.
- Sztupecki, W.; Rhazi, L.; Depeint, F.; Aussenac, T. Functional and Nutritional Characteristics of Natural or Modified Wheat Bran Non-Starch Polysaccharides: A Literature Review. Foods 2023, 12, 2693. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Mahfuz, S.; Piao, X. The Impact of Wheat Bran on the Morphology and Physiology of the Gastrointestinal Tract in Broiler Chickens. Animals 2020, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Yang, M.D.; Fan, X.F. Study on Modification of Dietary Fiber from Wheat Bran. Adv. Mater. Res. 2011, 183–185, 1268–1272. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A Sight to Wheat Bran: High Value-Added Products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R. Microbial Biofilms: Structural Plasticity and Emerging Properties. Microorganisms 2022, 10, 138. [Google Scholar] [CrossRef]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kuhar, S.; Sharma, K.K.; Shrivastava, B. Microorganisms and Enzymes Involved in Lignin Degradation Vis-à-vis Production of Nutritionally Rich Animal Feed: An Overview. In Biotechnology for Environmental Management and Resource Recovery; Kuhad, R., Singh, A., Eds.; Springer: New Delhi, India, 2013. [Google Scholar]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.W.; Charlton, A. Establishing Experimental Conditions to Produce Lignin-Degrading Enzymes on Wheat Bran by Trametes versicolor CM13 Using Solid State Fermentation. Waste 2023, 1, 711–723. [Google Scholar] [CrossRef]
- Li, P.; Liu, D.; Pei, Z.; Zhao, L.; Shi, F.; Yao, Z.; Liu, J. Evaluation of lignin inhibition in anaerobic digestion from the perspective of reducing the hydrolysis rate of holocellulose. Bioresour. Technol. 2021, 333, 125204. [Google Scholar] [CrossRef] [PubMed]
- Blair, E.M.; Dickson, K.L.; O’malley, M.A. Microbial communities and their enzymes facilitate degradation of recalcitrant polymers in anaerobic digestion. Curr. Opin. Microbiol. 2021, 64, 100–108. [Google Scholar] [CrossRef]
- Liu, W.; Sun, C.; Li, W.; Li, T.; Chen, Z.; Wang, J.; Ren, Z.; Wen, X. Sludge composition and characteristics shaped microbial community and further determined process performance: A study on full-scale thermal hydrolysis-anaerobic digestion processes. J. Environ. Sci. 2024, 137, 96–107. [Google Scholar] [CrossRef]
- Duan, N.; Kougias, P.G.; Campanaro, S.; Treu, L.; Angelidaki, I. Evolution of the microbial community structure in biogas reactors inoculated with seeds from different origin. Sci. Total Environ. 2021, 773, 144981. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).