Bacillus subtilis Strain YJ-15, Isolated from the Rhizosphere of Wheat Grown under Saline Conditions, Increases Soil Fertility and Modifies Microbial Community Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Location and Experimental Setup
2.2. Soil Sampling and Fertility Assessment
2.3. DNA Extraction and PCR
2.4. Illumina MiSeq Sequencing
2.5. Data Processing
2.6. Statistical Analysis
3. Results
3.1. Soil Fertility Variation
3.2. Sequencing Quality Evaluation
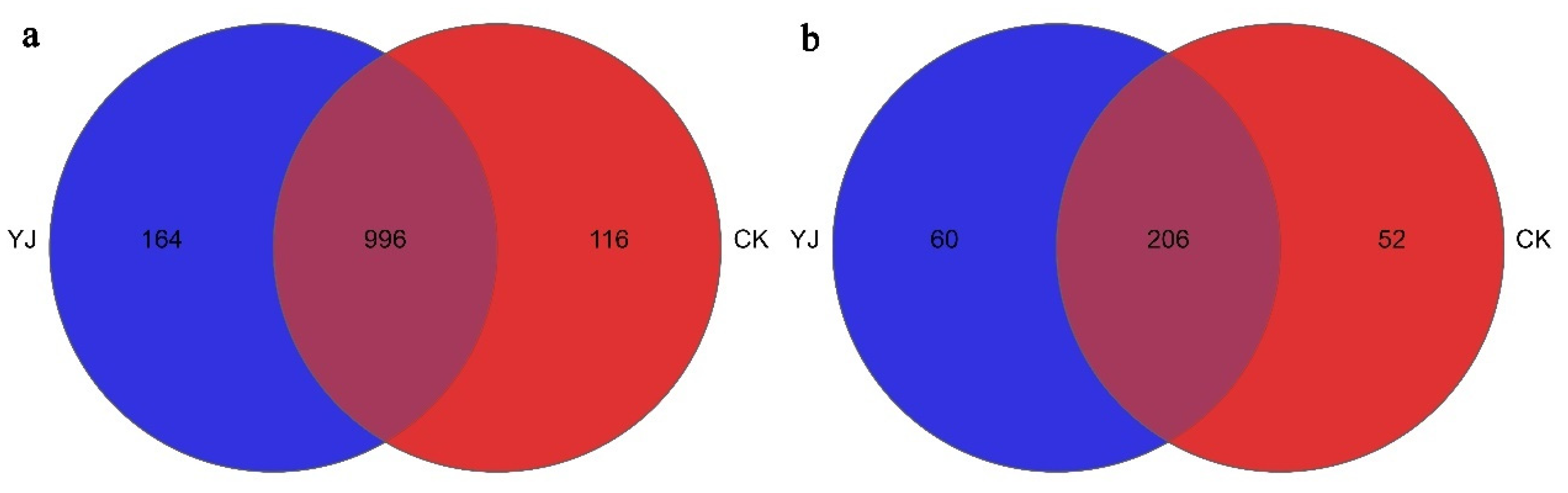
3.3. α-Diversity and β-Diversity Analysis
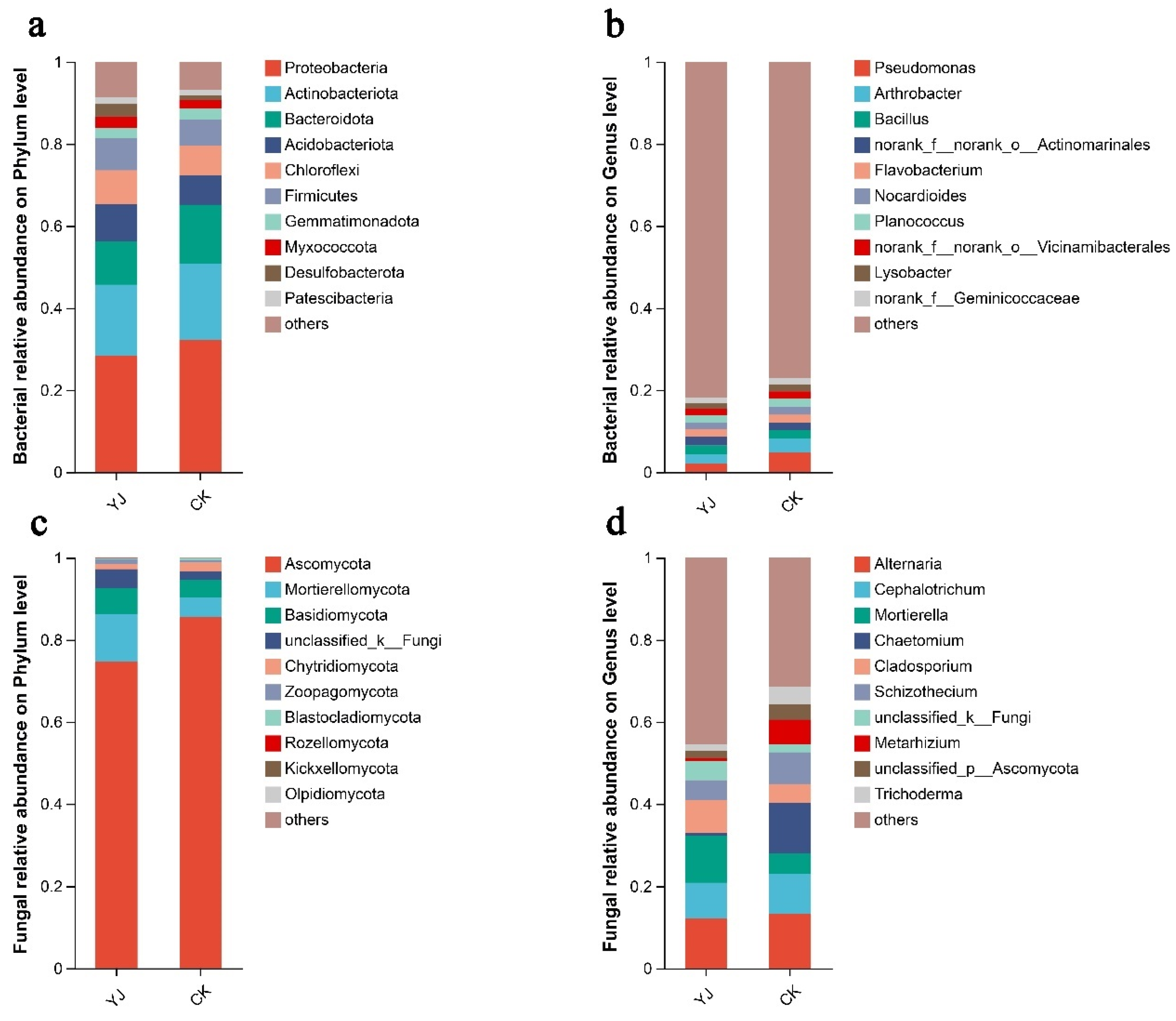
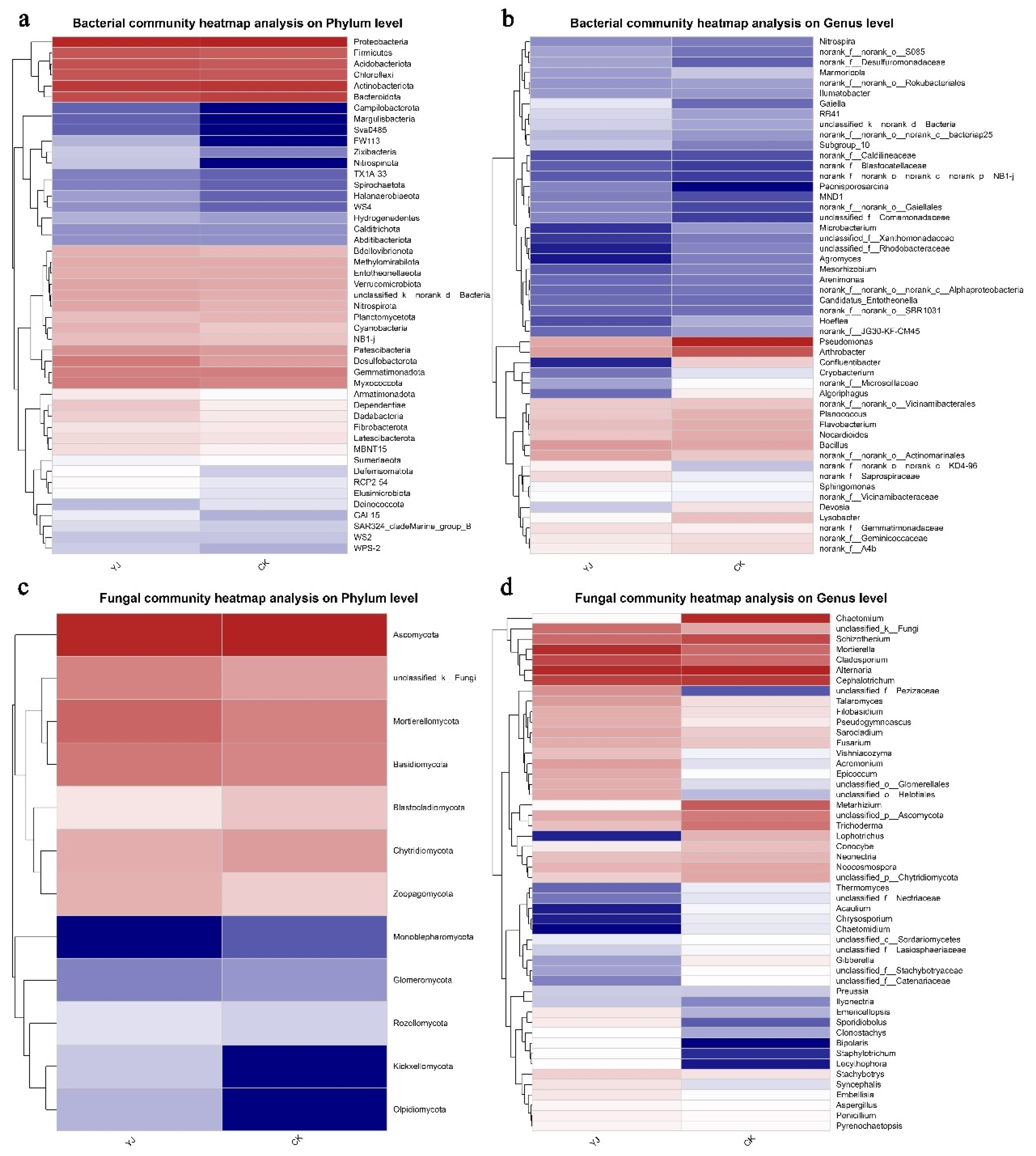
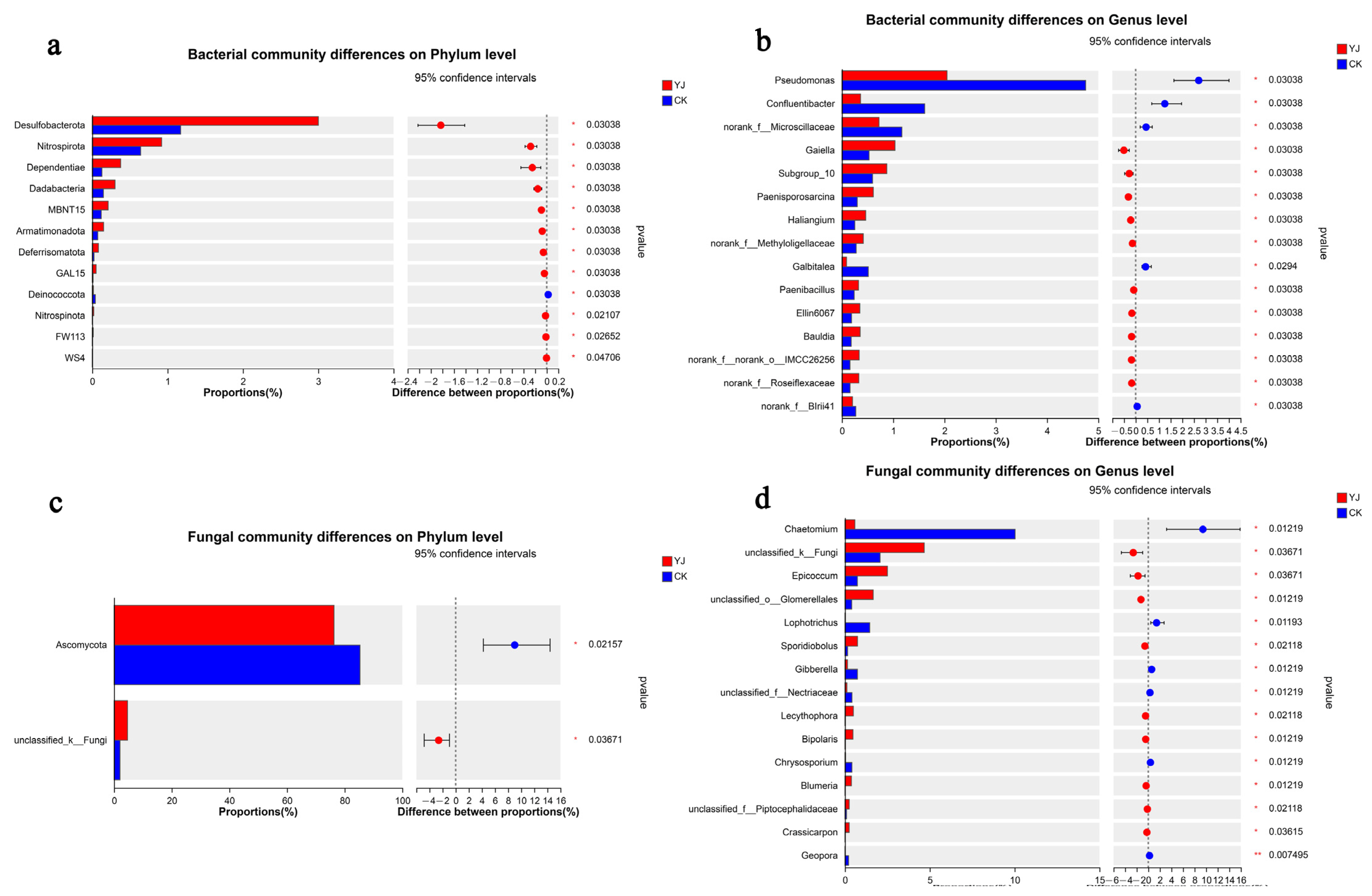
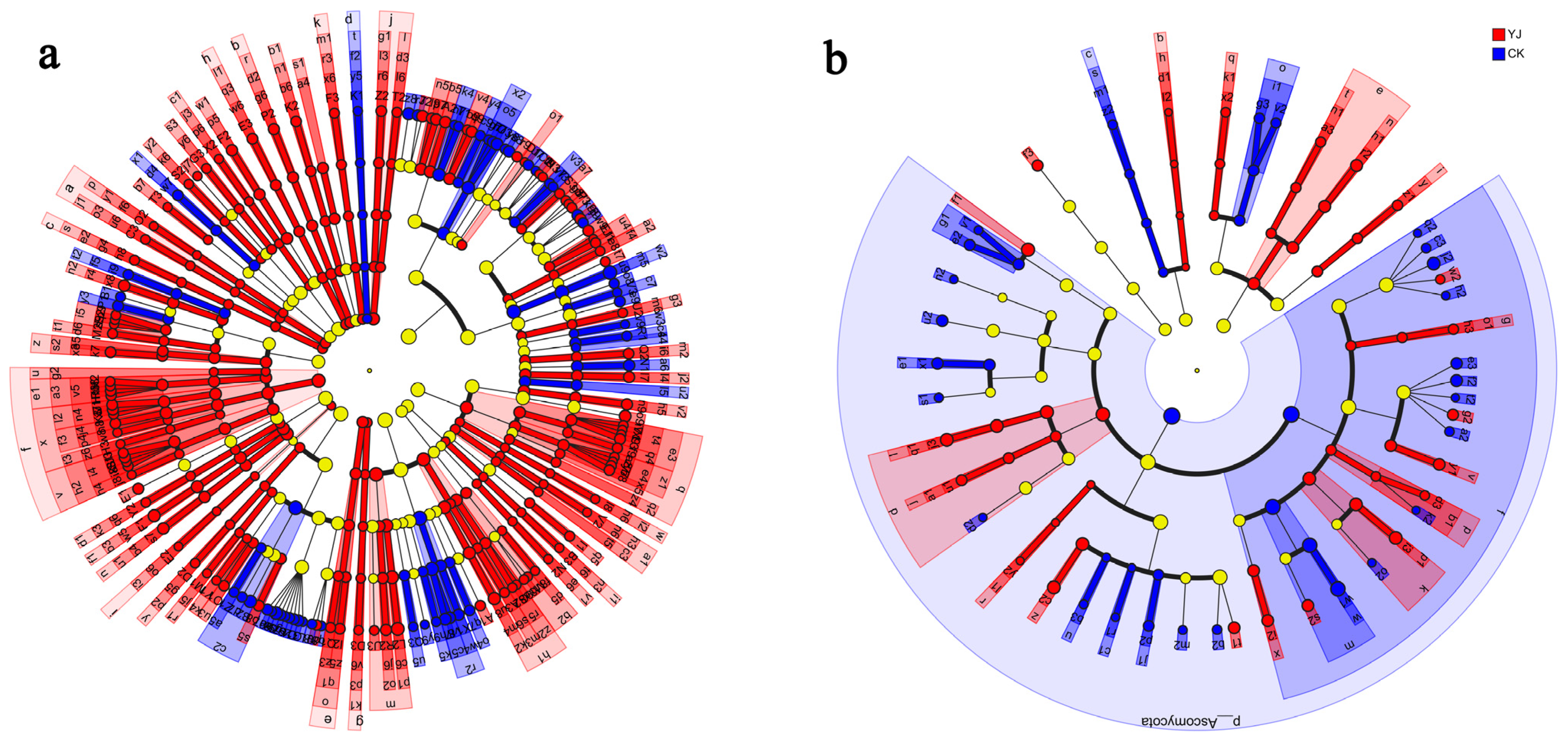
3.4. Composition and Structure of Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. Int. J. Mol. Sci. 2022, 23, 7036. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Han, G.L.; Qiao, Z.Q.; Li, Y.X.; Yang, Z.R.; Wang, B.S. Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings. Plants 2022, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Hou, D. Biochar for sustainable soil management. Soil Use Manag. 2021, 37, 2–6. [Google Scholar] [CrossRef]
- Li, G. A summary on soil salinization of Yellow River Delta. Anhui Agric. Sci. Bull. 2020, 26, 113–115. [Google Scholar]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Wang, S.; Guo, K.; Ameen, A.; Fang, D.; Li, X.; Liu, X.; Han, L. Evaluation of Different Shallow Groundwater Tables and Alfalfa Cultivars for Forage Yield and Nutritional Value in Coastal Saline Soil of North China. Life 2022, 12, 217. [Google Scholar] [CrossRef]
- Pei, L.; Wang, C.; Sun, L. Effects of Unconventional Water Agricultural Utilization on the Heavy Metals Accumulation in Typical Black Clay Soil around the Metallic Ore. Toxics 2022, 10, 476. [Google Scholar] [CrossRef]
- Zhu, W.; Gu, S.; Jiang, R.; Zhang, X.; Hatano, R. Saline–Alkali Soil Reclamation Contributes to Soil Health Improvement in China. Agriculture 2024, 14, 1210. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, K.; Yin, C.; Xu, X.; Yu, X.; Ye, B.; Sun, Z.; Dong, J.; Bi, A.; Zhao, X.; et al. Genetic basis of geographical differentiation and breeding selection for wheat plant architecture traits. Genome Biol. 2023, 24, 114. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, P.; Basak, N.; Kumar, A.; Yadav, R.; Singh, R.; Sharma, R.; Kumar, S.; Singh, R.K.; Sharma, P. Ameliorants and salt tolerant varieties improve rice-wheat production in soils undergoing sodification with alkali water irrigation in Indo–Gangetic Plains of India. Agric. Water Manag. 2021, 243, 106492. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Avliyakulov, M.A.; Kumari, M.; Rajabov, N.Q.; Durdiev, N.K. Characterization of soil salinity and its impact on wheat crop using space-borne hyperspectral data. Geoinf. Support Sustain. Dev. Territ. 2020, 26, 271–285. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Dai, Y.; Chen, Y.; Tian, Y.; Huo, Z. Isolation and screening of phosphorus solubilizing bacteria from saline alkali soil and their potential for Pb pollution remediation. Front. Bioeng. Biotechnol. 2023, 11, 1134310. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, B.; Lyu, H.; Duan, Y.; Yang, Y.; Wang, S.; Li, Z. Rhizosphere enzyme activities and microorganisms drive the transformation of organic and inorganic carbon in saline–alkali soil region. Sci. Rep. 2022, 12, 1314. [Google Scholar] [CrossRef]
- Xu, T.; Meng, S.; Zhu, X.; Di, J.; Zhu, Y.; Yang, X.; Yan, W. Integrated GWAS and transcriptomic analysis reveal the candidate salt-responding genes regulating Na+/K+ balance in barley (Hordeum vulgare L.). Front. Plant Sci. 2022, 13, 1004477. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Z.J.; Su, M.X.; Zhang, M. Transcriptomic Profile Analysis of Populus talassica × Populus euphratica Response and Tolerance under Salt Stress Conditions. Genes 2022, 13, 1032. [Google Scholar] [CrossRef]
- Han, M.; Cui, R.; Wang, D.; Huang, H.; Rui, C.; Malik, W.A.; Wang, J.; Zhang, H.; Xu, N.; Liu, X.; et al. Combined transcriptomic and metabolomic analyses elucidate key salt-responsive biomarkers to regulate salt tolerance in cotton. BMC Plant Biol. 2023, 23, 245. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, S.; Li, J.; Li, S.; Yu, Z.; Liu, C.; Li, X.; Liu, J.; Ren, Y.; Zhang, P.; et al. Development of Sequence-Tagged Site Marker Set for Identification of J, JS, and St Sub-genomes of Thinopyrum intermedium in Wheat Background. Front. Plant Sci. 2021, 12, 685216. [Google Scholar] [CrossRef]
- Hou, J.; Rusuli, Y. Estimation of soil salt content in the Bosten Lake watershed, Northwest China based on a support vector machine model and optimal spectral indices. PLoS ONE 2023, 18, e0273738. [Google Scholar] [CrossRef] [PubMed]
- Mengmeng, C.; Shirong, Z.; Lipeng, W.; Chao, F.; Xiaodong, D. Organic fertilization improves the availability and adsorptive capacity of phosphorus in saline-alkaline soils. J. Soil Sci. Plant Nutr. 2021, 21, 487–496. [Google Scholar] [CrossRef]
- Wang, S.; Gao, P.; Zhang, Q.; Shi, Y.; Guo, X.; Lv, Q.; Wu, W.; Zhang, X.; Li, M.; Meng, Q. Application of biochar and organic fertilizer to saline-alkali soil in the Yellow River Delta: Effects on soil water, salinity, nutrients, and maize yield. Soil. Use Manag. 2022, 38, 1679–1692. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, B.; Ren, P.; Chen, X.; Zhou, D.; Yao, S.; Fan, D.; Chen, X. Effects of Bacillus subtilis on cotton physiology and growth under water and salt stress. Agric. Water Manag. 2024, 303, 109038. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Mago, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, F.; Zhu, X.; Lamlom, S.F.; Zhao, K.; Zhang, B.; Wang, J. Manipulating rhizosphere microorganisms to improve crop yield in saline-alkali soil: A study on soybean growth and development. Front. Microbiol. 2023, 14, 1233351. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Jiang, L.; Hu, D.; Lv, G. Impacts of plant and soil stoichiometry on species diversity in a desert ecosystem. AoB PLANTS 2022, 14, plac034. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhang, Y.; Yu, H.; Zhang, Y.A. Ganoderma lucidum cultivation affect microbial community structure of soil, wood segments and tree roots. Sci. Rep. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Xie, J.; Xue, W.; Li, C.; Yan, Z.; Li, D.; Li, G.; Chen, X.; Chen, D. Water-soluble phosphorus contributes significantly to shaping the community structure of rhizospheric bacteria in rocky desertification areas. Sci. Rep. 2019, 9, 18408. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Yaling, H.; Beibei, Z.; Quanjiu, W. Effects of Bacillus subtilis on Evaporation of Soil Surface and Water and Salt Dsitritution in Saline-alkali Soil. J. Soil. Water Conserv. 2018, 32, 306–311. [Google Scholar]
- Duan, M.; Zhang, Y.; Zhou, B.; Qin, Z.; Wu, J.; Wang, Q.; Yin, Y. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure–straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Q.; Ning, S.; Lin, S.; Hu, X.; Song, Z. Application of Magnetized Ionized Water and Bacillus subtilis Improved Saline Soil Quality and Cotton Productivity. Plants 2024, 13, 2458. [Google Scholar] [CrossRef]
- Jabborova, D.; Narimanov, A.; Enakiev, Y.; Davranov, K. Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulg. J. Agric. Sci. 2020, 26, 744–747. [Google Scholar]
- Chang, F.; Wang, G.; Ji, H.; Zhang, H.; Pang, H.; Zhang, J.; Wang, J.; Zhang, X.; Li, Y. Effects of Microbial Agents on Physicochemical Properties and Microbial Flora of Rhizosphere Saline-alkali Soil. Ecol. Environ. 2022, 31, 1984–1992. [Google Scholar] [CrossRef]
- Ahsan, T.; Tian, P.-C.; Gao, J.; Wang, C.; Liu, C.; Huang, Y.-Q. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2024, 64, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Liu, W.; Ahammed, G.J.; Wen, W.; Guo, S.; Shu, S.; Sun, J. Auxin is involved in arbuscular mycorrhizal fungi-promoted tomato growth and NADP-malic enzymes expression in continuous cropping substrates. BMC Plant Biol. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Yun, C.; Yan, C.; Xue, Y.; Xu, Z.; Jin, T.; Liu, Q. Effects of exogenous microbial agents on soil nutrient and microbial community composition in greenhouse-derived vegetable straw composts. Sustainability 2021, 13, 2925. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Wang, L.; Zhang, X.; Song, X.; Li, J.; Yang, J.; Chen, S.; Zhang, J. Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones. Int. J. Environ. Res. Public Health 2022, 19, 6529. [Google Scholar] [CrossRef]
- Power, J.F.; Carere, C.R.; Lee, C.K.; Wakerley, G.L.J.; Evans, D.W.; Button, M.; White, D.; Climo, M.D.; Hinze, A.M.; Morgan, X.C.; et al. Microbial biogeography of 925 geothermal springs in New Zealand. Nat. Commun. 2018, 9, 2876. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Ma, J.; Rong, Z.; Chen, J.; Wang, Y.; Zheng, X.; Ye, W. Wheat Straw Return Influences Soybean Root-Associated Bacterial and Fungal Microbiota in a Wheat-Soybean Rotation System. Microorganisms 2022, 10, 667. [Google Scholar] [CrossRef]
- Liu, W.; Wang, N.; Yao, X.; He, D.; Sun, H.; Ao, X.; Wang, H.; Zhang, H.; St Martin, S.; Xie, F.; et al. Continuous-cropping-tolerant soybean cultivars alleviate continuous cropping obstacles by improving structure and function of rhizosphere microorganisms. Front. Microbiol. 2022, 13, 1048747. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Kang, F.; Yan, X.; Sun, L.; Wang, N.; Gong, Y.; Gao, X.; Huang, L. Microbial diversity composition of apple tree roots and resistance of apple Valsa canker with different grafting rootstock types. BMC Microbiol. 2022, 22, 148. [Google Scholar] [CrossRef]
- Aguirre de Cárcer, D. A conceptual framework for the phylogenetically constrained assembly of microbial communities. Microbiome 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Yu-Te, L.; Whitman, W.B.; Coleman, D.C.; Chih-Yu, C. Effects of reforestation on the structure and diversity of bacterial communities in subtropical low mountain forest soils. Front. Microbiol. 2018, 9, 1968. [Google Scholar]
- Bell, E.; Lamminmäki, T.; Alneberg, J.; Qian, C.; Xiong, W.; Hettich, R.L.; Frutschi, M.; Bernier-Latmani, R. Active anaerobic methane oxidation and sulfur disproportionation in the deep terrestrial subsurface. ISME J. 2022, 16, 1583–1593. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.Y.; Akter, S.; Huq, M.A. Probiotic-Mediated Biosynthesis of Silver Nanoparticles and Their Antibacterial Applications against Pathogenic Strains of Escherichia coli O157:H7. Polymers 2022, 14, 1834. [Google Scholar] [CrossRef]
- Mazhar, S.; Khokhlova, E.; Colom, J.; Simon, A.; Deaton, J.; Rea, K. In vitro and in silico assessment of probiotic and functional properties of Bacillus subtilis DE111(®). Front. Microbiol. 2022, 13, 1101144. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Niu, H.; Zhao, P.; Li, X.; Yan, F.; Wang, C.; Qiu, Z. Synergistic biocontrol of Bacillus subtilis and Pseudomonas fluorescens against early blight disease in tomato. Appl. Microbiol. Biotechnol. 2023, 107, 6071–6083. [Google Scholar] [CrossRef]
- Zheng, X.; Lv, W.; Song, K.; Li, S.; Zhang, H.; Bai, N.; Zhang, J. Effects of a vegetable-eel-earthworm integrated planting and breeding system on bacterial community structure in vegetable fields. Sci. Rep. 2018, 8, 9520. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; de Oliveira, F.S.; Carvalho-Silva, M.; Câmara, P.; Rosa, L.H. Soil Fungal Diversity and Ecology Assessed Using DNA Metabarcoding along a Deglaciated Chronosequence at Clearwater Mesa, James Ross Island, Antarctic Peninsula. Biology 2023, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zeng, X.; Li, M.; Weng, X.; Frey, B.; Yang, L.; Li, M. Influence of Different Vegetation Types on Soil Physicochemical Parameters and Fungal Communities. Microorganisms 2022, 10, 829. [Google Scholar] [CrossRef]
- Batta, Y. Entomopathogenic effect of Trichothecium roseum (Pers.) Link (Hypocreales: Ascomycota) against Pauropsylla buxtoni (Psylloidea: Hemiptera) infesting Ficus carica leaves and its potential use as biocontrol agent of the insect. J. Appl. Microbiol. 2020, 129, 400–410. [Google Scholar] [CrossRef]
- Patkowska, E. Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture 2021, 11, 347. [Google Scholar] [CrossRef]
- Besaury, L.; Martinet, L.; Mühle, E.; Clermont, D.; Rémond, C. Streptomyces silvae sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2021, 71, 005147. [Google Scholar] [CrossRef] [PubMed]
- Ramdass, A.C.; Rampersad, S.N. Diversity and Oil Degradation Potential of Culturable Microbes Isolated from Chronically Contaminated Soils in Trinidad. Microorganisms 2021, 9, 1167. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, S.-N.; Zhou, L.-L.; Sun, S.; Zhang, J.; Zhuang, L.-L. Combination of Biochar and Functional Bacteria Drives the Ecological Improvement of Saline–Alkali Soil. Plants 2023, 12, 284. [Google Scholar] [CrossRef]
- Zou, M.; Yu, K.; Liu, H.; Sheng, Q.; Zhang, Y. Effects of Bacillus subtilis on Rose Growth Promotion and Rhizosphere Microbial Community Changes under Saline–Alkaline Stress. Agronomy 2024, 14, 730. [Google Scholar] [CrossRef]



| TN g/kg | TP g/kg | TKg/kg | AP mg/kg | AK mg/kg | NN mg/kg | AN mg/kg | OC g/kg | MBC mg/kg | pH | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| YJ | 0.82 ± 0.08 A | 0.68 ± 0.10 a | 19.29 ± 0.39 A | 8.95 ± 1.91 A | 114.59 ± 4.99 A | 121.68 ± 25.04 A | 11.69 ± 1.83 a | 13.86 ± 2.28 a | 432.58 ± 106.84 a | 6.20 ± 0.12 A | 1.34 ± 0.11 B |
| CK | 0.62 ± 0.09 B | 0.54 ± 0.06 b | 18.03 ± 0.53 B | 5.21 ± 1.37 B | 78.63 ± 7.56 B | 94.33 ± 2.32 B | 11.21 ± 0.93 a | 10.18 ± 2.90 a | 328.59 ± 118.64 b | 5.78 ± 0.08 B | 2.64 ± 0.32 A |
| Sample | ACE | Chao | Sobs | Simpson | Shannon | Coverage | |
|---|---|---|---|---|---|---|---|
| Bacterial | YJ | 5672.20 ± 262.04 A | 5467.85 ± 228.87 A | 4646.40 ± 233.96 A | 0.0033 ± 0.0016 a | 7.10 ± 0.18 a | 0.9744 |
| CK | 4944.62 ± 230.38 B | 4757.75 ± 207.22 B | 4040.40 ± 214.15 B | 0.0065 ± 0.0034 a | 6.70 ± 0.21 b | 0.9772 | |
| Fungal | YJ | 494.92 ± 31.96 a | 493.95 ± 33.03 a | 484.00 ± 30.39 a | 0.073 ± 0.045 a | 3.83 ± 0.41 a | 0.9996 |
| CK | 516.60 ± 60.92 a | 516.85 ± 61.68 a | 492.00 ± 47.63 a | 0.093 ± 0.030 a | 3.46 ± 0.26 a | 0.9993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, J.; Wang, C.; Chu, P.; Ren, C.; Hou, F.; Zhang, Y.; Shang, X.; Zhao, Q.; Hua, X.; Zhang, H. Bacillus subtilis Strain YJ-15, Isolated from the Rhizosphere of Wheat Grown under Saline Conditions, Increases Soil Fertility and Modifies Microbial Community Structure. Microorganisms 2024, 12, 2023. https://doi.org/10.3390/microorganisms12102023
Sui J, Wang C, Chu P, Ren C, Hou F, Zhang Y, Shang X, Zhao Q, Hua X, Zhang H. Bacillus subtilis Strain YJ-15, Isolated from the Rhizosphere of Wheat Grown under Saline Conditions, Increases Soil Fertility and Modifies Microbial Community Structure. Microorganisms. 2024; 12(10):2023. https://doi.org/10.3390/microorganisms12102023
Chicago/Turabian StyleSui, Junkang, Chenyu Wang, Pengfei Chu, Changqing Ren, Feifan Hou, Yuxuan Zhang, Xueting Shang, Qiqi Zhao, Xuewen Hua, and Hengjia Zhang. 2024. "Bacillus subtilis Strain YJ-15, Isolated from the Rhizosphere of Wheat Grown under Saline Conditions, Increases Soil Fertility and Modifies Microbial Community Structure" Microorganisms 12, no. 10: 2023. https://doi.org/10.3390/microorganisms12102023






