Silver and Copper Nanoparticles Hosted by Carboxymethyl Cellulose Reduce the Infective Effects of Enterotoxigenic Escherichia coli:F4 on Porcine Intestinal Enterocyte IPEC-J2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cu0/CMC and Ag0/CMC
2.3. Characterization of MNP/CMC by Dynamic Light Scattering (DLS)
2.4. Study of MNP/CMC by ATR-FTIR Spectroscopy
2.5. Enterotoxigenic E. coli F4 Fimbriae (ETEC:F4) Bacteria Culture
2.6. Porcine Intestinal Enterocyte IPEC-J2 Culture
2.7. Bactericidal Activity of MNP/CMC
2.8. Disk Diffusion Assay
2.9. Cytotoxic Effect of MNP/CMC on IPEC-J2
2.10. Infection Assay and Cytotoxicity Assay of MNP/CMC on Infected IPEC-J2
2.11. Statistical Analysis
3. Results and Discussion
3.1. Dispersion of Cu0/CMC and of Ag0/CMC
3.2. Characterization of MNP/CMC–Carboxyl Interaction
3.3. Effect of Cu0/CMC and Ag0/CMC on IPEC-J2 Viability
3.4. Effect of MNP/CMC on ETEC:F4 Bacteria Viability
3.5. Cytotoxicity of MNP/CMC on the Proliferation of ETEC:F4 EcL8559 Strain on the Infected IPEC-J2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Zhang, W.; Zhao, M.; Ruesch, L.; Omot, A.; Francis, D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 2007, 123, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, A.-M.; Lundgren, A. Developments in Oral Enterotoxigenic Escherichia Coli Vaccines. Curr. Opin. Immunol. 2023, 84, 102372–102380. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar]
- Nguyet, L.T.Y.; Ounjai, P.; Kaeoket, K.; Ngamwongsatit, N. Feasibility of crude F4 fimbriae extract as a vaccine candidate for preventing Escherichia coli-induced diarrhea in piglets. Vet. World 2023, 16, 2063–2070. [Google Scholar] [CrossRef]
- de Lagarde, M.; Vanier, G.; Desmarais, G.; Kohan-Ghadr, H.R.; Arsenault, J.; Fairbrother, J.M. A new multidrug-resistant enterotoxigenic Escherichia coli pulsed-field gel electrophoresis cluster associated with enrofloxacin non-susceptibility in diseased pigs. J. Appl. Microbiol. 2021, 130, 707–721. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, X.; Chen, G.; Wang, F.; Ren, J. AuNPs-PCL Nanocomposite Accelerated Abdominal Wound Healing through Photothermal Effect and Improving Cell Adhesion. J. Biomater. Sci. Polym. Ed. 2018, 29, 2035–2049. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Metal-Based Antibacterial Substrates for Biomedical Applications. Biomacromolecules 2015, 16, 1873–1885. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Rdzawski, Z. Antibacterial Properties of Copper and Its Alloys. Arch. Mater. Sci. Eng. 2012, 56, 53–60. [Google Scholar]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Stabilisers. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 689–694. ISBN 978-0-12-818766-1. [Google Scholar]
- Ergun, R.; Guo, J.; Huebner-Keese, B. Cellulose. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 694–702. [Google Scholar]
- Ispas-Szabo, P.; De Koninck, P.; Calinescu, C.; Mateescu, M.A. Carboxymethyl Starch Excipients for Drug Chronodelivery. Aaps Pharmscitech 2017, 18, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.-Q.; Cui, F.-Z.; Kim, T.; Kim, J. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia Coli and Staphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Noori, F.; Megoura, M.; Labelle, M.-A.; Mateescu, M.A.; Azzouz, A. Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation. Antibiotics 2022, 11, 439. [Google Scholar] [CrossRef]
- El Riz, A.; Neree, A.T.; Mousavifar, L.; Roy, R.; Chorfi, Y.; Mateescu, M.A. Metallo-Glycodendrimeric Materials against Enterotoxigenic Escherichia coli. Microorganisms 2024, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Jayaramudu, T.; Varaprasad, K.; Sadiku, R.; Ray, S.S.; Raju, K.M. Cellulose–Polymer–Ag Nanocomposite Fibers for Antibacterial Fabrics/Skin Scaffolds. Carbohydr. Polym. 2013, 93, 553–560. [Google Scholar] [CrossRef]
- de Britto, D.; Assis, O.B. Thermal Degradation of Carboxymethylcellulose in Different Salty Forms. Thermochim. Acta 2009, 494, 115–122. [Google Scholar] [CrossRef]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–134. [Google Scholar]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia Coli in Postweaning Diarrhea in Pigs: An Update on Bacterial Types, Pathogenesis, and Prevention Strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 1–19. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, É. Colibacillosis. Dis. Swine 2019, 807–834. [Google Scholar]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of Lactate Dehydrogenase for Cell Viability Testing Using Cell Lines and Primary Cultured Astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2.26.1–2.26.10. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.-L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Skomorokhova, E.A.; Sankova, T.P.; Orlov, I.A.; Savelev, A.N.; Magazenkova, D.N.; Pliss, M.G.; Skvortsov, A.N.; Sosnin, I.M.; A Kirilenko, D.; Grishchuk, I.V.; et al. Size-Dependent Bioactivity of Silver Nanoparticles: Antibacterial Properties, Influence on Copper Status in Mice, and Whole-Body Turnover. Nanotechnol. Sci. Appl. 2020, 13, 137–157. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, W.; Ding, R.; Wang, J.; Yao, L. Mechanism of low concentrations of polystyrene microplastics influence the cytotoxicity of Ag ions to Escherichia coli. Chemosphere 2020, 253, 126705. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Li, Z.; Zhong, Z.; Ruan, Y.; Sun, L.; Zuo, F.; Li, L.; Hou, S. Size-dependent enhancement on conjugative transfer of antibiotic resistance genes by micro/nanoplastics. J. Hazard. Mater. 2022, 431, 128561–128573. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Salamanca-Cardona, L.; Ashe, C.S.; Stipanovic, A.J.; Nomura, C.T. Enhanced production of polyhydroxyalkanoates (PHAs) from beechwood xylan by recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Xu, W.; Shi, D.; Chen, K.; Palmer, J.; Popovich, D.G. An improved MTT colorimetric method for rapid viable bacteria counting. J. Microbiol. Methods 2023, 214, 106830. [Google Scholar] [CrossRef]
- Benov, L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS ONE 2019, 14, e0219713. [Google Scholar] [CrossRef]
- Geens, M.M.; Niewold, T.A. Preliminary Characterization of the Transcriptional Response of the Porcine Intestinal Cell Line IPEC-J2 to Enterotoxigenic Escherichia Coli, Escherichia Coli, and E. Coli Lipopolysaccharide. Int. J. Genom. 2010, 2010, 469583–469594. [Google Scholar]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2011, 156, 229–237. [Google Scholar] [CrossRef]
- Noori, F.; Neree, A.T.; Megoura, M.; Mateescu, M.A.; Azzouz, A. Insights into the metal retention role in the antibacterial behavior of montmorillonite and cellulose tissue-supported copper and silver nanoparticles. RSC Adv. 2021, 11, 24156–24171. [Google Scholar] [CrossRef]
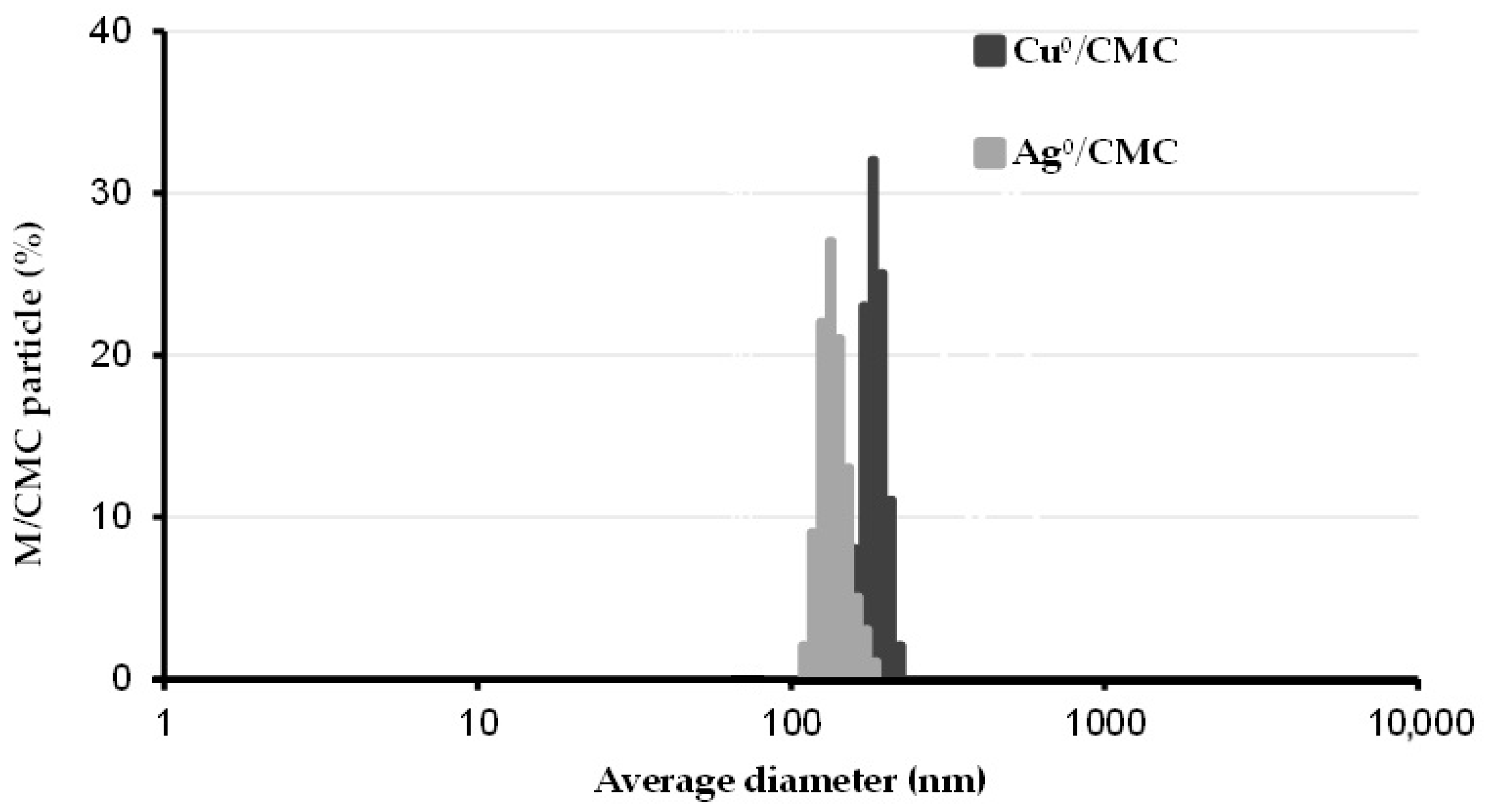


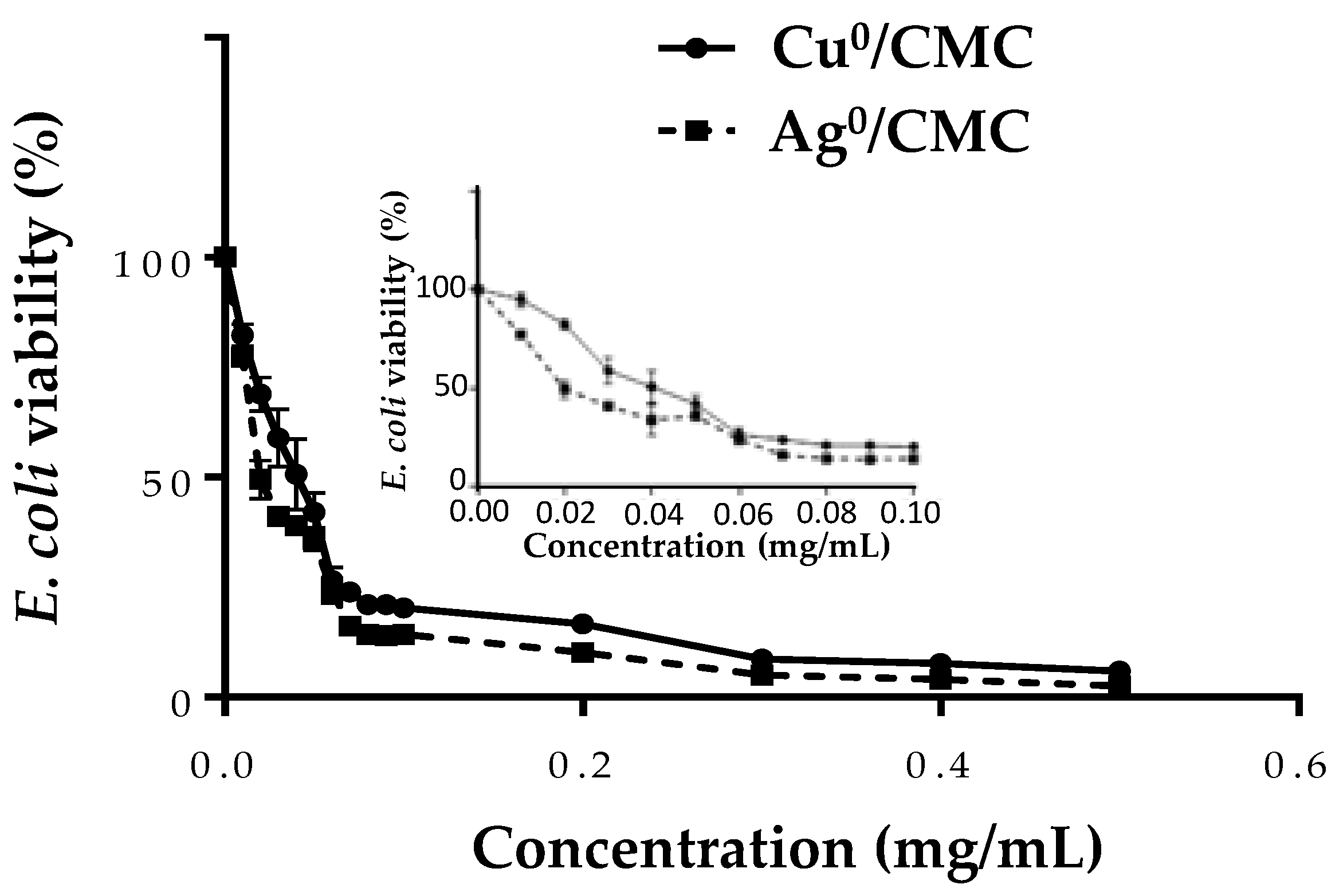
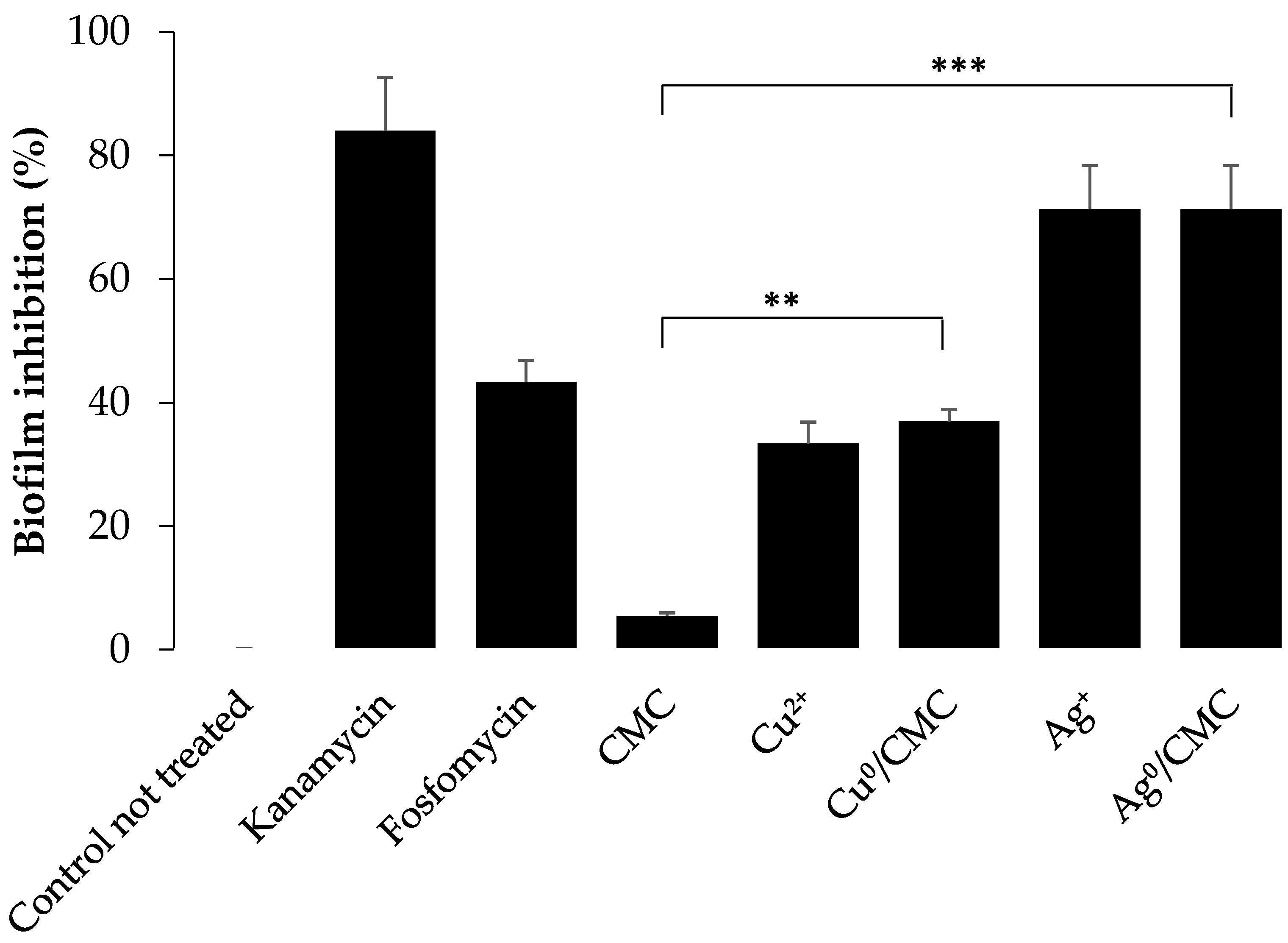
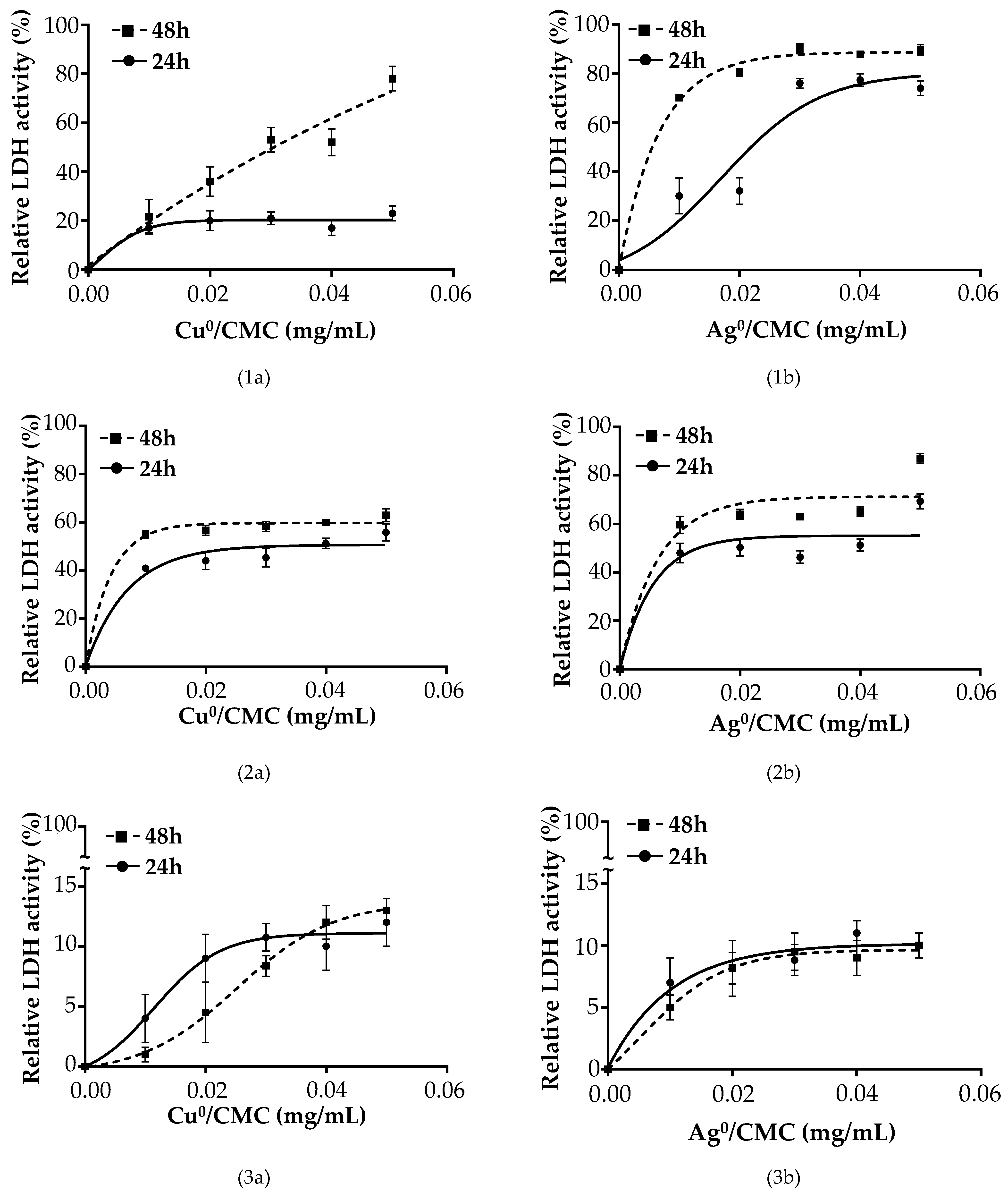
| Characteristics | Measured Parameters * | CMC | Cu0/CMC | Ag0/CMC |
|---|---|---|---|---|
| Particle size and dispersion | Z average (nm) | 367 ± 39 | 218 ± 17 | |
| Average diameter (nm) | 369 ± 23 | 210 ± 11 | ||
| PDI | 0.96 ± 0.06 | 0.25 ± 0.02 | ||
| Main IR vibration bands (cm−1) | O-H vibration | 3255 | 3343 | 3371 |
| C-H stretching of -CH2 group | 2872 | 2872 | 2882 | |
| C=O stretching of polysaccharides | 1591 | 1583 | 1588 | |
| O-H bending carboxylic group | 1411 | 1409 | 1409 | |
| O-H bending of polysaccharide chains | 1323 | 1325 | 1326 | |
| C-O bending and C-O-C stretching | 1021 | 1015 | 1110 |
| Antibacterial Agents | Inhibition Diameter (cm) | |
|---|---|---|
| Human-used antibacterial agent | Kanamycin | 2.6 ± 0.33 |
| Porcine-used antibacterial agent | Fosfomycin | 1.2 ± 0.21 |
| CMC | - | |
| Metal nanoparticles | Cu0/CMC | 1.0 ± 0.1 |
| Ag0/CMC | 2.2 ± 0.3 | |
| Metal ions * | Cu2+ | 1.2 ± 0.1 |
| Ag+ | 2.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchoumi Neree, A.; Noori, F.; Azzouz, A.; Costa, M.; Fairbrother, J.M.; Mateescu, M.A.; Chorfi, Y. Silver and Copper Nanoparticles Hosted by Carboxymethyl Cellulose Reduce the Infective Effects of Enterotoxigenic Escherichia coli:F4 on Porcine Intestinal Enterocyte IPEC-J2. Microorganisms 2024, 12, 2026. https://doi.org/10.3390/microorganisms12102026
Tchoumi Neree A, Noori F, Azzouz A, Costa M, Fairbrother JM, Mateescu MA, Chorfi Y. Silver and Copper Nanoparticles Hosted by Carboxymethyl Cellulose Reduce the Infective Effects of Enterotoxigenic Escherichia coli:F4 on Porcine Intestinal Enterocyte IPEC-J2. Microorganisms. 2024; 12(10):2026. https://doi.org/10.3390/microorganisms12102026
Chicago/Turabian StyleTchoumi Neree, Armelle, Farzaneh Noori, Abdelkrim Azzouz, Marcio Costa, John Morris Fairbrother, Mircea Alexandru Mateescu, and Younes Chorfi. 2024. "Silver and Copper Nanoparticles Hosted by Carboxymethyl Cellulose Reduce the Infective Effects of Enterotoxigenic Escherichia coli:F4 on Porcine Intestinal Enterocyte IPEC-J2" Microorganisms 12, no. 10: 2026. https://doi.org/10.3390/microorganisms12102026








