A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste
Abstract
:1. Introduction
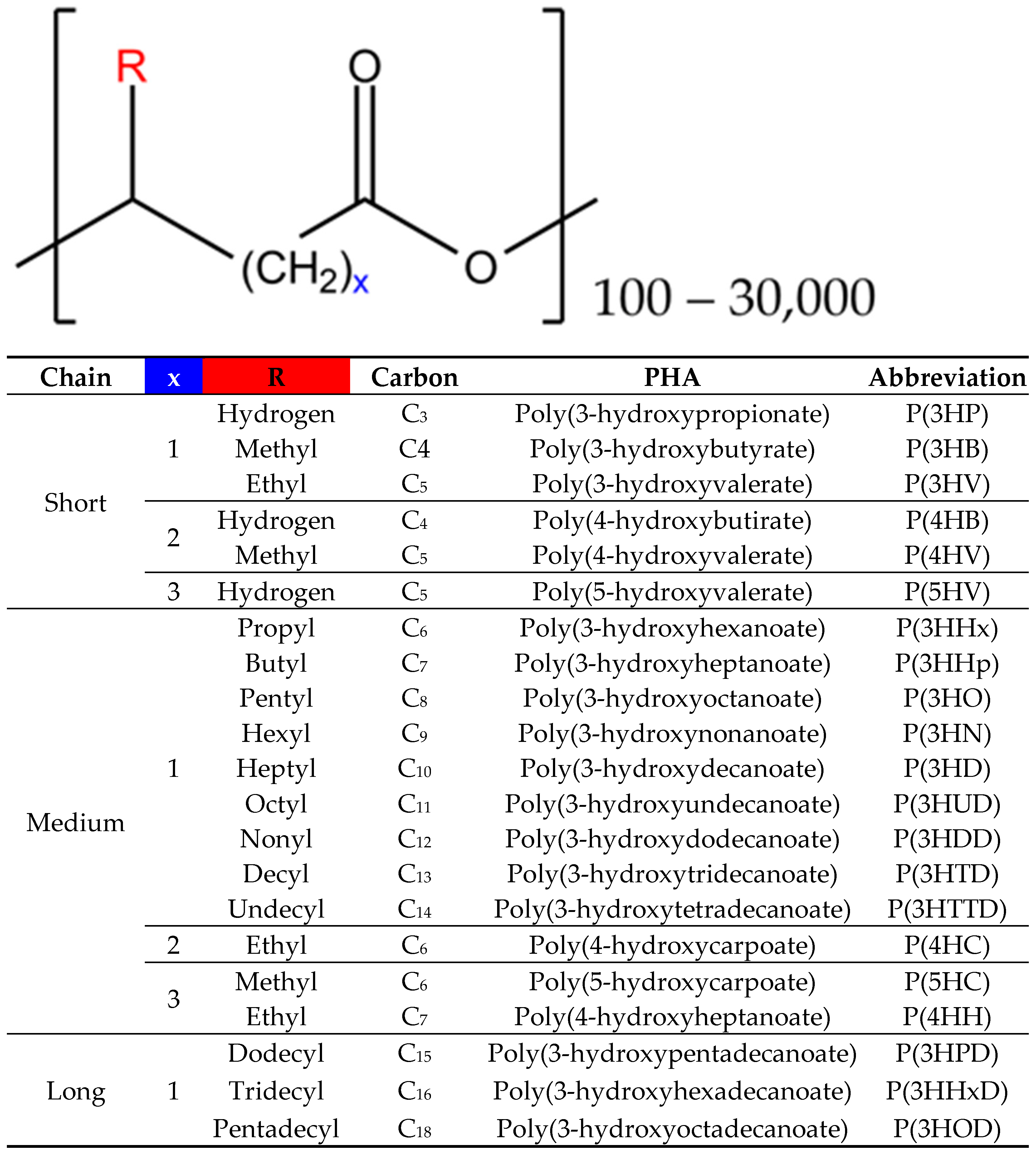
2. Characteristics and Applications of PHAs
3. Molecular Mechanisms
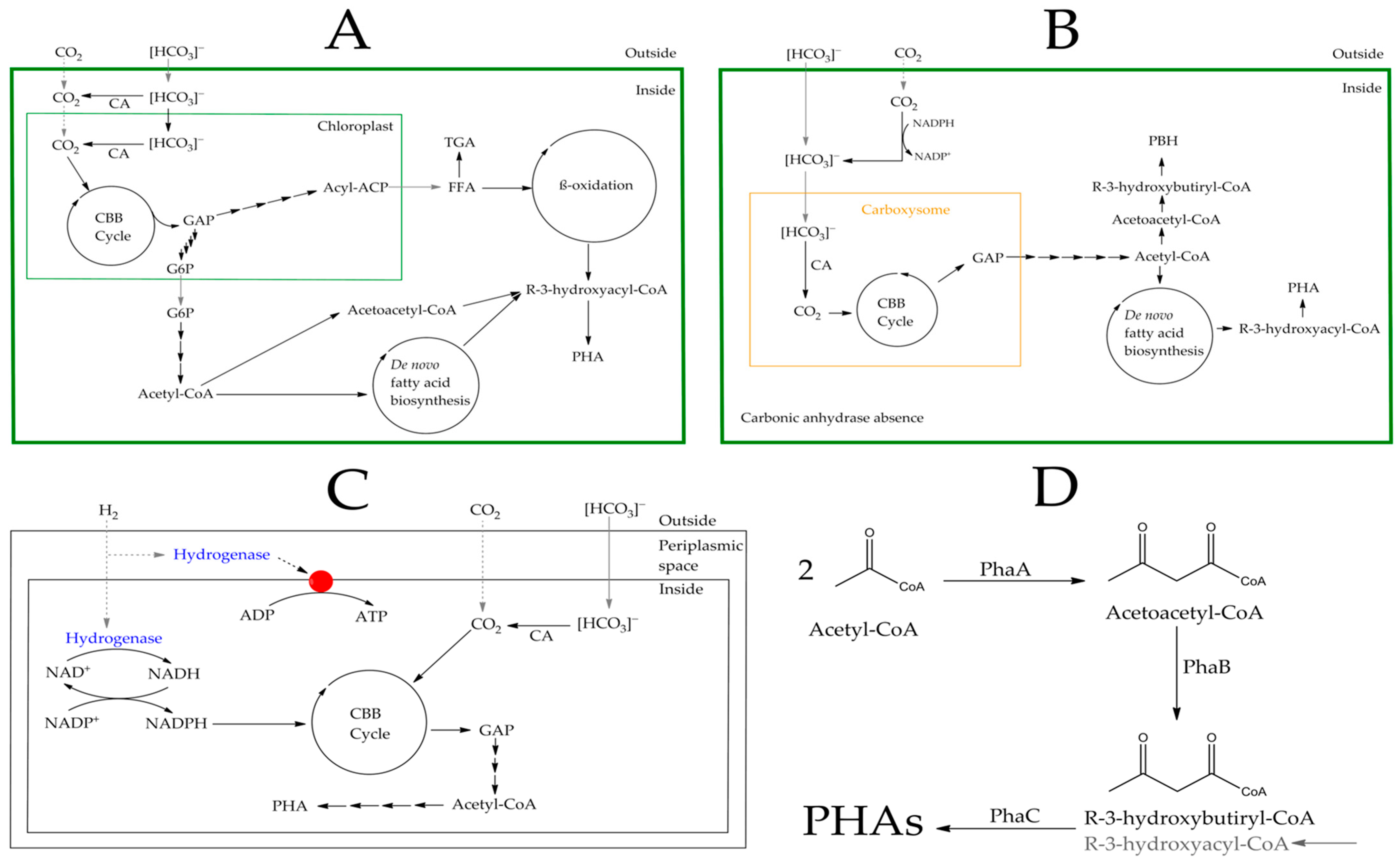
3.1. Autotrophic Biosynthesis
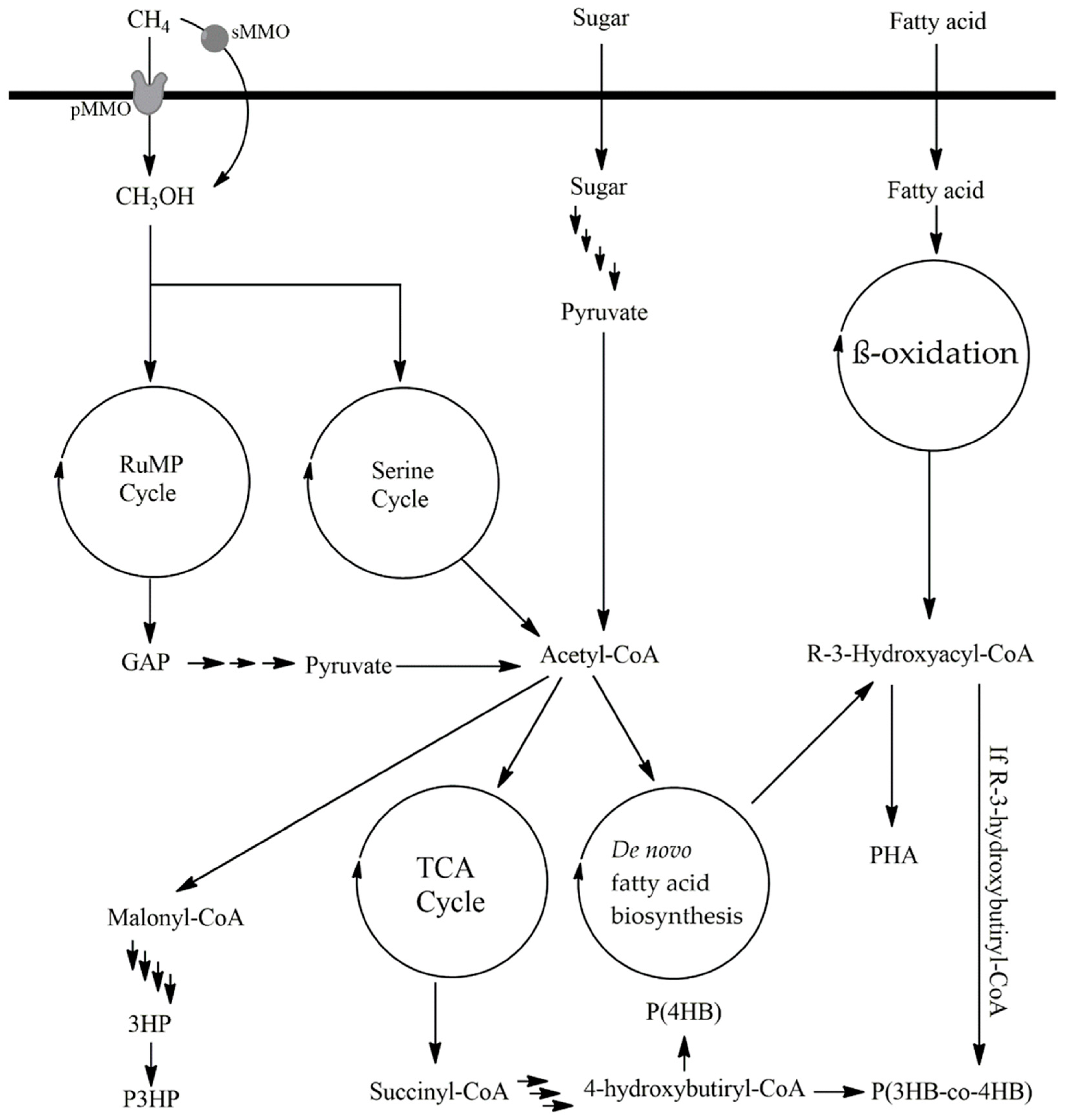
3.2. Heterotrophic Biosynthesis
4. Waste as a Resource to Produce Bioplastics
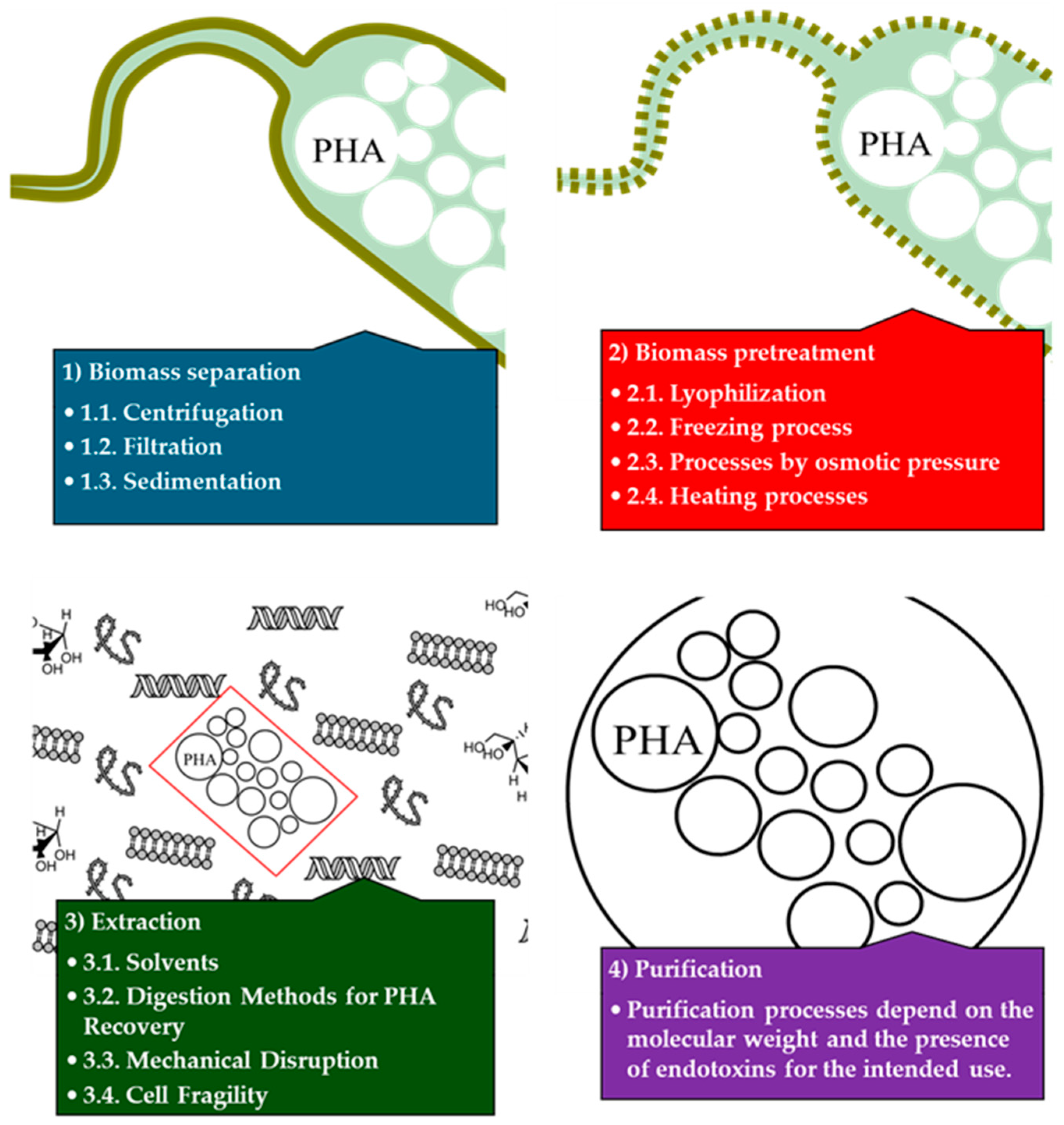
5. PHAs Extraction and Purification Process
5.1. Biomass Separation
5.2. Biomass Pretreatment
5.3. Extraction
5.4. Purification
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi. PHA-Based Bioplastic: A Potential Alternative to Address Microplastic Pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef] [PubMed]
- Vandi, L.-J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Wood-PHA Composites: Mapping Opportunities. Polymers 2018, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts 2023; Plastics Europe AISBL: Brussels, Belgium, 2023; Available online: https://plasticseurope.org/es/plastics-europe-publica-plastics-the-fast-facts-2023/ (accessed on 26 August 2024).
- Hundertmark, T.; Mayer, M.; McNally, C.; Simons, T.J.; Witte, C. How Plastics Waste Recycling Could Transform the Chemical Industry. McKinsey & Company. 2018. Available online: https://www.mckinsey.com/~/media/McKinsey/Industries/Chemicals/Our%20Insights/How%20plastics%20waste%20recycling%20could%20transform%20the%20chemical%20industry/How-plastics-waste-recycling-could-transform.pdf (accessed on 26 August 2024).
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotechnol. 2023, 15, 100254. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 88. [Google Scholar] [CrossRef]
- Diniz, M.S.d.F.; Mourão, M.M.; Xavier, L.P.; Santos, A.V. Recent Biotechnological Applications of Polyhydroxyalkanoates (PHA) in the Biomedical Sector—A Review. Polymers 2023, 15, 4405. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Colwell, J.; Pratt, S.; Lant, P.; Laycock, B. Hazardous state lifetimes of biodegradable plastics in natural environments. Sci. Total Environ. 2023, 894, 165025. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Oluwoye, I.; Machuca, L.L.; Higgins, S.; Suh, S.; Galloway, T.S.; Halley, P.; Tanaka, S.; Iannuzzi, M. Degradation and lifetime prediction of plastics in subsea and offshore infrastructures. Sci. Total Environ. 2023, 904, 166719. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Ali, S.; Bukhari, D.A.; Rehman, A. Call for biotechnological approach to degrade plastic in the era of COVID-19 pandemic. Saudi J. Biol. Sci. 2023, 30, 103583. [Google Scholar] [CrossRef]
- Oliveira, J.; Almeida, P.L.; Sobral, R.G.; Lourenço, N.D.; Gaudêncio, S.P. Marine-Derived Actinomycetes: Biodegradation of Plastics and Formation of PHA Bioplastics—A Circular Bioeconomy Approach. Mar. Drugs 2022, 20, 760. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Fusco, A.; Al-Asmar, A.; Mariniello, L. Microbial Transglutaminase as a Tool to Improve the Features of Hydrocolloid-Based Bioplastics. Int. J. Mol. Sci. 2020, 21, 3656. [Google Scholar] [CrossRef]
- Fukala, I.; Kučera, I. Natural Polyhydroxyalkanoates—An Overview of Bacterial Production Methods. Molecules 2024, 29, 2293. [Google Scholar] [CrossRef]
- Costa, A.; Encarnação, T.; Tavares, R.; Todo Bom, T.; Mateus, A. Bioplastics: Innovation for Green Transition. Polymers 2023, 15, 517. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koller, M. Microbial PolyHydroxyAlkanoate (PHA) Biopolymers—Intrinsically Natural. Bioengineering 2023, 10, 855. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef]
- Miu, D.-M.; Eremia, M.C.; Moscovici, M. Polyhydroxyalkanoates (PHAs) as Biomaterials in Tissue Engineering: Production, Isolation, Characterization. Materials 2022, 15, 1410. [Google Scholar] [CrossRef]
- Alves, A.A.; Siqueira, E.C.; Barros, M.P.S.; Silva, P.E.C.; Houllou, L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2023, 20, 3409–3420. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Ishak, K.A.; Velayutham, T.S.; Annuar, M.S.M.; Sirajudeen, A.A.O. Structure-property interpretation of biological polyhydroxyalkanoates with different monomeric composition: Dielectric spectroscopy investigation. Int. J. Biol. Macromol. 2021, 169, 311–320. [Google Scholar] [CrossRef]
- Koller, M. Chemical and Biochemical Engineering Approaches in Manufacturing Polyhydroxyalkanoate (PHA) Biopolyesters of Tailored Structure with Focus on the Diversity of Building Blocks. Chem. Biochem. Eng. Q. 2018, 32, 413–438. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, R.; Wang, Z.; Deng, Y.; Chen, G.-Q. Application of (R)-3-Hydroxyalkanoate Methyl Esters Derived from Microbial Polyhydroxyalkanoates as Novel Biofuels. Biomacromolecules 2009, 10, 707–711. [Google Scholar] [CrossRef]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Reddy, V.U.N.; Ramanaiah, S.V.; Reddy, M.V.; Chang, Y.-C. Review of the Developments of Bacterial Medium-Chain-Length Polyhydroxyalkanoates (mcl-PHAs). Bioengineering 2022, 9, 225. [Google Scholar] [CrossRef]
- Basnett, P.; Lukasiewicz, B.; Marcello, E.; Gura, H.K.; Knowles, J.C.; Roy, I. Production of a novel medium chain length poly(3-hydroxyalkanoate) using unprocessed biodiesel waste and its evaluation as a tissue engineering scaffold. Microb. Biotechnol. 2017, 10, 1384–1399. [Google Scholar] [CrossRef] [PubMed]
- Aldor, I.S.; Keasling, J.D. Process design for microbial plastic factories: Metabolic engineering of polyhydroxyalkanoates. Curr. Opin. Biotechnol. 2003, 14, 475–483. [Google Scholar] [CrossRef]
- Song, H.M.; Joo, J.C.; Lim, S.H.; Lim, H.J.; Lee, S.; Park, S.J. Production of polyhydroxyalkanoates containing monomers conferring amorphous and elastomeric properties from renewable resources: Current status and future perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Van Thuoc, D.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently from Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef]
- Abe, H.; Doi, Y. Side-chain effect of second monomer units on crystalline morphology, thermal properties, and enzymatic degradability for random copolyesters of (R)-3-hydroxybutyric acid with (R)-3-hydroxyalkanoic acids. Biomacromolecules 2002, 3, 133–138. [Google Scholar] [CrossRef]
- Hanik, N.; Utsunomia, C.; Arai, S.; Matsumoto, K.I.; Zinn, M. Influence of Unusual Co-substrates on the Biosynthesis of Medium-Chain-Length Polyhydroxyalkanoates Produced in Multistage Chemostat. Front. Bioeng. Biotechnol. 2019, 7, 301. [Google Scholar] [CrossRef]
- Chen, G.G.-Q. Plastics from Bacteria: Natural Functions and Applications. Plast. Bact. Nat. Funct. Appl. 2010, 14, 1–450. [Google Scholar] [CrossRef]
- Guzik, M.; Witko, T.; Steinbüchel, A.; Wojnarowska, M.; Sołtysik, M.; Wawak, S. What Has Been Trending in the Research of Polyhydroxyalkanoates? A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Snoch, W.; Wnuk, D.; Witko, T.; Staroń, J.; Bojarski, A.J.; Jarek, E.; Plou, F.J.; Guzik, M. In Search of Effective Anticancer Agents—Novel Sugar Esters Based on Polyhydroxyalkanoate Monomers. Int. J. Mol. Sci. 2021, 22, 7238. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, H.W.; Chung, M.G.; Rhee, Y.H. Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates. J. Microbiol. 2007, 45, 87–97. [Google Scholar] [PubMed]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Pandey, A.; Adama, N.; Adjallé, K.; Blais, J.-F. Sustainable applications of polyhydroxyalkanoates in various fields: A critical review. Int. J. Biol. Macromol. 2022, 221, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Thamarai, P.; Vickram, A.S.; Saravanan, A.; Deivayanai, V.C.; Evangeline, S. Recent advancements in biosynthesis, industrial production, and environmental applications of polyhydroxyalkanoates (PHAs): A review. Bioresour. Technol. Rep. 2024, 27, 101957. [Google Scholar] [CrossRef]
- Bano, S.; Aslam, A.A.; Khan, A.; Shabbir, A.; Qayyum, F.; Wahab, N.; Jabar, A.; Ul Islam, I.; Ng, S.L. A mini-review on polyhydroxyalkanoates: Synthesis, extraction, characterization, and applications. Process Biochem. 2024, 146, 250–261. [Google Scholar] [CrossRef]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of polyhydroxyalkanoates from renewable resources: A review on prospects, challenges and applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and its efficient production: An eco-friendly approach towards development. 3 Biotech 2020, 10, 549. [Google Scholar] [CrossRef]
- Xiong, Y.-C.; Yao, Y.-C.; Zhan, X.-Y.; Chen, G.-Q. Application of Polyhydroxyalkanoates Nanoparticles as Intracellular Sustained Drug-Release Vectors. J. Biomater. Sci. Polym. Ed. 2010, 21, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N. A tool for biomedical application: Synthesis and modification of polyhydroxyalkanoates. Sustain. Chem. Pharm. 2023, 32, 101041. [Google Scholar] [CrossRef]
- Lukasiewicz, B.; Basnett, P.; Nigmatullin, R.; Matharu, R.; Knowles, J.C.; Roy, I. Binary polyhydroxyalkanoate systems for soft tissue engineering. Acta Biomater. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef] [PubMed]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A.; González-Martínez, C.; Vargas, M. Production of Polyhydroxyalkanoates for Biodegradable Food Packaging Applications Using Haloferax mediterranei and Agrifood Wastes. Foods 2024, 13, 950. [Google Scholar] [CrossRef]
- González, E.; Herencias, C.; Prieto, M.A. A polyhydroxyalkanoate-based encapsulating strategy for ‘bioplasticizing’ microorganisms. Microb. Biotechnol. 2020, 13, 185–198. [Google Scholar] [CrossRef]
- Riera, M.A.; Palma, R.R. Multicriteria Analysis in the Selection of Agro-Industrial Waste for the Production of Biopolymers. In Advances in the Domain of Environmental Biotechnology: Microbiological Developments in Industries, Wastewater Treatment and Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 335–356. [Google Scholar]
- Kim, S.; Jang, Y.J.; Gong, G.; Lee, S.-M.; Um, Y.; Kim, K.H.; Ko, J.K. Engineering Cupriavidus necator H16 for enhanced lithoautotrophic poly(3-hydroxybutyrate) production from CO2. Microb. Cell Factories 2022, 21, 231. [Google Scholar] [CrossRef]
- Windhagauer, M.; Doblin, M.A.; Signal, B.; Kuzhiumparambil, U.; Fabris, M.; Abbriano, R.M. Metabolic response to a heterologous poly-3-hydroxybutyrate (PHB) pathway in Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2024, 108, 104. [Google Scholar] [CrossRef]
- Afreen, R.; Tyagi, S.; Singh, G.P.; Singh, M. Challenges and Perspectives of Polyhydroxyalkanoate Production from Microalgae/Cyanobacteria and Bacteria as Microbial Factories: An Assessment of Hybrid Biological System. Front. Bioeng. Biotechnol. 2021, 9, 624885. [Google Scholar] [CrossRef]
- Simonazzi, M.; Pezzolesi, L.; Galletti, P.; Gualandi, C.; Pistocchi, R.; De Marco, N.; Paganelli, Z.; Samorì, C. Production of polyhydroxybutyrate by the cyanobacterium cf. Anabaena sp. Int. J. Biol. Macromol. 2021, 191, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Sundaram, S.; Sinha, S.; Rahman, M.A.; Kapur, S. Recent advances in CO2 uptake and fixation mechanism of cyanobacteria and microalgae. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1297–1323. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; Assis, D.d.J.; Souza, C.O.; de Morais, M.G.; Vieira Costa, J.A.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Rueda, E.; Altamira-Algarra, B.; García, J. Process optimization of the polyhydroxybutyrate production in the cyanobacteria Synechocystis sp. and Synechococcus sp. Bioresour. Technol. 2022, 356, 127330. [Google Scholar] [CrossRef]
- Kharwar, S.; Mishra, A.K. Unraveling the complexities underlying sulfur deficiency and starvation in the cyanobacterium Anabaena sp. PCC 7120. Environ. Exp. Bot. 2020, 172, 103966. [Google Scholar] [CrossRef]
- García, G.; Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Iqbal, H.; Parra-Saldívar, R. Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions. Polymers 2021, 13, 131. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef]
- Bacha, S.; Arous, F.; Chouikh, E.; Jaouani, A.; Gtari, M.; Charradi, K.; Attia, H.; Ghorbel, D. Exploring Bacillus amyloliquefaciens strain OM81 for the production of polyhydroxyalkanoate (PHA) bioplastic using olive mill wastewater. 3 Biotech 2023, 13, 415. [Google Scholar] [CrossRef]
- Sabirova, J.S.; Ferrer, M.; Lünsdorf, H.; Wray, V.; Kalscheuer, R.; Steinbüchel, A.; Timmis, K.N.; Golyshin, P.N. Mutation in a “tesB-like” hydroxyacyl-coenzyme a-specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J. Bacteriol. 2006, 188, 8452–8459. [Google Scholar] [CrossRef]
- Page, W.J.; Cornish, A. Growth of Azotobacter vinelandii UWD in Fish Peptone Medium and Simplified Extraction of Poly-β-Hydroxybutyrate. Appl. Environ. Microbiol. 1993, 59, 4236–4244. [Google Scholar] [CrossRef]
- Andler, R.; González-Arancibia, F.; Vilos, C.; Sepulveda-Verdugo, R.; Castro, R.; Mamani, M.; Valdés, C.; Arto-Paz, F.; Díaz-Barrera, A.; Martínez, I. Production of poly-3-hydroxybutyrate (PHB) nanoparticles using grape residues as the sole carbon source. Int. J. Biol. Macromol. 2024, 261, 129649. [Google Scholar] [CrossRef] [PubMed]
- San Miguel-González, G.d.J.; Alemán-Huerta, M.E.; Martínez-Herrera, R.E.; Quintero-Zapata, I.; de la Torre-Zavala, S.; Avilés-Arnaut, H.; Gandarilla-Pacheco, F.L.; de Luna-Santillana, E.d.J. Alkaline-Tolerant Bacillus cereus 12GS: A Promising Polyhydroxybutyrate (PHB) Producer Isolated from the North of Mexico. Microorganisms 2024, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.S.; Shamala, T.R. Extractability of polyhydroxyalkanoate synthesized by Bacillus flexus cultivated in organic and inorganic nutrient media. Indian J. Microbiol. 2010, 50, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of desugarized sugar beet molasses for the production of poly(3-hydroxybutyrate) by halophilic Bacillus megaterium uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Madi, F.; Hachicha, R.; Gamero, J.E.R.; Gupte, A.P.; Gronchi, N.; Haddad, M.; Favaro, L.; Casella, S.; Basaglia, M. Exploitation of spoilage dates as biomass for the production of bioethanol and polyhydroxyalkanoates. Renew. Energy 2024, 220, 119655. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Rodriguez, J.E.G.; Morelli, A.; Ibraheem, O.; Pizzocchero, V.; Casella, S. Bacterial Production of PHAs from Lipid-Rich by-Products. Appl. Food Biotechnol. 2019, 6, 45–52. [Google Scholar] [CrossRef]
- Ramos, L.H.; Cisneros-Yupanqui, M.; Santisteban Soto, D.V.; Lante, A.; Favaro, L.; Casella, S.; Basaglia, M. Exploitation of Cocoa Pod Residues for the Production of Antioxidants, Polyhydroxyalkanoates, and Ethanol. Fermentation 2023, 9, 843. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar Beet Molasses as a Potential C-Substrate for PHA Production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Giovanna Romanelli, M.; Povolo, S.; Favaro, L.; Fontana, F.; Basaglia, M.; Casella, S. Engineering Delftia acidovorans DSM39 to produce polyhydroxyalkanoates from slaughterhouse waste. Int. J. Biol. Macromol. 2014, 71, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mozejko-Ciesielska, J.; Moraczewski, K.; Czaplicki, S.; Singh, V. Production and characterization of polyhydroxyalkanoates by Halomonas alkaliantarctica utilizing dairy waste as feedstock. Sci. Rep. 2023, 13, 22289. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, M.S.; Pai, J.S.; Ganesh, M. Production and recovery of poly-3-hydroxybutyrate from Methylobacterium sp V49. J. Chem. Technol. Biotechnol. 2002, 77, 444–448. [Google Scholar] [CrossRef]
- Tyagi, B.; Takkar, S.; Meena, R.; Thakur, I.S. Production of polyhydroxybutyrate (PHB) by Parapedobacter sp. ISTM3 isolated from Mawsmai cave utilizing molasses as carbon source. Environ. Technol. Innov. 2021, 24, 101854. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, M.; Cai, C.; Chen, S.; Jin, M. Microbial polyhydroxyalkanoate production from lignin by Pseudomonas putida NX-1. Bioresour. Technol. 2021, 319, 124210. [Google Scholar] [CrossRef] [PubMed]
- Drakonaki, A.; Mathioudaki, E.; Geladas, E.D.; Konsolaki, E.; Vitsaxakis, N.; Chaniotakis, N.; Xie, H.; Tsiotis, G. Production of Polyhydroxybutyrate by Genetically Modified Pseudomonas sp. phDV1: A Comparative Study of Utilizing Wine Industry Waste as a Carbon Source. Microorganisms 2023, 11, 1592. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef]
- Koch, M.; Berendzen, K.W.; Forchhammer, K. On the Role and Production of Polyhydroxybutyrate (PHB) in the Cyanobacterium Synechocystis sp. PCC 6803. Life 2020, 10, 47. [Google Scholar] [CrossRef]
- Li, D.; Yin, F.; Ma, X. Towards biodegradable polyhydroxyalkanoate production from wood waste: Using volatile fatty acids as conversion medium. Bioresour. Technol. 2020, 299, 122629. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Su, Y.; Yan, X.; Wang, F.; Yu, L.; Ma, X. Efficient and economical production of polyhydroxyalkanoate from sustainable rubber wood hydrolysate and xylose as co-substrate by mixed microbial cultures. Bioresour. Technol. 2022, 355, 127238. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Calvo, M.V.; Sciarria, T.P.; Scaglia, B.; Kizito, S.S.; D’Imporzano, G.; Adani, F. Biohydrogen and polyhydroxyalkanoates (PHA) as products of a two-steps bioprocess from deproteinized dairy wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Panich, J.; Fong, B.; Singer, S.W. Metabolic Engineering of Cupriavidus necator H16 for Sustainable Biofuels from CO2. Trends Biotechnol. 2021, 39, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Kimura, Y.; Ishii, D.; Nakaoki, T. Biosynthesis of Poly(3-hydroxyalkanoate) from Amino Acids in Medium with Nitrogen, Phosphate, and Magnesium, or Some Combination of These Nutrients. J. Polym. Environ. 2014, 22, 488–493. [Google Scholar] [CrossRef]
- Li, H.-L.; Deng, R.-X.; Wang, W.; Liu, K.-Q.; Hu, H.-B.; Huang, X.-Q.; Zhang, X.-H. Biosynthesis and Characterization of Medium-Chain-Length Polyhydroxyalkanoate with an Enriched 3-Hydroxydodecanoate Monomer from a Pseudomonas chlororaphis Cell Factory. J. Agric. Food Chem. 2021, 69, 3895–3903. [Google Scholar] [CrossRef]
- Chau, T.H.T.; Nguyen, A.D.; Lee, E.Y. Engineering type I methanotrophic bacteria as novel platform for sustainable production of 3-hydroxybutyrate and biodegradable polyhydroxybutyrate from methane and xylose. Bioresour. Technol. 2022, 363, 127898. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Chapter 6-Microbial polymers produced from methane: Overview of recent progress and new perspectives. In Microbial and Natural Macromolecules; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 117–142. [Google Scholar]
- Sakai, Y.; Yurimoto, H.; Shima, S. Methane monooxygenases; physiology, biochemistry and structure. Catal. Sci. Technol. 2023, 13, 6342–6354. [Google Scholar] [CrossRef]
- Zhu, Y.; Koo, C.W.; Cassidy, C.K.; Spink, M.C.; Ni, T.; Zanetti-Domingues, L.C.; Bateman, B.; Martin-Fernandez, M.L.; Shen, J.; Sheng, Y.; et al. Structure and activity of particulate methane monooxygenase arrays in methanotrophs. Nat. Commun. 2022, 13, 5221. [Google Scholar] [CrossRef]
- Ahmadi, F.; Bodraya, T.; Lackner, M. Methane Biofiltration Processes: A Summary of Biotic and Abiotic Factors. Methane 2024, 3, 122–148. [Google Scholar] [CrossRef]
- Eggink, G.; de Waard, P.; Huijberts, G.N.M. The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in Pseudomonas putida. FEMS Microbiol. Lett. 1992, 103, 159–163. [Google Scholar] [CrossRef]
- Huijberts, G.N.M.; Eggink, G.; de Waard, P.; Huisman, G.W.; Witholt, B. Pseudomonas putida KT2442 Cultivated on Glucose Accumulates Poly(3-Hydroxyalkanoates) Consisting of Saturated and Unsaturated Monomers. Appl. Environ. Microbiol. 1992, 58, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Yuan, X.-X.; Shi, Z.-Y.; Meng, D.-C.; Jiang, W.-J.; Wu, L.-P.; Chen, J.-C.; Chen, G.-Q. Hyperproduction of poly(4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb. Cell Fact. 2012, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, J.-W.; Zhang, X.; Huang, W.; Zhang, Z.; Lin, Y.; Zhang, G.; Wu, F.; Wang, Z.; Wu, Q.; et al. Effective production of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by engineered Halomonas bluephagenesis grown on glucose and 1,4-Butanediol. Bioresour. Technol. 2022, 355, 127270. [Google Scholar] [CrossRef] [PubMed]
- Andreeßen, B.; Lange, A.B.; Robenek, H.; Steinbüchel, A. Conversion of Glycerol to Poly(3-Hydroxypropionate) in Recombinant Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2021, 16, e2000165. [Google Scholar] [CrossRef]
- Poirier, Y.; Erard, N.; Petétot, J.M.-C. Synthesis of Polyhydroxyalkanoate in the Peroxisome of Saccharomyces cerevisiae by using intermediates of fatty acid β-oxidation. Appl. Environ. Microbiol. 2001, 67, 5254–5260. [Google Scholar] [CrossRef]
- Arias, S.; Sandoval, A.; Arcos, M.; Cañedo, L.M.; Maestro, B.; Sanz, J.M.; Naharro, G.; Luengo, J.M. Poly-3-hydroxyalkanoate synthases from Pseudomonas putida U: Substrate specificity and ultrastructural studies. Microb. Biotechnol. 2008, 1, 170–176. [Google Scholar] [CrossRef]
- Yasin, A.R.; Al-Mayaly, I.K. Study of The Fermentation Conditions of the Bacillus cereus Strain ARY73 to Produce Polyhydroxyalkanoate from Glucose. J. Ecol. Eng. 2021, 22, 41–53. [Google Scholar] [CrossRef]
- Dabrowska, D.; Mozejko-Ciesielska, J.; Pokój, T.; Ciesielski, S. Transcriptome Changes in Pseudomonas putida KT2440 during Medium-Chain-Length Polyhydroxyalkanoate Synthesis Induced by Nitrogen Limitation. Int. J. Mol. Sci. 2021, 22, 152. [Google Scholar] [CrossRef]
- Montiel-Jarillo, G.; Carrera, J.; Eugenia Suárez-Ojeda, M. Enrichment of a mixed microbial culture for polyhydroxyalkanoates production: Effect of pH and N and P concentrations. Sci. Total Environ. 2017, 583, 300–307. [Google Scholar] [CrossRef]
- Chalermthai, B.; Ashraf, M.T.; Bastidas-Oyanedel, J.-R.; Olsen, B.D.; Schmidt, J.E.; Taher, H. Techno-Economic Assessment of Whey Protein-Based Plastic Production from a Co-Polymerization Process. Polymers 2020, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Francini, G.; Lombardi, L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Environmental life cycle assessment of polyhydroxyalkanoates production from cheese whey. Waste Manag. 2021, 132, 31–43. [Google Scholar] [CrossRef]
- Tesfaye, W.; Suarez-Lepe, J.A.; Loira, I.; Palomero, F.; Morata, A. Dairy and Nondairy-Based Beverages as a Vehicle for Probiotics, Prebiotics, and Symbiotics: Alternatives to Health Versus Disease Binomial Approach through Food. In Milk-Based Beverages, Vol 9: The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; Volume 9, pp. 473–520. [Google Scholar]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valor. 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Khdair, A.I.; Abu-Rumman, G.; Khdair, S.I. Pollution estimation from olive mills wastewater in Jordan. Heliyon 2019, 5, e02386. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ma, X.; Li, J.; Sun, B. Insights into enhanced polyhydroxyalkanoate production by the synergistic use of waste wood hydrolysate and volatile fatty acids by mixed microbial cultures. Bioresour. Technol. 2021, 337, 125488. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Pereira, J.; Martins, M.; Torres-Acosta, M.A.; Dias, A.C.R.V.; Lemos, P.C.; Ventura, S.P.M.; Eisele, G.; Alekseeva, A.; Adani, F.; et al. Recovering PHA from mixed microbial biomass: Using non-ionic surfactants as a pretreatment step. Sep. Purif. Technol. 2020, 253, 117521. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Eugenia Suárez-Ojeda, M. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; Pablo López-Gómez, J.; Roy, I. A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Koller, M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. EuroBiotech J. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.-K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Banu, J.R.; Yoon, J.-J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, N.; Oh, S.J.; Hwang, J.H.; Kim, H.J.; Bhatia, S.K.; Yun, J.; Kim, J.-S.; Yang, Y.-H. A strategy to promote the convenient storage and direct use of polyhydroxybutyrate-degrading Bacillus sp. JY14 by lyophilization with protective reagents. Microb. Cell Fact. 2023, 22, 184. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Krzyzanek, V.; Mravec, F.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Marova, I. Accumulation of Poly(3-hydroxybutyrate) Helps Bacterial Cells to Survive Freezing. PLoS ONE 2016, 11, e0157778. [Google Scholar] [CrossRef]
- Munoz Escalona, A.; Rodriguez Varela, F.; Marcilla Gomis, A.; Gomis, A.M.; Escalona, A.M.; Varela, F.R. Procedure for Extraction of Polyhydroxyalkanoates from Halophilic Bacteria Which Contain Them. U.S. Patent US5536419A, 28 April 1994. Available online: https://patents.google.com/patent/US5536419A/en?oq=US5536419A (accessed on 18 August 2024).
- Boily, S.; Gaudet, J.; Blouin, M.; Masaro, L.; Lapointe, P.; Sabin, B.; Julie, G.; Marco, B.; Laurent, M.; Patrick, L. Method of Drying Biodegradable Polymers. U.S. Patent US2005223587A1, 22 January 2003. Available online: https://patents.google.com/patent/US20050223587A1/en?oq=US2005223587-A1 (accessed on 18 August 2024).
- Werker, A.; Pei, R.; Kim, K.; Moretto, G.; Estevez-Alonso, A.; Vermeer, C.; Arcos-Hernandez, M.; Dijkstra, J.; de Vries, E. Thermal pre-processing before extraction of polyhydroxyalkanoates for molecular weight quality control. Polym. Degrad. Stab. 2023, 209, 110277. [Google Scholar] [CrossRef]
- Yan, X.; Li, D.; Ma, X.; Li, J. Bioconversion of renewable lignocellulosic biomass into multicomponent substrate via pressurized hot water pretreatment for bioplastic polyhydroxyalkanoate accumulation. Bioresour. Technol. 2021, 339, 125667. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C.; Ramsay, B.A. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 1994, 8, 589–594. [Google Scholar] [CrossRef]
- Barham, P.J. Physical-Properties of Poly(Hydroxybutyrate) and Poly(Hydroxybutyrate-Co-Hydroxyvalerate). Nov. Biodegrad. Microb. Polym. 1990, 186, 81–96. [Google Scholar]
- Narasimhan, K.; Noda, I.; Satkowski, M.M.; Cearley, A.C.; Gibson, M.S.; Welling, S.J.; Satkowski, M.; Cearley, A.; Gibson, M.; Welling, S. Process for the Extraction of Polyhydroxyalkanoates from Biomass. U.S. Patent US7,118,897 B2, 10 October 2006. Available online: https://patents.google.com/patent/US7118897B2/en?oq=US7%2c118%2c897+B2 (accessed on 18 August 2024).
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Hecht, S.E.; Niehoff, R.L.; Narasimhan, K.; Neal, C.W.; Forshey, P.A.; Phan, D.V.; Brooker, A.D.M.; Combs, K.H.; Neal, C.; Combs, K. Extracting Biopolymers from a Biomass Using Ionic Liquids. U.S. Patent US7763715-B2, 19 April 2006. Available online: https://patents.google.com/patent/US7763715B2/en?oq=US7763715-B2 (accessed on 18 August 2024).
- Poliakoff, M.; Licence, P. Supercritical fluids: Green solvents for green chemistry? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20150018. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Chang, Y.K.; Kim, B.S.; Lee, K.M.; Chang, H.N. The recovery of poly(3-hydroxybutyrate) by using dispersions of sodium hypochlorite solution and chloroform. Biotechnol. Tech. 1993, 7, 209–212. [Google Scholar] [CrossRef]
- Heinrich, D.; Madkour, M.H.; Al-Ghamdi, M.A.; Shabbaj, I.I.; Steinbuchel, A. Large scale extraction of poly(3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2012, 2, 59. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassan, M.A.; Shirai, Y.; Man, H.C.; Ariffin, H.; Yee, L.-N.; Mumtaz, T.; Chong, M.-L.; Phang, L.-Y. Separation and Purification of Polyhydroxyalkanoates from Newly Isolated Comamonas sp. EB172 by Simple Digestion with Sodium Hydroxide. Sep. Sci. Technol. 2012, 47, 534–541. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassan, M.A.; Phang, L.-Y.; Shirai, Y.; Man, H.C.; Ariffin, H.; Amirul, A.A.; Syairah, S.N. Efficient Polyhydroxyalkanoate Recovery from Recombinant Cupriavidus necator by Using Low Concentration of NaOH. Environ. Eng. Sci. 2012, 29, 783–789. [Google Scholar] [CrossRef]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of several methods for the separation of poly(3-hydroxybutyrate) from Cupriavidus necator H16 cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 3. Bioengineering 2022, 9, 328. [Google Scholar] [CrossRef]
- de Koning, G.J.M.; Witholt, B. A process for the recovery of poly(hydroxyalkanoates) from Pseudomonads Part 1: Solubilization. Bioprocess Eng. 1997, 17, 7–13. [Google Scholar] [CrossRef]
- Kapritchkoff, F.M.; Viotti, A.P.; Alli, R.C.P.; Zuccolo, M.; Pradella, J.G.C.; Maiorano, A.E.; Miranda, E.A.; Bonomi, A. Enzymatic recovery and purification of polyhydroxybutyrate produced by Ralstonia eutropha. J. Biotechnol. 2006, 122, 453–462. [Google Scholar] [CrossRef]
- Murugan, P.; Han, L.; Gan, C.-Y.; Maurer, F.H.J.; Sudesh, K. A new biological recovery approach for PHA using mealworm, Tenebrio molitor. J. Biotechnol. 2016, 239, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tamer, I.M.; Moo-Young, M.; Chisti, Y. Disruption of Alcaligenes latus for recovery of poly(β-hydroxybutyric acid): Comparison of high-pressure homogenization, bead milling, and chemically induced lysis. Ind. Eng. Chem. Res. 1998, 37, 1807–1814. [Google Scholar] [CrossRef]
- Gutt, B.; Kehl, K.; Ren, Q.; Boesel, L.F. Using ANOVA Models To Compare and Optimize Extraction Protocols of P3HBHV from Cupriavidus necator. Ind. Eng. Chem. Res. 2016, 55, 10355–10365. [Google Scholar] [CrossRef]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. Microbial production of polyhydroxybutyrate with tailor-made properties: An integrated modelling approach and experimental validation. Biotechnol. Adv. 2012, 30, 329–337. [Google Scholar] [CrossRef]
- Samorì, C.; Abbondanzi, F.; Galletti, P.; Giorgini, L.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Extraction of polyhydroxyalkanoates from mixed microbial cultures: Impact on polymer quality and recovery. Bioresour. Technol. 2015, 189, 195–202. [Google Scholar] [CrossRef]
- Mohandessi, M.; Bandara, K.; Wan, C. Chapter Six-Green technologies for recovery of polyhydroxyalkanoates: Opportunities and perspectives. In Advances in Bioenergy; Li, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 9, pp. 335–381. [Google Scholar]
- Divyashree, M.S.; Shamala, T.R. Effect of gamma irradiation on cell lysis and polyhydroxyalkanoate produced by Bacillus flexus. Radiat. Phys. Chem. 2009, 78, 147–152. [Google Scholar] [CrossRef]
- Schumann, D.; Mueller, R.A. Method for Obtaining Polyhydroxyalkanoates (PHA) and the Copolymers Thereof. U.S. Patent US7070966-B2, 13 March 2000. Available online: https://patents.google.com/patent/US7070966B2/en?oq=US7070966-B2 (accessed on 18 August 2024).
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Choi, J.-I.; Lee, S.Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Roy, I. Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P.; Horowitz, D.M.; Peoples, O.P. PHA applications: Addressing the price performance issue I. Tissue engineering. Int. J. Biol. Macromol. 1999, 25, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Wang, Q.; Smith, T.R.; Hurst, W.E.; Sulpizio, T. Endotoxin removal using a synthetic adsorbent of crystalline calcium silicate hydrate. Biotechnol. Prog. 2005, 21, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, D.M.; Brennan, E.M.; Brennan, E. Method of Purifying Polyhydroxy-Alkanoate Compounds Derived from Biomass. U.S. Patent US6368836-B2, 13 March 2001. Available online: https://patents.google.com/patent/US6368836B2/en?oq=US6368836-B2 (accessed on 18 August 2024).
- Wampfler, B.; Ramsauer, T.; Kehl, K.; Zinn, M.; Thöny-Meyer, L. Application of Activated Charcoal in the Downstream Processing of Bacterial Olefinic Poly(3-hydroxyalkanoates). Chimia 2010, 64, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Hassan, M.A.; Phang, L.-Y.; Shirai, Y.; Man, H.C.; Ariffin, H. Intracellular polyhydroxyalkanoates recovery by cleaner halogen-free methods towards zero emission in the palm oil mill. J. Clean. Prod. 2012, 37, 353–360. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Kleerebezem, R.; Cuellar, M.C.; Ramirez, A. Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: Techno-economic analysis and ex-ante environmental assessment. Bioresour. Technol. 2015, 185, 368–377. [Google Scholar] [CrossRef]
| Plastic | Applications | Usage Time | Degradation Time * |
|---|---|---|---|
| PET (Polyethylene terephthalate) | Bottles and other plastic containers | 1–3 years | 500–1000 years |
| HDPE (high-density polyethylene) | Pipelines, bottles | 5–35 years | 250–5000 years |
| LDPE (low-density polyethylene) | Plastic wrappers and bags | 1–3 years | 150 years |
| PVC (Polyvinyl chloride) | Pipelines and other uses in construction | 35 years | >1000 years |
| PP (Polypropylene) | Textiles, packaging, automotive components | 5–15 years | 50–800 years |
| PHAs (Polyhidroxyalkanoates) | Bags, packaging, medical implants | - | <1 year |
| Classification | Properties | Applications | Examples | References |
|---|---|---|---|---|
| Short-chain PHAs (4–5 carbons) | Fragile High melting point Greater biodegradability Biocompatibility Rigidity High crystallinity | Biofuel production Tissue engineering Drugs encapsulation | Poly(3-hydroxybutyrate) Poly(3-hydroxyvalerate) | [26,29,30,31] |
| Medium-chain PHAs (6–14 carbons) | More elastic Low melting point Biodegradability Biocompatibility Semi-crystalline or amorphous | Fertilizer encapsulation Adhesives Coatings Soft tissue engineering | Poly(3-hydroxyoctanoate) Poly(3-hydroxyhexanoate) | [32,33,34,35] |
| Long-chain PHAs (>14 carbons) | Elastic Low melting point Low glass transition temperature Low crystallinity Low tensile strength | Packaging materials | Poly(3-hydroxyhexadecanoate) | [36] |
| Phylum | Microorganism | Carbon Source | Treatment | PHA Type | PHA Concentration | % PHA (CDM) | Yield | Reference |
|---|---|---|---|---|---|---|---|---|
| Archaea | Haloferax mediterranei DSM 1411 | Vinasse (25% V/V) | P(3HB-co-3HV) | 19.7 g/L | 70% | 0.87 | [68] | |
| Bacteria | Klebsiella oxytoca | Glucose (20 g/L) | PHAs | 0.08 ± 0.21 g/L | 22.6 ± 0.1% | n.d. | [69] | |
| Alcanivorax borkumensis SK2 | Pyruvate (1.5% w/V) | P(3HB) | 6.5 ± 1.2 mg/L | n.d. | n.d. | [70] | ||
| Octadecane (1.5% w/V) | P(3HH-co-3HO-co-3HD-co-3HDD) | 18.0 ± 3.8 mg/L | n.d. | n.d. | ||||
| Pyruvate (1.5% w/V) | Genetically modified strain super-producer of PHAs | P(3HB-co-3HV) | 112 ± 16.8 mg/L | n.d. | n.d. | |||
| Octadecane (1.5% w/V) | P(3HH-co-3HO-co-3HD-co-3HDD) | 2.560 ± 165.1 mg/L | n.d. | n.d. | ||||
| Azotobacter vinelandii | Fish peptone (0.1% w/V) | P(3HB) | 14–25 g/L | 74–86% | 0.29–0.65 | [71] | ||
| Sugars from grape residues (20 g/L) | P(3HB) | n.d. | 37.7 ± 2.15% | 0.07 ± 0.01 | [72] | |||
| Bacillus amyloliquefaciens | Glucose (20 g/L) | PHAs | 0.14 ± 0.61 g/L | 30.2 ± 0.3% | n.d. | [69] | ||
| Olive mill wastewater (25% V/V) | PHAs | 0.06 ± 0.3 g/L | 5.6 ± 0.1% | n.d. | ||||
| Olive mill wastewater (50% V/V) | PHAs | 0.18 ± 0.4 g/L | 5.4 ± 0.4% | n.d. | ||||
| Olive mill wastewater (75% V/V) | PHAs | 0.14 ± 0.2 g/L | 11.2 ± 0.3% | n.d. | ||||
| Olive mill wastewater (100% V/V) | PHAs | 0.16 ± 0.1 g/L | 7.6 ± 0.4% | n.d. | ||||
| Bacillus cereus | Glucose (20 g/L) | PHAs | 0.07 ± 0.08 g/L | 18.9 ± 0.0% | n.d. | [69] | ||
| Glucose (15 g/L) | PHB | 1.91 ± 0.1 g/L | 87.2% | n.d. | [73] | |||
| Bacillus flexus | Sucrose (0.2 g/L) | P(HB-co-HV) | 1.0 ± 0.42 g/L | 47.67 ± 4.04% | n.d. | [74] | ||
| Sucrose (0.2 g/L) | Yeast extract supplementation (2.5 g/L) | P(HB-co-HV) | 1.6 ± 0.28 g/L | 22.00 ± 15.59% | n.d. | |||
| Sucrose (0.2 g/L) | Beef extract supplementation (2.5 g/L) | P(HB-co-HV) | 0.7 ± 0.21 g/L | 42.33 ± 2.89% | n.d. | |||
| Sucrose (0.2 g/L) | Peptone supplementation (5 g/L) | P(HB-co-HV) | 1.6 ± 0.28 g/L | 30.5 ± 10.61% | n.d. | |||
| Bacillus megaterium uyuni S29 | Desugarized sugar beet molasses (20% w/w) | Two different batches of beet molasses | P(3HB) | 9.2 ± 0.05 g/L 10.2 ± 0.04 g/L | 55 ± 0.61% 60 ± 0.08% | n.d. | [75] | |
| Bacillus thioparans | Glucose (20 g/L) | PHAs | 0.14 ± 0.13 g/L | 27.5 ± 0.1% | n.d. | [69] | ||
| Bacillus thuringiensis | Glucose (20 g/L) | PHAs | 0.03 ± 0.14 g/L | 13.6 ± 0.1% | n.d. | [69] | ||
| Cupriavidus necator | Grape sugar extract (20 g/L) | PHB | 1.9 ± 1.5 g/L | 47.2 ± 2.1% | n.d. | [76] | ||
| Spoilage dates | Equivalent to 15.5 g/L glucose and 15.5 g/L fructose | P(3HB) | 0.93 ± 0.10 g/L | 73.20 ± 4.67% | 0.049 | [77] | ||
| Bacon rind (3% w/V) | Finely minced | P(3HB-co-3HV) | n.d. | 11 ± 2% | n.d. | [78] | ||
| Udder (3% w/V) | Finely minced | P(3HB-co-3HV) | n.d. | 55 ± 13% | n.d. | |||
| Tallow (3% w/V) | Finely minced | P(3HB-co-3HV) | n.d. | 23 ± 2% | n.d. | |||
| Waste oil (3% w/V) | P(3HB-co-3HV) | n.d. | 21 ± 7% | n.d. | ||||
| Enzymatic digested waste oil (3% w/V) | P(3HB-co-3HV) | n.d. | 81 ± 17% | n.d. | ||||
| Cocoa Pod Residue | 30 mL/L of alkaline pretreated cocoa pod husks | P(3HB) | n.d. | 51.30 ± 2.83% | n.d. | [79] | ||
| Fructose 20 g/L | PHB | 5.5 ± 0.5 g/L | 61.6 ± 1.2% | n.d. | [76] | |||
| Grape sugar extract (20 g/L) | PHB | 1.9 ± 1.5 g/L | 47.2 ± 2.1% | n.d. | ||||
| Purified grape seeds oil (20 g/L) | PHB | 6.4 ± 1.9 g/L | 76.8 ± 5.8% | n.d. | ||||
| Spent coffee grounds oil (20 g/L) | PHB | 6.5 ± 0.7 g/L | 65.3 ± 1.0% | n.d. | ||||
| Waste fried sunflower oil (20 g/L) | PHB | 6.1 ± 0.1 g/L | 70.4 ± 2.4% | n.d. | ||||
| Hydrolysates of beet molasses | Phosphorous addition | P(3HB-co-3HV) | n.d. | 80% | n.d. | [80] | ||
| Beer brewery wastewater containing maltose | P(3HB) | n.d. | 14–79% | 0.01–0.33 | [81] | |||
| Delftia acidovorans | Myristic acid (2% w/V) | P(3HB-co-4HB-co-3HV) | n.d. | 42.03 ± 9.06% | n.d. | [82] | ||
| Oleic acid (2% w/V) | P(3HB-co-3HV) | n.d. | 22.26 ± 6.85% | n.d. | ||||
| Stearic acid (2% w/V) | PHAs | n.d. | 0.26 ± 0.02% | n.d. | ||||
| Palmitic acid (2% w/V) | P(3HB-co-4HB-co-3HV) | n.d. | 32.22 ± 5.26% | n.d. | ||||
| Corn oil (2% w/V) | Recombinant strain carring lipH and lipC from Pseudomonas stutzeri | P(3HB-co-4HB-co-3HV) | n.d. | 26.72 ± 6.66% | n.d. | |||
| Udder (2% w/V) | Recombinant strain carring lipH and lipC from Pseudomonas stutzeri | P(3HB-co-4HB-co-3HV) | n.d. | 26.72 ± 6.66% | n.d. | |||
| Lard (2% w/V) | Recombinant strain carring lipH and lipC from Pseudomonas stutzeri | P(3HB-co-4HB-co-3HV) | n.d. | 39.33 ± 1.04% | n.d. | |||
| Tallow (2% w/V) | Recombinant strain carring lipH and lipC from Pseudomonas stutzeri | P(3HB-co-4HB-co-3HV) | n.d. | 15.33 ± 6.11% | n.d. | |||
| Halomonas alkaliantarctica | Cheese whey mother liquor (110 g/L) | P(3HB-co-3HV) | 0.42 g/L | 20.1% | n.d. | [83] | ||
| Halomonas halophila | Glucose (20 g/L) | P(3HB) | 3.7 ± 0.6 g/L | 72.5 ± 0.9% | n.d. | [76] | ||
| Grape sugar extract (20 g/L) | P(3HB) | 1.8 ± 0.6 g/L | 57.0 ± 1.0% | n.d. | ||||
| Halomonas organivorans | glucose (20 g/L) | P(3HB) | 3.9 ± 8.2 g/L | 66.0 ± 9.2% | n.d. | [76] | ||
| Grape sugar extract (20 g/L) | P(3HB) | 2.1 ± 0.4 g/L | 55.4 ± 1.4% | n.d. | ||||
| Methylobacterium sp V49 | Methanol (0.5%) | P(3HB) | 0.08 g/L | 11% | n.d. | [84] | ||
| Glucose (15 g/L) | P(3HB) | 0.80 g/L | 53% | n.d. | ||||
| Fructose (15 g/L) | P(3HB) | 0.3 g/L | 25% | n.d. | ||||
| Sucrose (15 g/L) | P(3HB) | 0.5 g/L | 28% | n.d. | ||||
| Lactose (15 g/L) | P(3HB) | 0.11 g/L | 15% | n.d. | ||||
| Parapedobacter sp. ISTM3 | Molasses (5% V/V) | Nitrogen-limiting medium | PHB | 0.47 ± 0.02 g/L | 55.62 ± 0.44% | n.d. | [85] | |
| Pseudomonas oleovorans | Bacon rind (3% w/V) | co-polymers containing 3HV | n.d. | 8 ± 2% | n.d. | [78] | ||
| Udder (3% w/V) | co-polymers containing 3HV | n.d. | 15 ± 3% | n.d. | ||||
| Tallow (3% w/V) | co-polymers containing 3HV | n.d. | 13 ± 2% | n.d. | ||||
| Waste oil (3% w/V) | co-polymers containing 3HV | n.d. | 21 ± 6% | n.d. | ||||
| Enzymatic digested waste oil (3% w/V) | co-polymers containing 3HV | n.d. | 76 ± 14% | n.d. | ||||
| Pseudomonas putida NX-1 | Kraft lignin (10 g/L) | mcl-PHAs | 114.16 mg/L | 37.64% | n.d. | [86] | ||
| Pseudomonas sp. PhDV1 | Grape pomace (1% V/V) | ΔphaZ strain | P(3HB) | n.d. | 16% | n.d. | [87] | |
| Cyanobacteria | Anabaena sp. | CO2 and acetate (1 g/L) | Mixotrophic growth phosphorous limitation | P(3HB) | 0.07 g/L | 37.4 ± 3.0% | n.d. | [62] |
| Aulosira fertilissima | Acetate + Citrate + CO2 | P(3HB) | 31.7 ± 0.23 mg/L | 6.4 ± 0.05% | n.d. | [88] | ||
| Citrate (0.5% w/V) | Dark conditions and phosphorous deficiency | P(3HB) | 73.1 ± 0.82 mg/L | 44.6 ± 0.19% | n.d. | |||
| Acetate (0.5 w/V) | Phosphorous deficiency | P(3HB) | 160.1 ± 1.02 mg/L | 77.2 ± 1.89% | n.d. | |||
| Citrate (0.26% w/V) + acetate (0.28% w/V) | 5.58 mg/L K2HPO4 | P(3HB) | n.d. | 85.1 ± 0.94% | n.d. | |||
| Nostoc muscorum | CO2 + citrate (0.012 mM) | PHB | n.d. | 26.37% | n.d. | [89] | ||
| Synechocystis sp. | NaHCO3 (5 mM) | PHAs | n.d. | n.d. | n.d. | [90] | ||
| Microalgae | Scenedesmus sp. | Glucose (1–4 g/L) | PHAs | 0.007–0.239 g/L | 0.831–29.92% | [67] | ||
| Mixed microbial consortia | Mixed microbial consortia | Wood hydrolysis-enzymatic hydrolysate | Anaerobiosis; fatty acid proportion even/odd = 88:12) | PHAs | n.d. | 50.3 ± 0.7% | n.d. | [91] |
| Anaerobiosis; fatty acid proportion (even/odd = 63:37) | PHAs | n.d. | 44.9 ± 0.5% | n.d. | ||||
| Anaerobiosis; fatty acid proportion (even/odd = 54:46) | PHAs | n.d. | 44.5 ± 0.6% | n.d. | ||||
| Anaerobiosis; fatty acid proportion (even/odd = 48:52) | PHAs | n.d. | 44.7 ± 0.8% | n.d. | ||||
| Rubber wood hydrolysate (1 g/L) | Xylose supplemented up to 1:1 ratio with glucose from hydrolysate | P(3HB-co-3HV) | n.d. | 16.9–43.6% | n.d. | [92] | ||
| Cheese whey | P(3HB) | n.d. | 55.1–62% | n.d. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getino, L.; Martín, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. https://doi.org/10.3390/microorganisms12102028
Getino L, Martín JL, Chamizo-Ampudia A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms. 2024; 12(10):2028. https://doi.org/10.3390/microorganisms12102028
Chicago/Turabian StyleGetino, Luis, José Luis Martín, and Alejandro Chamizo-Ampudia. 2024. "A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste" Microorganisms 12, no. 10: 2028. https://doi.org/10.3390/microorganisms12102028
APA StyleGetino, L., Martín, J. L., & Chamizo-Ampudia, A. (2024). A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms, 12(10), 2028. https://doi.org/10.3390/microorganisms12102028






