Interplay between Lung Diseases and Viral Infections: A Comprehensive Review
Abstract
1. Introduction
2. Impact of Viral Infections on Chronic Lung Diseases
2.1. Chronic Obstructive Pulmonary Disease (COPD)
2.2. Asthma
2.3. Interstitial Lung Diseases (ILDs)
2.4. Pulmonary Hypertension (PH)
3. Mechanisms of Disease Exacerbation
3.1. Immune Dysregulation in CLDs
3.2. Inflammatory Pathways and Viral Exacerbation of CLD
4. Impact of Cigarette Smoke and Viral Infections on Inflammatory Responses
5. IL-6 as a Biomarker and Therapeutic Target
6. Inflammatory Mediators
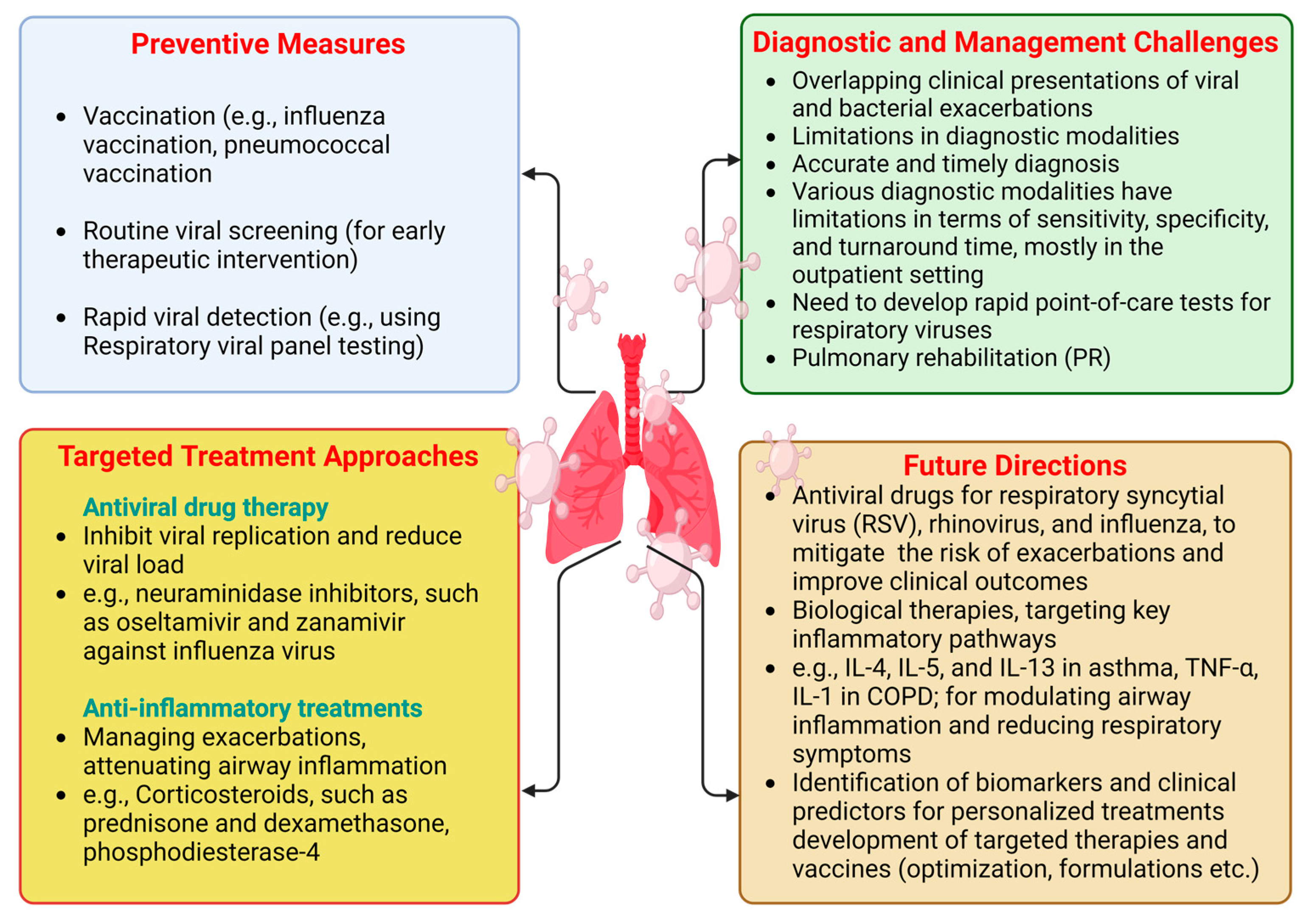
7. Preventive Measures and Early Detection
8. Preventive Strategies for Managing CLDs
9. Targeted Treatment Approaches
9.1. Antiviral Treatments
9.2. Anti-Inflammatory Treatments
10. Diagnostic and Management Challenges
11. Future Directions
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Britto, C.J.; Brady, V.; Lee, S.; Dela Cruz, C.S. Respiratory Viral Infections in Chronic Lung Diseases. Clin. Chest Med. 2017, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Alzaabi, A.; Casas Herrera, A.; Polatli, M.; Rabahi, M.F.; Cortes Telles, A.; Aggarwal, B.; Acharya, S.; Hasnaoui, A.E.; Compton, C. Understanding the Gaps in the Reporting of COPD Exacerbations by Patients: A Review. COPD J. Chronic Obstr. Pulm. Dis. 2024, 21, 2316594. [Google Scholar] [CrossRef]
- Barnes, P.J.; Anderson, G.P.; Fagerås, M.; Belvisi, M.G. Chronic lung diseases: Prospects for regeneration and repair. Eur. Respir. Rev. 2021, 30, 200213. [Google Scholar] [CrossRef]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148. [Google Scholar] [CrossRef]
- Pragman, A.A.; Berger, J.P.; Williams, B.J. Understanding persistent bacterial lung infections: Clinical implications informed by the biology of the microbiota and biofilms. Clin. Pulm. Med. 2016, 23, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cai, C.; Sun, M.; Lv, D.; Zhao, Y. Analyses of Factors Associated with Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Review. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2707–2723. [Google Scholar] [CrossRef]
- Mohanty, P.; Pande, B.; Acharya, R.; Bhaskar, L.V.K.S.; Verma, H.K. Unravelling the Triad of Lung Cancer, Drug Resistance, and Metabolic Pathways. Diseases 2024, 12, 93. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Russell, R.J.; Boulet, L.P.; Brightling, C.E.; Pavord, I.D.; Porsbjerg, C.; Dorscheid, D.; Sverrild, A. The airway epithelium: An orchestrator of inflammation, a key structural barrier and a therapeutic target in severe asthma. Eur. Respir. J. 2024, 63, 2301397. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006, 129, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ganjian, H.; Rajput, C.; Elzoheiry, M.; Sajjan, U. Rhinovirus and Innate Immune Function of Airway Epithelium. Front. Cell. Infect. Microbiol. 2020, 10, 277. [Google Scholar] [CrossRef]
- Nishioki, T.; Sato, T.; Okajima, A.; Motomura, H.; Takeshige, T.; Watanabe, J.; Yae, T.; Koyama, R.; Kido, K.; Takahashi, K. Impact of the COVID-19 pandemic on COPD exacerbations in Japanese patients: A retrospective study. Sci. Rep. 2024, 14, 2792. [Google Scholar] [CrossRef]
- Perakakis, N.; Harb, H.; Hale, B.G.; Varga, Z.; Steenblock, C.; Kanczkowski, W.; Alexaki, V.I.; Ludwig, B.; Mirtschink, P.; Solimena, M.; et al. Mechanisms and clinical relevance of the bidirectional relationship of viral infections with metabolic diseases. Lancet Diabetes Endocrinol. 2023, 11, 675–693. [Google Scholar] [CrossRef]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86014. [Google Scholar] [CrossRef]
- Li, X.; Shao, M.; Zeng, X.; Qian, P.; Huang, H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct. Target. Ther. 2021, 6, 367. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Swaroop Vanka, K.; Chavelier, A.; Shastri, M.D.; Tambuwala, M.M.; Bakshi, H.A.; Pabreja, K.; Mahmood, M.Q.; O’Toole, R.F. Chapter 1—Chronic respiratory diseases: An introduction and need for novel drug delivery approaches. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Dua, K., Hansbro, P.M., Wadhwa, R., Haghi, M., Pont, L.G., Williams, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–31. [Google Scholar] [CrossRef]
- Laupèze, B.; Del Giudice, G.; Doherty, M.T.; Van der Most, R. Vaccination as a preventative measure contributing to immune fitness. npj Vaccines 2021, 6, 93. [Google Scholar] [CrossRef]
- Meckawy, R.; Stuckler, D.; Mehta, A.; Al-Ahdal, T.; Doebbeling, B.N. Effectiveness of early warning systems in the detection of infectious diseases outbreaks: A systematic review. BMC Public Health 2022, 22, 2216. [Google Scholar] [CrossRef]
- Chetty, A.; Guse, T.; Malema, M. Integrated vs non-integrated treatment outcomes in dual diagnosis disorders: A systematic review. Health SA 2023, 28, a2094. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; Guo-Parke, H.; Coyle, P.V.; Fairley, D.; McAuley, D.F.; Taggart, C.C.; Kidney, J. Respiratory viral infection: A potential “missing link” in the pathogenesis of COPD. Eur. Respir. Rev. 2019, 28, 180063. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Taborda-Barata, L.; Cruz, Á.A.; Viegi, G.; Maricoto, T. COPD treatment—A conceptual review based on critical endpoints. Pulmonology 2023, 29, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, 00103-20. [Google Scholar] [CrossRef]
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.M.; Caramori, G.; Stefano, A.D. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef]
- Guo-Parke, H.; Linden, D.; Weldon, S.; Kidney, J.C.; Taggart, C.C. Deciphering Respiratory-Virus-Associated Interferon Signaling in COPD Airway Epithelium. Medicina 2022, 58, 121. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Hewitt, R.; Farne, H.; Ritchie, A.; Luke, E.; Johnston, S.L.; Mallia, P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther. Adv. Respir. Dis. 2016, 10, 158–174. [Google Scholar] [CrossRef]
- Gavala, M.L.; Bertics, P.J.; Gern, J.E. Rhinoviruses, allergic inflammation, and asthma. Immunol. Rev. 2011, 242, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Owuor, N.; Nalamala, N.; Gimenes, J.A., Jr.; Sajjan, U.S. Rhinovirus and COPD airway epithelium. Pulm. Crit. Care Med. 2017, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Bizot, E.; Bousquet, A.; Charpié, M.; Coquelin, F.; Lefevre, S.; Le Lorier, J.; Patin, M.; Sée, P.; Sarfati, E.; Walle, S.; et al. Rhinovirus: A Narrative Review on Its Genetic Characteristics, Pediatric Clinical Presentations, and Pathogenesis. Front. Pediatr. 2021, 9, 643219. [Google Scholar] [CrossRef]
- Sawa, T.; Akaike, T. What triggers inflammation in COVID-19? Elife 2022, 11, e76231. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Ortega, H.; Nickle, D.; Carter, L. Rhinovirus and asthma: Challenges and opportunities. Rev. Med. Virol. 2021, 31, e2193. [Google Scholar] [CrossRef]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.A.; Hansbro, P.M. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Bomsel, M.; Alfsen, A. Entry of viruses through the epithelial barrier: Pathogenic trickery. Nat. Rev. Mol. Cell Biol. 2003, 4, 57–68. [Google Scholar] [CrossRef]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental exposures and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Wagner, C.; Wegmann, M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017, 367, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Azadeh, N.; Limper, A.H.; Carmona, E.M.; Ryu, J.H. The Role of Infection in Interstitial Lung Diseases: A Review. Chest 2017, 152, 842–852. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Galvin, J.R.; Burke, A.P.; Atamas, S.P.; Todd, N.W. Interstitial Lung Disease and Pulmonary Fibrosis: A Practical Approach for General Medicine Physicians with Focus on the Medical History. J. Clin. Med. 2018, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Wollin, L.; Fischer, A.; Quaresma, M.; Stowasser, S.; Harari, S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019, 28, 180100. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Wight, T.N.; Potter-Perigo, S. The extracellular matrix: An active or passive player in fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G950–G955. [Google Scholar] [CrossRef]
- Fukihara, J.; Kondoh, Y. COVID-19 and interstitial lung diseases: A multifaceted look at the relationship between the two diseases. Respir. Investig. 2023, 61, 601–617. [Google Scholar] [CrossRef]
- Prasad, M.; Leon, M.; Lerman, L.O.; Lerman, A. Viral Endothelial Dysfunction: A Unifying Mechanism for COVID-19. Mayo Clin. Proc. 2021, 96, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A. Fighting cytokine storm and immunomodulatory deficiency: By using natural products therapy up to now. Front. Pharmacol. 2023, 14, 1111329. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.C.; Stampfli, M.R. The immune system as a victim and aggressor in chronic obstructive pulmonary disease. J. Thorac. Dis. 2018, 10, S2011–S2017. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial⁻Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 99–109. [Google Scholar] [CrossRef]
- Kumar, A.; Mahajan, A.; Salazar, E.A.; Pruitt, K.; Guzman, C.A.; Clauss, M.A.; Almodovar, S.; Dhillon, N.K. Impact of human immunodeficiency virus on pulmonary vascular disease. Glob. Cardiol. Sci. Pract. 2021, 2021, e202112. [Google Scholar] [CrossRef]
- Su, Y.C.; Jalalvand, F.; Thegerström, J.; Riesbeck, K. The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae. Front. Immunol. 2018, 9, 2530. [Google Scholar] [CrossRef]
- Karnati, S.; Seimetz, M.; Kleefeldt, F.; Sonawane, A.; Madhusudhan, T.; Bachhuka, A.; Kosanovic, D.; Weissmann, N.; Krüger, K.; Ergün, S. Chronic Obstructive Pulmonary Disease and the Cardiovascular System: Vascular Repair and Regeneration as a Therapeutic Target. Front. Cardiovasc. Med. 2021, 8, 649512. [Google Scholar] [CrossRef]
- Kash, J.C.; Taubenberger, J.K. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am. J. Pathol. 2015, 185, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Muscente, F.; De Caterina, R. Causal relationship between influenza infection and risk of acute myocardial infarction: Pathophysiological hypothesis and clinical implications. Eur. Heart J. Suppl. 2020, 22, E68–E72. [Google Scholar] [CrossRef] [PubMed]
- Latreille, E.; Lee, W.L. Interactions of Influenza and SARS-CoV-2 with the Lung Endothelium: Similarities, Differences, and Implications for Therapy. Viruses 2021, 13, 161. [Google Scholar] [CrossRef]
- Barberà, J.A.; Peinado, V.I.; Santos, S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 21, 892–905. [Google Scholar] [CrossRef]
- McIntosh, K.; Perlman, S. Coronaviruses, Including Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier Inc.: Philadelphia, PA, 2015; pp. 1928–1936.e22. [Google Scholar] [CrossRef]
- Ambrosino, P.; Calcaterra, I.L.; Mosella, M.; Formisano, R.; D’Anna, S.E.; Bachetti, T.; Marcuccio, G.; Galloway, B.; Mancini, F.P.; Papa, A.; et al. Endothelial Dysfunction in COVID-19: A Unifying Mechanism and a Potential Therapeutic Target. Biomedicines 2022, 10, 812. [Google Scholar] [CrossRef]
- Hsu, R.J.; Yu, W.C.; Peng, G.R.; Ye, C.H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.C.; Yu, S.H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef]
- Carlin, C.R. New Insights to Adenovirus-Directed Innate Immunity in Respiratory Epithelial Cells. Microorganisms 2019, 7, 216. [Google Scholar] [CrossRef]
- Partovian, C.; Adnot, S.; Raffestin, B.; Louzier, V.; Levame, M.; Mavier, I.M.; Lemarchand, P.; Eddahibi, S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 2000, 23, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Franczuk, P.; Tkaczyszyn, M.; Kulak, M.; Domenico, E.; Ponikowski, P.; Jankowska, E.A. Cardiovascular Complications of Viral Respiratory Infections and COVID-19. Biomedicines 2022, 11, 71. [Google Scholar] [CrossRef]
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef]
- Arnbjörnsson, E.; Asp, N.G.; Westin, S.I. Decreasing incidence of acute appendicitis, with special reference to the consumption of dietary fiber. Acta Chir. Scand. 1982, 148, 461–464. [Google Scholar] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Panzica, L.; Kalathil, S.G.; Thanavala, Y. Immune Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S169–S175. [Google Scholar] [CrossRef]
- Ueha, R.; Ueha, S.; Kondo, K.; Nishijima, H.; Yamasoba, T. Effects of Cigarette Smoke on the Nasal Respiratory and Olfactory Mucosa in Allergic Rhinitis Mice. Front. Neurosci. 2020, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Hohmann, M.S.; Yao, C. Cellular Senescence: Pathogenic Mechanisms in Lung Fibrosis. Int. J. Mol. Sci. 2021, 22, 6214. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.H.; Cardani, A.; Braciale, T.J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 2016, 38, 471–482. [Google Scholar] [CrossRef]
- Kayongo, A.; Robertson, N.M.; Siddharthan, T.; Ntayi, M.L.; Ndawula, J.C.; Sande, O.J.; Bagaya, B.S.; Kirenga, B.; Mayanja-Kizza, H.; Joloba, M.L.; et al. Airway microbiome-immune crosstalk in chronic obstructive pulmonary disease. Front. Immunol. 2022, 13, 1085551. [Google Scholar] [CrossRef]
- Manohar, P.; Loh, B.; Athira, S.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary Bacterial Infections During Pulmonary Viral Disease: Phage Therapeutics as Alternatives to Antibiotics? Front. Microbiol. 2020, 11, 1434. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Innate Immune Responses by Respiratory Viruses, Including Rhinovirus, During Asthma Exacerbation. Front. Immunol. 2022, 13, 865973. [Google Scholar] [CrossRef]
- Larenas-Linnemann, D.; Rodríguez-Pérez, N.; Arias-Cruz, A.; Blandón-Vijil, M.V.; Del Río-Navarro, B.E.; Estrada-Cardona, A.; Gereda, J.E.; Luna-Pech, J.A.; Navarrete-Rodríguez, E.M.; Onuma-Takane, E.; et al. Enhancing innate immunity against virus in times of COVID-19: Trying to untangle facts from fictions. World Allergy Organ. J. 2020, 13, 100476. [Google Scholar] [CrossRef]
- Guo-Parke, H.; Linden, D.; Weldon, S.; Kidney, J.C.; Taggart, C.C. Mechanisms of Virus-Induced Airway Immunity Dysfunction in the Pathogenesis of COPD Disease, Progression, and Exacerbation. Front. Immunol. 2020, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- El-Shimy, W.S.; El-Dib, A.S.; Nagy, H.M.; Sabry, W. A study of IL-6, IL-8, and TNF-α as inflammatory markers in COPD patients. Egypt. J. Bronchol. 2014, 8, 91–99. [Google Scholar] [CrossRef]
- Britt, R.D., Jr.; Ruwanpathirana, A.; Ford, M.L.; Lewis, B.W. Macrophages Orchestrate Airway Inflammation, Remodeling, and Resolution in Asthma. Int. J. Mol. Sci. 2023, 24, 451. [Google Scholar] [CrossRef]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ludden, T.M.; Beal, S.L.; Sheiner, L.B. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J. Pharmacokinet. Biopharm. 1994, 22, 431–445. [Google Scholar] [CrossRef]
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Velez, T.E.; Upadhyay, V.; Vazquez, S.E.; Lee, C.T.; Selvan, K.C.; Law, C.S.; Blaine, K.M.; Hollinger, M.K.; Decker, D.C.; et al. Antigenic responses are hallmarks of fibrotic interstitial lung diseases independent of underlying etiologies. medRxiv 2023, 11, 23289640. [Google Scholar] [CrossRef]
- Moore, M.W.; Herzog, E.L. Regulation and Relevance of Myofibroblast Responses in Idiopathic Pulmonary Fibrosis. Curr. Pathobiol. Rep. 2013, 1, 199–208. [Google Scholar] [CrossRef]
- Smelter, D.F.; Sathish, V.; Thompson, M.A.; Pabelick, C.M.; Vassallo, R.; Prakash, Y.S. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J. Immunol. 2010, 185, 3035–3040. [Google Scholar] [CrossRef]
- Riera-Martínez, L.; Cànaves-Gómez, L.; Iglesias, A.; Martin-Medina, A.; Cosío, B.G. The Role of IL-33/ST2 in COPD and Its Future as an Antibody Therapy. Int. J. Mol. Sci. 2023, 24, 8702. [Google Scholar] [CrossRef]
- Hsu, A.T.; Gottschalk, T.A.; Tsantikos, E.; Hibbs, M.L. The Role of Innate Lymphoid Cells in Chronic Respiratory Diseases. Front. Immunol. 2021, 12, 733324. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Thur, L.A.; Gnjatic, S.; Chapuis, A.; Milano, F.; Hill, J.A. Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy. J. Immunother. Cancer 2021, 9, e002392. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Polinova, A.I.; Drutskaya, M.S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef]
- Mehta, P.; Fajgenbaum, D.C. Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate. Curr. Opin. Rheumatol. 2021, 33, 419–430. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From arthritis to CAR-T-cell therapy and COVID-19. Int. Immunol. 2021, 33, 515–519. [Google Scholar] [CrossRef]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Liew, K.Y.; Koh, S.K.; Hooi, S.L.; Ng, M.K.L.; Chee, H.Y.; Harith, H.H.; Israf, D.A.; Tham, C.L. Rhinovirus-Induced Cytokine Alterations with Potential Implications in Asthma Exacerbations: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 782936. [Google Scholar] [CrossRef]
- Michi, A.N.; Love, M.E.; Proud, D. Rhinovirus-Induced Modulation of Epithelial Phenotype: Role in Asthma. Viruses 2020, 12, 1328. [Google Scholar] [CrossRef]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Potena, A.; Caramori, G.; Casolari, P.; Contoli, M.; Johnston, S.L.; Papi, A. Pathophysiology of viral-induced exacerbations of COPD. Int. J. Chron. Obstr. Pulm. Dis. 2007, 2, 477–483. [Google Scholar]
- van Geffen, W.H.; Tan, D.J.; Walters, J.A.; Walters, E.H. Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2023, 12, Cd011600. [Google Scholar] [CrossRef]
- Sha, Q.; Truong-Tran, A.Q.; Plitt, J.R.; Beck, L.A.; Schleimer, R.P. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef]
- Wei, L.; Mousawi, F.; Li, D.; Roger, S.; Li, J.; Yang, X.; Jiang, L.H. Adenosine Triphosphate Release and P2 Receptor Signaling in Piezo1 Channel-Dependent Mechanoregulation. Front. Pharmacol. 2019, 10, 1304. [Google Scholar] [CrossRef]
- Yoo, J.K.; Kim, T.S.; Hufford, M.M.; Braciale, T.J. Viral infection of the lung: Host response and sequelae. J. Allergy Clin. Immunol. 2013, 132, 1263–1276, quiz 1277. [Google Scholar] [CrossRef]
- Basil, M.C.; Katzen, J.; Engler, A.E.; Guo, M.; Herriges, M.J.; Kathiriya, J.J.; Windmueller, R.; Ysasi, A.B.; Zacharias, W.J.; Chapman, H.A.; et al. The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell 2020, 26, 482–502. [Google Scholar] [CrossRef]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Kurai, D.; Saraya, T.; Ishii, H.; Takizawa, H. Virus-induced exacerbations in asthma and COPD. Front. Microbiol. 2013, 4, 293. [Google Scholar] [CrossRef]
- Artucio, H.; Hurtado, J.; Zimet, L.; de Paula, J.; Beron, M. PEEP-induced tricuspid regurgitation. Intensive Care Med. 1997, 23, 836–840. [Google Scholar] [CrossRef]
- Ortiz-Zapater, E.; Signes-Costa, J.; Montero, P.; Roger, I. Lung Fibrosis and Fibrosis in the Lungs: Is It All about Myofibroblasts? Biomedicines 2022, 10, 1423. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Kuchibhotla, V.N.S.; Roffel, M.P.; Maes, T.; Knight, D.A.; Sayers, I.; Nawijn, M.C. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020, 75, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Wenzel, S. Clinical relevance of airway remodelling in airway diseases. Eur. Respir. J. 2007, 30, 134–155. [Google Scholar] [CrossRef]
- Samarelli, A.V.; Tonelli, R.; Marchioni, A.; Bruzzi, G.; Gozzi, F.; Andrisani, D.; Castaniere, I.; Manicardi, L.; Moretti, A.; Tabbì, L.; et al. Fibrotic Idiopathic Interstitial Lung Disease: The Molecular and Cellular Key Players. Int. J. Mol. Sci. 2021, 22, 8952. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.R.; Lindsay, M.A. Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs. Int. J. Mol. Sci. 2020, 21, 524. [Google Scholar] [CrossRef]
- Eenjes, E.; Tibboel, D.; Wijnen, R.M.H.; Rottier, R.J. Lung epithelium development and airway regeneration. Front. Cell Dev. Biol. 2022, 10, 1022457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Guo, L.; Qian, J.; Ge, J.; Sinner, D.; Ding, H.; Califano, A.; Cardoso, W.V. Airway basal cells show regionally distinct potential to undergo metaplastic differentiation. Elife 2022, 11, e80083. [Google Scholar] [CrossRef]
- Khedoe, P.; Wu, X.; Gosens, R.; Hiemstra, P.S. Repairing damaged lungs using regenerative therapy. Curr. Opin. Pharmacol. 2021, 59, 85–94. [Google Scholar] [CrossRef]
- El Agha, E.; Kosanovic, D.; Schermuly, R.T.; Bellusci, S. Role of fibroblast growth factors in organ regeneration and repair. Semin. Cell Dev. Biol. 2016, 53, 76–84. [Google Scholar] [CrossRef]
- Yen, T.T.; Thao, D.T.; Thuoc, T.L. An overview on keratinocyte growth factor: From the molecular properties to clinical applications. Protein Pept. Lett. 2014, 21, 306–317. [Google Scholar] [CrossRef]
- Clark, J.N.; Whiting, A.; McCaffery, P. Retinoic acid receptor-targeted drugs in neurodegenerative disease. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1097–1108. [Google Scholar] [CrossRef]
- Basil, M.C.; Alysandratos, K.D.; Kotton, D.N.; Morrisey, E.E. Lung repair and regeneration: Advanced models and insights into human disease. Cell Stem Cell 2024, 31, 439–454. [Google Scholar] [CrossRef]
- Raffaele, M.; Vinciguerra, M. The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Fazleen, A.; Wilkinson, T. Early COPD: Current evidence for diagnosis and management. Ther. Adv. Respir. Dis. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Swartz, T.H.; Palermo, A.S.; Masur, S.K.; Aberg, J.A. The Science and Value of Diversity: Closing the Gaps in Our Understanding of Inclusion and Diversity. J. Infect. Dis. 2019, 220, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Murgia, N.; Gambelunghe, A. Occupational COPD-The most under-recognized occupational lung disease? Respirology 2022, 27, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.M.A.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fagerås, M.; et al. At the Root: Defining and Halting Progression of Early Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Ji, Z.; Jareño-Esteban, J.J.; de Miguel-Díez, J. Role of Vaccines in COPD Patients. Open Respir. Arch. 2022, 4, 100191. [Google Scholar] [CrossRef]
- Bao, W.; Li, Y.; Wang, T.; Li, X.; He, J.; Wang, Y.; Wen, F.; Chen, J. Effects of influenza vaccination on clinical outcomes of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 68, 101337. [Google Scholar] [CrossRef]
- Aliberti, S.; Mantero, M.; Mirsaeidi, M.; Blasi, F. The role of vaccination in preventing pneumococcal disease in adults. Clin. Microbiol. Infect. 2014, 20 (Suppl. S5), 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Gordon, A.; Foxman, B. The role of respiratory viruses in the etiology of bacterial pneumonia: An ecological perspective. Evol. Med. Public Health 2016, 2016, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, A.D.; Mousa, J.J. Diverse Mechanisms of Protective Anti-Pneumococcal Antibodies. Front. Cell. Infect. Microbiol. 2022, 12, 824788. [Google Scholar] [CrossRef] [PubMed]
- Koul, P.A.; Mir, H.; Akram, S.; Potdar, V.; Chadha, M.S. Respiratory viruses in acute exacerbations of chronic obstructive pulmonary disease. Lung India 2017, 34, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kherad, O.; Kaiser, L.; Bridevaux, P.O.; Sarasin, F.; Thomas, Y.; Janssens, J.P.; Rutschmann, O.T. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 2010, 138, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Demmler-Harrison, G.J. Healthcare-Associated Viral Infections: Considerations for Nosocomial Transmission and Infection Control. In Healthcare-Associated Infections in Children: A Guide to Prevention and Management; McNeil, J.C., Campbell, J.R., Crews, J.D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 229–257. [Google Scholar] [CrossRef]
- Blasi, F. Lung Diseases: Chronic Respiratory Infections. Int. J. Mol. Sci. 2018, 19, 3051. [Google Scholar] [CrossRef]
- Koul, P.A.; Vora, A.C.; Jindal, S.K.; Ramasubramanian, V.; Narayanan, V.; Tripathi, S.K.; Bahera, D.; Chandrashekhar, H.B.; Mehta, R.; Raval, N.; et al. Expert panel opinion on adult pneumococcal vaccination in the post-COVID era (NAP- EXPO Recommendations-2024). Lung India 2024, 41, 307–317. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Hodel, K.V.S.; Fonseca, L.; Pires, V.C.; Mascarenhas, L.A.B.; da Silva Andrade, L.P.C.; Moret, M.A.; Badaró, R. The Importance of Vaccination in the Context of the COVID-19 Pandemic: A Brief Update Regarding the Use of Vaccines. Vaccines 2022, 10, 591. [Google Scholar] [CrossRef]
- Im, H.; Ser, J.; Sim, U.; Cho, H. Promising Expectations for Pneumococcal Vaccination during COVID-19. Vaccines 2021, 9, 1507. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef]
- Andreas, S.; Hering, T.; Mühlig, S.; Nowak, D.; Raupach, T.; Worth, H. Smoking cessation in chronic obstructive pulmonary disease: An effective medical intervention. Dtsch. Arztebl. Int. 2009, 106, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ghodeshwar, G.K.; Dube, A.; Khobragade, D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus 2023, 15, e42616. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Miravitlles, M.; de la Rosa Carrillo, D.; Cantón, R.; Soler-Cataluña, J.J.; Martinez-Garcia, M.A. Current Challenges in Chronic Bronchial Infection in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 1639. [Google Scholar] [CrossRef]
- Rosenwasser, Y.; Berger, I.; Loewy, Z.G. Therapeutic Approaches for Chronic Obstructive Pulmonary Disease (COPD) Exacerbations. Pathogens 2022, 11, 1513. [Google Scholar] [CrossRef]
- Shahani, L.; Ariza-Heredia, E.J.; Chemaly, R.F. Antiviral therapy for respiratory viral infections in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2017, 15, 401–415. [Google Scholar] [CrossRef]
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Jones, M.A.; Doshi, P.; Del Mar, C.B.; Hama, R.; Thompson, M.J.; Spencer, E.A.; Onakpoya, I.; Mahtani, K.R.; Nunan, D.; et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst. Rev. 2014, 2014, Cd008965. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza virus-related critical illness: Prevention, diagnosis, treatment. Crit. Care 2019, 23, 214. [Google Scholar] [CrossRef]
- Lahham, A.; Holland, A.E. The Need for Expanding Pulmonary Rehabilitation Services. Life 2021, 11, 1236. [Google Scholar] [CrossRef]
- Holmes, E.C.; Hurt, A.C.; Dobbie, Z.; Clinch, B.; Oxford, J.S.; Piedra, P.A. Understanding the Impact of Resistance to Influenza Antivirals. Clin. Microbiol. Rev. 2021, 34, 2. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Okada, H.; Ohnishi, T.; Hirashima, M.; Fujita, J.; Yamaji, Y.; Takahara, J.; Todani, T. Anti-asthma effect of an antiviral drug, acyclovir: A clinical case and experimental study. Clin. Exp. Allergy 1997, 27, 431–437. [Google Scholar] [CrossRef]
- Linden, D.A.; Guo-Parke, H.; McKelvey, M.C.; Einarsson, G.G.; Lee, A.J.; Fairley, D.J.; Brown, V.; Lundy, G.; Campbell, C.; Logan, D.; et al. Valaciclovir for Epstein-Barr Virus Suppression in Moderate-to-Severe COPD: A Randomized Double-Blind Placebo-Controlled Trial. Chest 2023, 164, 625–636. [Google Scholar] [CrossRef]
- Beigel, J.H.; Bao, Y.; Beeler, J.; Manosuthi, W.; Slandzicki, A.; Dar, S.M.; Panuto, J.; Beasley, R.L.; Perez-Patrigeon, S.; Suwanpimolkul, G.; et al. Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: A multicentre, double-blind, randomised phase 2 trial. Lancet Infect. Dis. 2017, 17, 1255–1265. [Google Scholar] [CrossRef]
- Mertowska, P.; Smolak, K.; Mertowski, S.; Grywalska, E. Immunomodulatory Role of Interferons in Viral and Bacterial Infections. Int. J. Mol. Sci. 2023, 24, 10115. [Google Scholar] [CrossRef]
- Mihaescu, G.; Chifiriuc, M.C.; Filip, R.; Bleotu, C.; Ditu, L.M.; Constantin, M.; Cristian, R.-E.; Grigore, R.; Bertesteanu, S.V.; Bertesteanu, G.; et al. Role of interferons in the antiviral battle: From virus-host crosstalk to prophylactic and therapeutic potential in SARS-CoV-2 infection. Front. Immunol. 2024, 14, 1273604. [Google Scholar] [CrossRef]
- Singanayagam, A.; Loo, S.L.; Calderazzo, M.; Finney, L.J.; Trujillo Torralbo, M.B.; Bakhsoliani, E.; Girkin, J.; Veerati, P.; Pathinayake, P.S.; Nichol, K.S.; et al. Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L893–L903. [Google Scholar] [CrossRef]
- Nieto-Fontarigo, J.J.; Tillgren, S.; Cerps, S.; Sverrild, A.; Hvidtfeldt, M.; Ramu, S.; Menzel, M.; Sander, A.F.; Porsbjerg, C.; Uller, L. Imiquimod Boosts Interferon Response, and Decreases ACE2 and Pro-Inflammatory Response of Human Bronchial Epithelium in Asthma. Front. Immunol. 2021, 12, 743890. [Google Scholar] [CrossRef]
- Gillissen, A.; Paparoupa, M. Inflammation and infections in asthma. Clin. Respir. J. 2015, 9, 257–269. [Google Scholar] [CrossRef]
- Folkerts, G.; Busse, W.W.; Nijkamp, F.P.; Sorkness, R.; Gern, J.E. Virus-induced airway hyperresponsiveness and asthma. Am. J. Respir. Crit. Care Med. 1998, 157, 1708–1720. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Quint, J.K.; Ariel, A.; Barnes, P.J. Rational use of inhaled corticosteroids for the treatment of COPD. npj Prim. Care Respir. Med. 2023, 33, 27. [Google Scholar] [CrossRef]
- Mkorombindo, T.; Dransfield, M.T. Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease: Benefits and Risks. Clin. Chest Med. 2020, 41, 475–484. [Google Scholar] [CrossRef]
- Ramsahai, J.M.; Hansbro, P.M.; Wark, P.A.B. Mechanisms and Management of Asthma Exacerbations. Am. J. Respir. Crit. Care Med. 2019, 199, 423–432. [Google Scholar] [CrossRef]
- Kawamatawong, T. Phosphodiesterase-4 Inhibitors for Non-COPD Respiratory Diseases. Front. Pharmacol. 2021, 12, 518345. [Google Scholar] [CrossRef]
- Kawamatawong, T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J. Thorac. Dis. 2017, 9, 1144–1154. [Google Scholar] [CrossRef]
- Pollock, J.; Chalmers, J.D. The immunomodulatory effects of macrolide antibiotics in respiratory disease. Pulm. Pharmacol. Ther. 2021, 71, 102095. [Google Scholar] [CrossRef]
- Barstow, C.; Forbes, D. Respiratory Conditions: Chronic Obstructive Pulmonary Disease. FP Essent. 2019, 486, 26–32. [Google Scholar]
- George, S.N.; Garcha, D.S.; Mackay, A.J.; Patel, A.R.; Singh, R.; Sapsford, R.J.; Donaldson, G.C.; Wedzicha, J.A. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur. Respir. J. 2014, 44, 87–96. [Google Scholar] [CrossRef]
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.D.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; Montes de Oca, M.; et al. Differential Diagnosis of Suspected Chronic Obstructive Pulmonary Disease Exacerbations in the Acute Care Setting: Best Practice. Am. J. Respir. Crit. Care Med. 2023, 207, 1134–1144. [Google Scholar] [CrossRef]
- Huang, Y.; Ai, L.; Wang, X.; Sun, Z.; Wang, F. Review and Updates on the Diagnosis of Tuberculosis. J. Clin. Med. 2022, 11, 5826. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, M.; Dua, R.; Saini, L.K.; Sindhwani, G. Management of chronic respiratory diseases during viral pandemics: A concise review of guidance and recommendations. J. Fam. Med. Prim. Care 2022, 11, 6633–6639. [Google Scholar] [CrossRef]
- Vegivinti, C.T.R.; Evanson, K.W.; Lyons, H.; Akosman, I.; Barrett, A.; Hardy, N.; Kane, B.; Keesari, P.R.; Pulakurthi, Y.S.; Sheffels, E.; et al. Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials. BMC Infect. Dis. 2022, 22, 107. [Google Scholar] [CrossRef]
- Kopsaftis, Z.A.; Sulaiman, N.S.; Mountain, O.D.; Carson-Chahhoud, K.V.; Phillips, P.A.; Smith, B.J. Short-acting bronchodilators for the management of acute exacerbations of chronic obstructive pulmonary disease in the hospital setting: Systematic review. Syst. Rev. 2018, 7, 213. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, D.; Xu, Y.; Wu, L.; Lou, L. Effect of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2022, 54, 262–273. [Google Scholar] [CrossRef]
- Aguilaniu, B. Impact of bronchodilator therapy on exercise tolerance in COPD. Int. J. Chron. Obstr. Pulm. Dis. 2010, 5, 57–71. [Google Scholar] [CrossRef][Green Version]
- Rajaram, S.; Canaday, L.M.; Ochayon, D.E.; Rangel, K.M.; Ali, A.; Gyurova, I.E.; Krishnamurthy, D.; Fletcher, J.S.; Reighard, S.D.; Cox, A.; et al. The Promise and Peril of Natural Killer Cell Therapies in Pulmonary Infection. Immunity 2020, 52, 887–889. [Google Scholar] [CrossRef]
- Aoki, F.Y. Antiviral Drugs for Influenza and Other Respiratory Virus Infections. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 531–545.e5. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Katschnig, H. Modern medicine and the one-size-fits-all approach: A clinician’s comment to Alexandra Pârvan’s “Mind Electric” article. J. Eval. Clin. Pract. 2018, 24, 1079–1083. [Google Scholar] [CrossRef]
- Montoya, S.; Soong, D.; Nguyen, N.; Affer, M.; Munamarty, S.P.; Taylor, J. Targeted Therapies in Cancer: To Be or Not to Be, Selective. Biomedicines 2021, 9, 1591. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Koppelman, G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef]
- Rodrigues, S.O.; Cunha, C.; Soares, G.M.V.; Silva, P.L.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Mechanisms, Pathophysiology and Currently Proposed Treatments of Chronic Obstructive Pulmonary Disease. Pharmaceuticals 2021, 14, 979. [Google Scholar] [CrossRef]
- Tan, K.S.; Lim, R.L.; Liu, J.; Ong, H.H.; Tan, V.J.; Lim, H.F.; Chung, K.F.; Adcock, I.M.; Chow, V.T.; Wang, Y. Respiratory Viral Infections in Exacerbation of Chronic Airway Inflammatory Diseases: Novel Mechanisms and Insights from the Upper Airway Epithelium. Front. Cell Dev. Biol. 2020, 8, 99. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Gopallawa, I.; Dehinwal, R.; Bhatia, V.; Gujar, V.; Chirmule, N. A four-part guide to lung immunology: Invasion, inflammation, immunity, and intervention. Front. Immunol. 2023, 14, 1119564. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bibi, S.; Rahaman, M.S.; Rahman, F.; Islam, F.; Khan, M.S.; Hasan, M.M.; Parvez, A.; Hossain, M.A.; Maeesa, S.K.; et al. Natural therapeutics and nutraceuticals for lung diseases: Traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022, 150, 113041. [Google Scholar] [CrossRef]
- Kakavas, S.; Kotsiou, O.S.; Perlikos, F.; Mermiri, M.; Mavrovounis, G.; Gourgoulianis, K.; Pantazopoulos, I. Pulmonary function testing in COPD: Looking beyond the curtain of FEV1. npj Prim. Care Respir. Med. 2021, 31, 23. [Google Scholar] [CrossRef]

| Disease/ Characteristic | COPD | Asthma | Interstitial Lung Disease | Pulmonary Hypertension |
|---|---|---|---|---|
| Disease Exacerbation Mechanism | Viral infections increase serum IL-6 and fibrinogen, intensifying inflammation and tissue damage. | Viral infections trigger eosinophilic inflammation, increasing Th2 cytokines (IL-4, IL-5, IL-13). | Viral-induced dysregulation leads to pro-fibrotic cytokine release (TGF-β, PDGF), causing fibrosis. | Viral infections exacerbate vascular remodeling, causing increased pulmonary vascular resistance and pressure. |
| Immune Dysregulation | Pollutants and cigarette smoke attract CD8/CD4 T cells and neutrophils, leading to tissue remodeling and emphysema. | Compromised epithelial barriers and mucociliary clearance impaired cytokine signalling worsened viral outcomes | Dysregulated immune responses cause progressive fibrotic remodelling. | Altered immune cell function and increased pro-inflammatory cytokines exacerbate vascular remodelling. |
| Inflammatory Pathways | Pro-inflammatory cytokines (IL-6, IL-8, TNF-α) drive neutrophil and macrophage activation, worsening inflammation. | IL-33 releaseslead to Th2 cytokine production and airway inflammation. | Pro-fibrotic cytokines (TGF-β, PDGF) cause myofibroblast activation, collagen deposition, and fibrosis. | Inflammatory mediators (IL-6, IL-8) contribute to pulmonary vascular remodelling and right ventricular strain. |
| IL-6 as a Biomarker | Elevated IL-6 correlates with severe disease and exacerbations, making it a potential therapeutic target. | IL-6 is a key cytokine in the inflammatory response and a potential target in severe asthma exacerbations. | IL-6 contributes to fibrosis progression and may serve as a biomarker for disease severity. | Elevated IL-6 levels linked to PH progression and severity; potential biomarker for disease monitoring |
| Inflammatory Mediators | IL-1, IL-6, and IL-8 exacerbate respiratory inflammation, leading to tissue damage and mucus hypersecretion. | Similar pathways between IL-1 and IL-33 induce inflammation and increase mucus production and airway constriction. | TGF-β and VEGF contribute to airway remodelling and fibrosis, leading to worsening lung function. | Inflammatory mediators induce vascular inflammation, promoting progression to advanced PH. |
| Preventive Measures | Annual influenza and pneumococcal vaccination are crucial to prevent exacerbations. | Early detection and vaccination | Early vaccination to prevent viral-induced exacerbations and fibrotic progression | Vaccination to reduce the risk of viral infections exacerbating PH and overall lung function |
| Targeted Treatment Approaches | Antivirals like oseltamivir; anti-inflammatories like corticosteroids and PDE4 inhibitors | ICS and corticosteroids to control inflammation; antivirals for reducing viral load | Antifibrotic and anti-inflammatory treatments to manage exacerbations and progression | Antiviral and anti-inflammatory therapies to mitigate vascular inflammation and PH progression |
| Diagnostic Challenges | Difficulty distinguishing viral from bacterial exacerbations; need for rapid diagnostic tests | Challenges distinguishing viral from bacterial exacerbations; rapid and accurate viral identification needed | Challenges differentiating viral-induced exacerbations; reliance on advanced diagnostics | Complicated diagnosis due to overlapping symptoms; importance of accurate viral detection |
| Preventive Strategies | Early diagnosis, vaccination, and targeted treatment to reduce exacerbation frequency and severity | Focus on early detection, vaccination, and maintaining airway function through preventive strategies | Preventive measures aimed at reducing fibrosis progression and maintaining lung function | Emphasis on early diagnosis and treatment to prevent exacerbation and progression to severe PH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, C.; Pande, B.; Sahithi, L.S.; Sahu, T.; Verma, H.K. Interplay between Lung Diseases and Viral Infections: A Comprehensive Review. Microorganisms 2024, 12, 2030. https://doi.org/10.3390/microorganisms12102030
Suri C, Pande B, Sahithi LS, Sahu T, Verma HK. Interplay between Lung Diseases and Viral Infections: A Comprehensive Review. Microorganisms. 2024; 12(10):2030. https://doi.org/10.3390/microorganisms12102030
Chicago/Turabian StyleSuri, Chahat, Babita Pande, Lakkakula Suhasini Sahithi, Tarun Sahu, and Henu Kumar Verma. 2024. "Interplay between Lung Diseases and Viral Infections: A Comprehensive Review" Microorganisms 12, no. 10: 2030. https://doi.org/10.3390/microorganisms12102030
APA StyleSuri, C., Pande, B., Sahithi, L. S., Sahu, T., & Verma, H. K. (2024). Interplay between Lung Diseases and Viral Infections: A Comprehensive Review. Microorganisms, 12(10), 2030. https://doi.org/10.3390/microorganisms12102030






