Laboratory-Simulated Inhibitory Effects of the Floating-Bed Plants on Microcystis aeruginosa and Their Microbial Communities’ Responses to Microcystins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Sample Collection and Measurement
2.4. Data Analysis
3. Results and Analysis
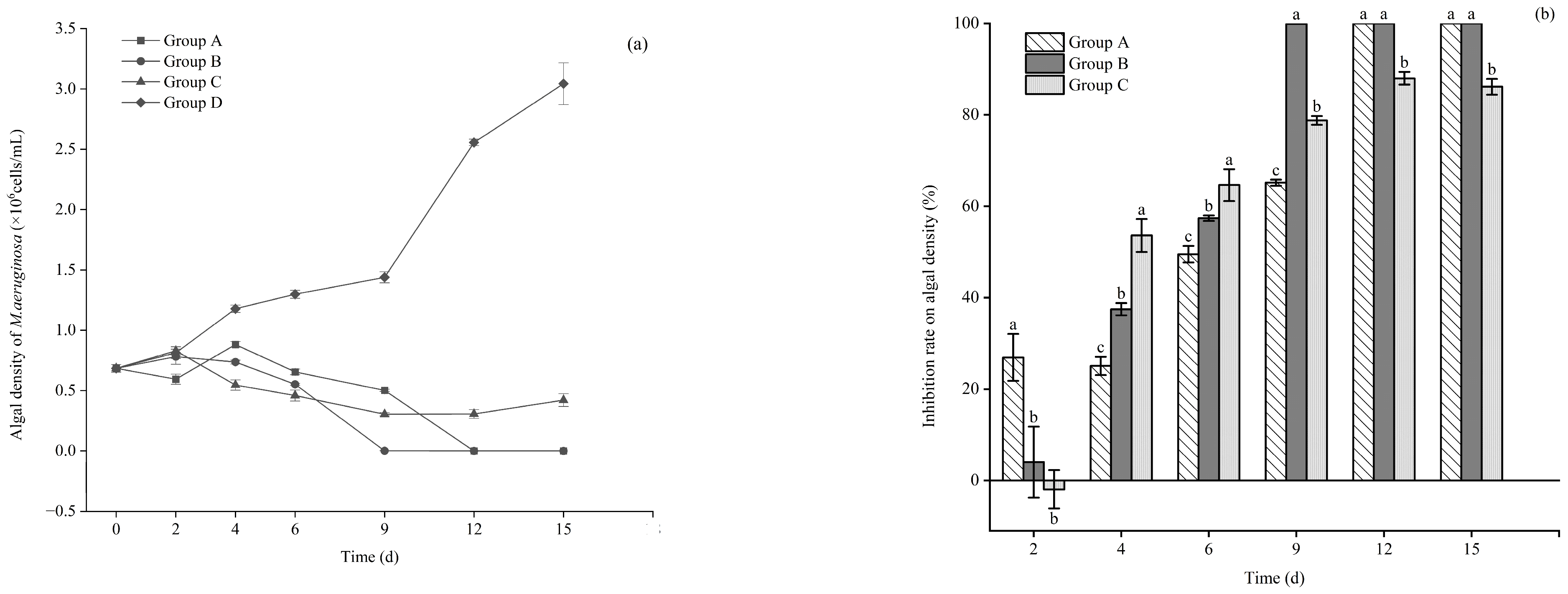
3.1. Effects of Different Floating Bed Plants on the Growth of M. aeruginosa in the Control and Each Treatment
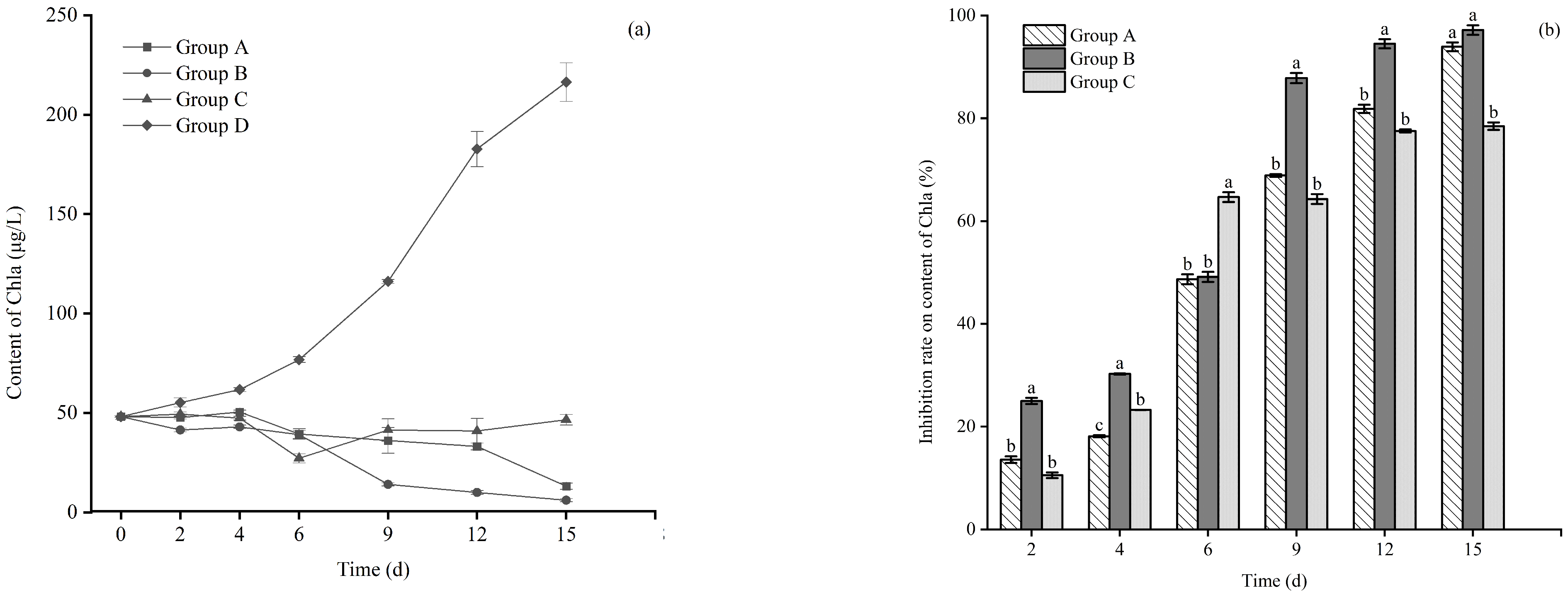
3.2. Effect of Different Floating Bed Plants on the Chl a (Chlorophyll a) Content of M. aeruginosa
3.3. The Antioxidant Response of Roots, Stems, and Leaves of Different Plants to the Toxic M. aeruginosa
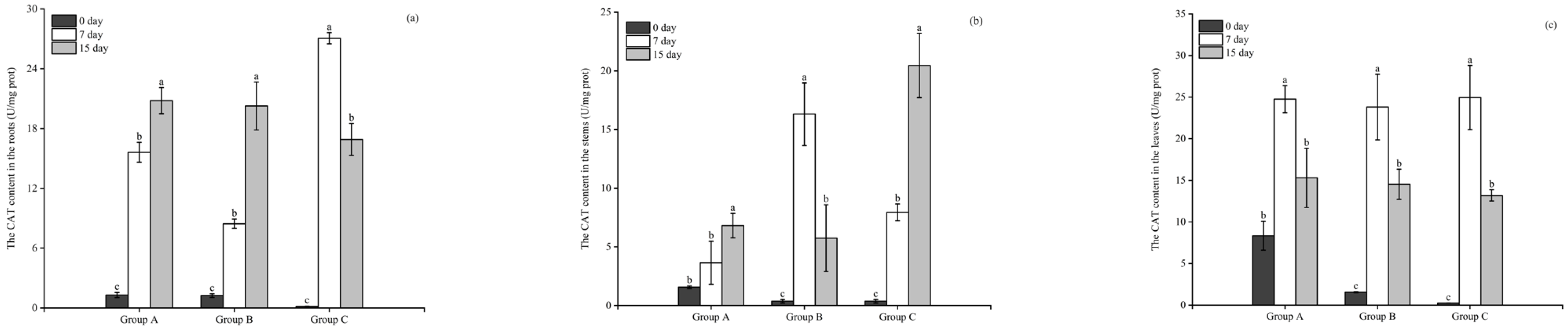
3.3.1. The Activity of CAT
3.3.2. The Activity of GSH
3.3.3. The Activity of SOD
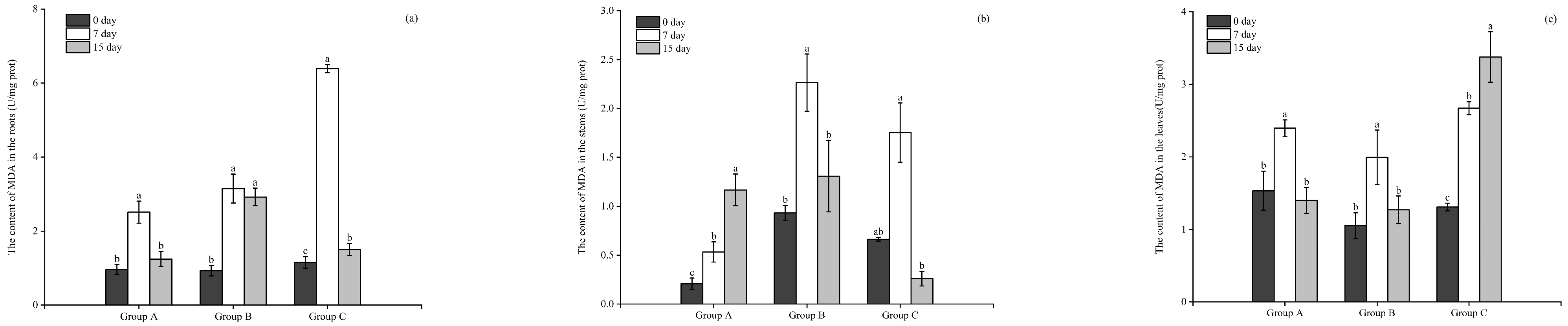
3.3.4. The Content of MDA
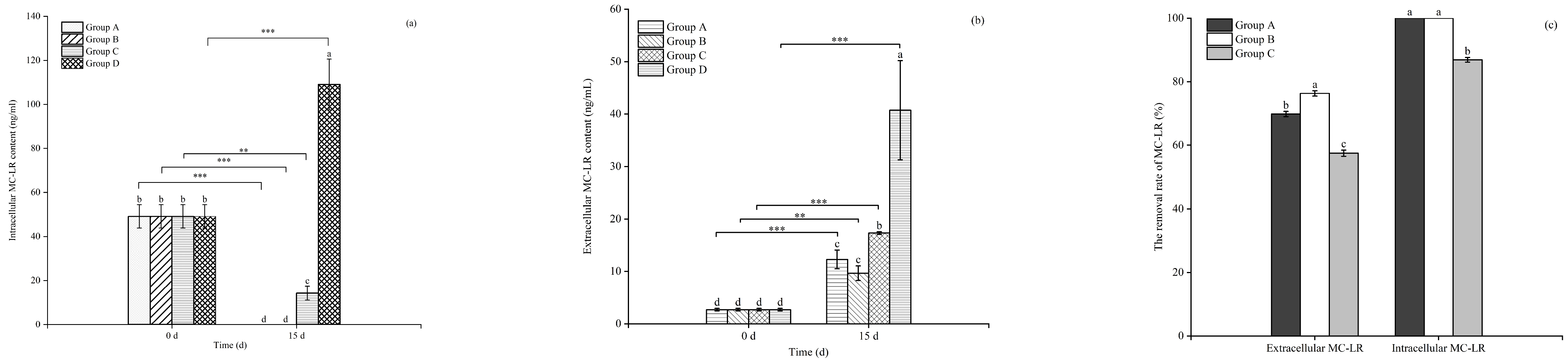
3.4. Effects of Floating Bed Plants on the MC-LR Content of the Toxic M. aeruginosa
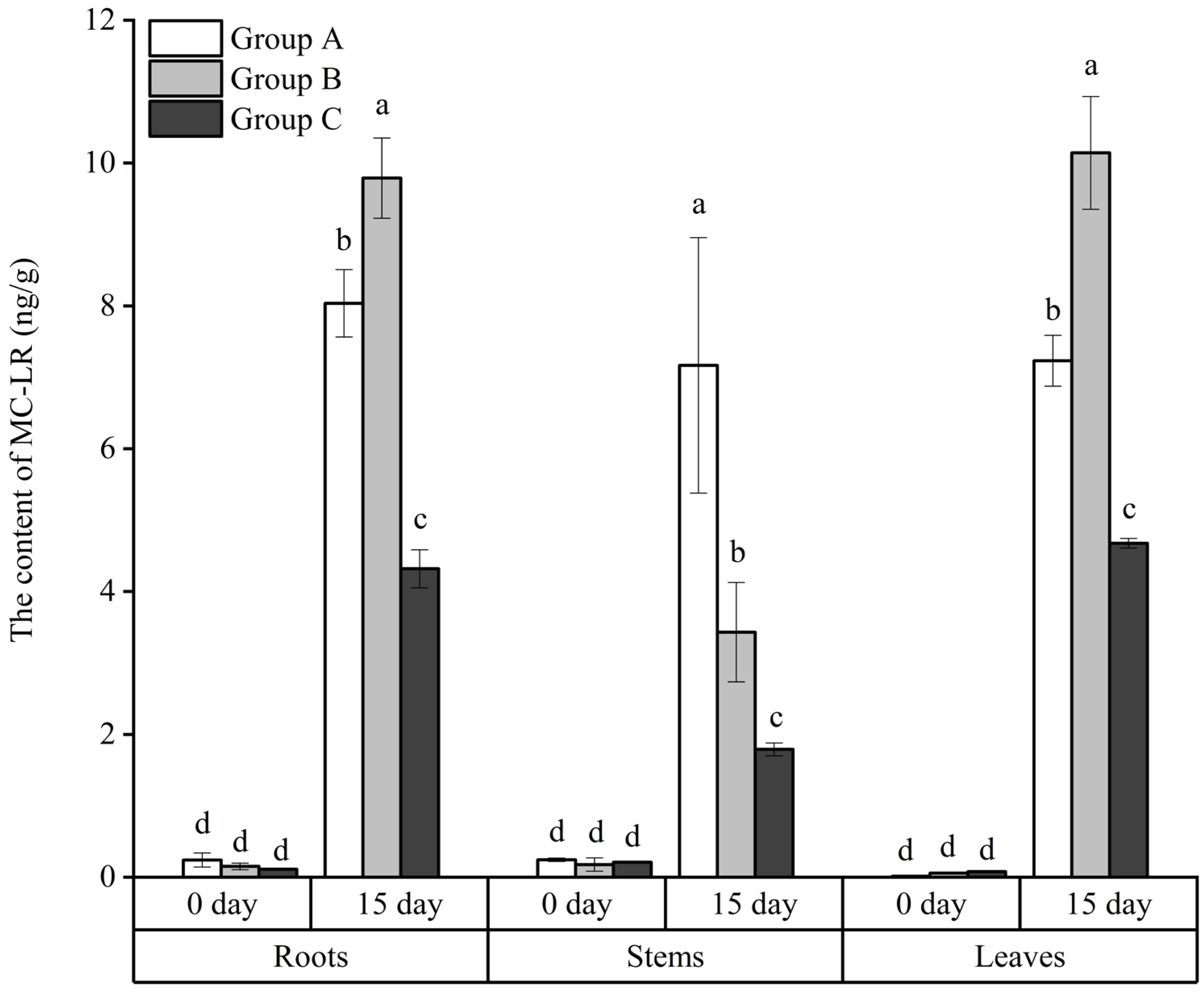
3.5. The Absorbance of MC-LR by the Roots, Stems, and Leaves of the Floating Bed Plants
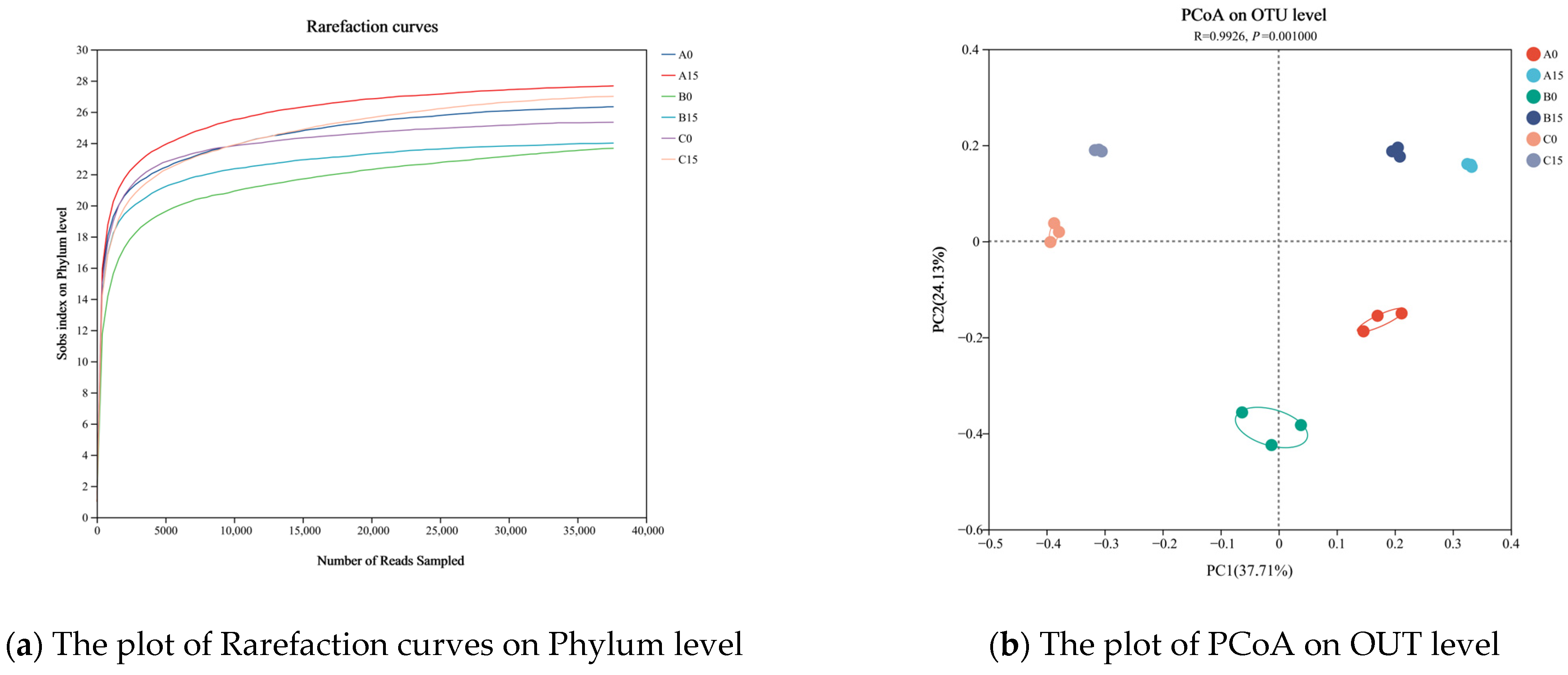
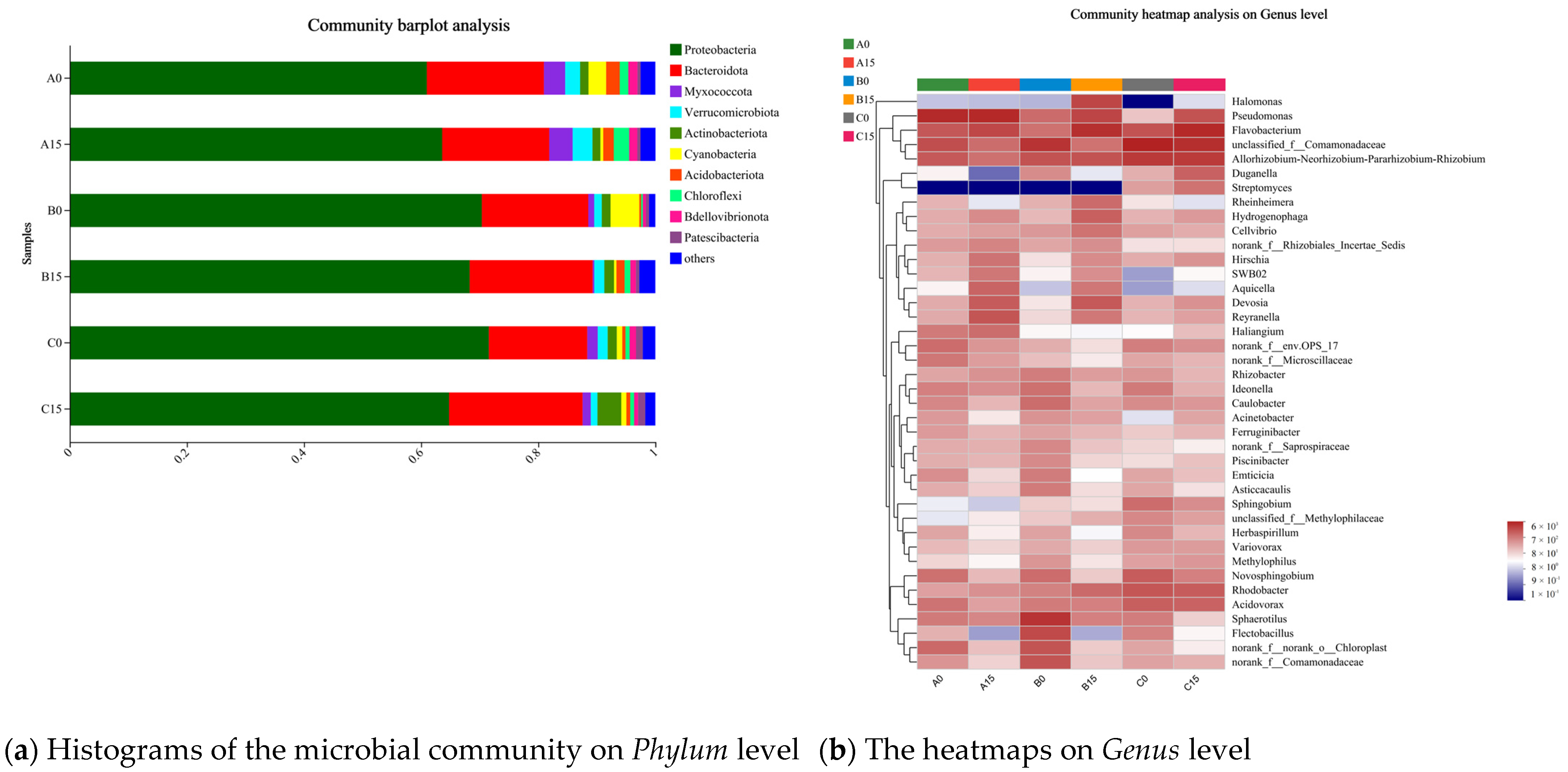
3.6. Analysis of the Microbial Communities in the Roots of the Plants
4. Discussion
4.1. Microalgae Suppression Effects on M. aeruginosa by Floating Bed Plants
4.2. Antioxidant Response of the Floating-Bed Plants to Toxic M. aeruginosa
4.3. The MCs Removal and Microbial Response of the Plants to MCs
4.4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, S.; Zaid, A.; Per, T.S.; Nisar, B.; Majeed, L.R.; Rafiq, S.; Wagay, N.A.; Shah, N.U.; Rather, M.A.; Zulfiqar, F.; et al. A critical review on phytoremediation of environmental contaminants in aquatic ecosystem. Rendiconti Lincei. Sci. Fis. Nat. 2023, 34, 749–766. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Toporowska, M. How to mitigate cyanobacterial blooms and cyanotoxin production in eutrophic water reservoirs? Hydrobiologia 2016, 778, 45–59. [Google Scholar] [CrossRef]
- Harke, M.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, Y.; Jung, J.; Jeon, C.O.; Toyofuku, M.; Lee, J.; Park, W. Biological and Chemical Approaches for Controlling Harmful Microcystis Blooms. J. Microbiol. 2024, 62, 249–260. [Google Scholar] [CrossRef]
- Kardinaal, W.E.A.; Janse, I.; Agterveld, M.K.; Meima, M.; Snoek, J.; Mur, L.R.; Huisman, J.; Zwart, G.; Visser, P.M. Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aqua. Microb. Ecol. 2007, 48, 1–12. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, R.; Liang, D.; Wang, F.; Han, Y.; Luo, Y.; Gao, P.; Wang, Q.; Wang, Q.; Yu, C.; et al. Comparison of the Advantages and Disadvantages of Algae Removal Technology and Its Development Status. Water 2023, 15, 1104. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Li, H.; Liu, J.; Jiang, T.; Yan, D.; Tong, J.; Li, D. Review on Ecological Response of Aquatic Plants to Balanced Harvesting. Sustainability 2022, 14, 12451. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H. Aquatic plant allelochemicals inhibit the growth of microalgae and cyanobacteria in aquatic environments. Environ. Sci. Pollut. Res. 2023, 30, 105084–105098. [Google Scholar] [CrossRef]
- Li, F.; Hu, H. Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl. Environ. Microbiol. 2005, 71, 6545–6553. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Han, Z.; Ye, J.; Liu, Z. The influence of aquatic macrophytes on Microcystis aeruginosa growth. Ecol. Eng. 2012, 42, 130–133. [Google Scholar] [CrossRef]
- Abed, S.N.; Almuktar, S.; Scholz, M. Remediation of synthetic greywater in mesocosm—Scale floating treatment wetlands. Ecol. Eng. 2017, 102, 303–319. [Google Scholar] [CrossRef]
- Song, J.; Li, Q.; Wang, X.C. Superposition effect of floating and fixed beds in series for enhancing nitrogen and phosphorus removal in a multistage pond system. Sci. Total Environ. 2019, 695, 133678. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ghamkhar, R.; Ashton, W.; Hicks, A.L. Sustainable Seafood and Vegetable Production: Aquaponics as a Potential Opportunity in Urban Areas. Integr. Environ. Assess. Manag. 2019, 15, 832–843. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S. Ecological floating bed (EFB) for decontamination of polluted water bodies: Design, mechanism and performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of nitrogen and phosphorus removal by six macrophytes from eutrophic water. Int. J. Phytoremediation 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, H.; Luo, X.; Zhang, J.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: Antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci. Total Environ. 2020, 699, 134325. [Google Scholar] [CrossRef]
- Loise de Morais Calado, S.; Esterhuizen-Londt, M.; Cristina Silva de Assis, H.; Pflugmacher, S. Phytoremediation: Green technology for the removal of mixed contaminants of a water supply reservoir. Int. J. Phytoremediation 2019, 21, 372–379. [Google Scholar] [CrossRef]
- Rojo, C.; Segura, M.; Cortés, F.; Rodrigo, M.A. Allelopathic effects of microcystin-LR on the germination, growth andmetabolism of five charophyte species and a submerged angiosperm. Aquat. Toxicol. 2013, 144–145, 1–10. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, Y.; Wang, N.; Xu, L.; Zheng, Z.; Zhang, J. Allelopathic effects of harmful algal extracts and exudates on biofilms on leaves of Vallisneria natans. Sci. Total Environ. 2018, 655, 823–830. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Z.; Jin, Y. Physiological Mechanism of Eichhornia crassipes in Inhibiting the Growth of Microcytisaeruginosa. Russ. J. Plant Physiol. 2019, 66, 433–439. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Ye, J.; Zhong, B. Study on the release routes of allelochemicals from Pistia stratiotes Linn., and its anti-cyanobacteria mechanisms on Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2015, 22, 18994–19001. [Google Scholar] [CrossRef] [PubMed]
- Wijewickrama, M.M.; Manage, P.M. Accumulation of Microcystin-LR in Grains of Two Rice Varieties (Oryza sativa L.) and a Leafy Vegetable, Ipomoea aquatica. Toxins 2019, 11, 432. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.Y.; Hu, W.R.; Song, M.M. A multi-technique approach for the quantification of Microcystis aeruginosa FACHB-905 biomass during high algae-laden periods. Environ. Technol. 2012, 33, 1773–1779. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Dai, D.; Wang, F.; Zhang, Y.; Chen, Y.; Yuan, J.; Guo, C.; Gao, Y.; Zhang, M.; et al. Response of submerged macrophyte Ceratophyllum demersum to the exponential phase (EP) and declining phase (DP) of toxic Microcystis aeruginosa. Hydrobiologia 2022, 849, 3581–3596. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, L.; Cao, J.; Chang, J. Allelopathic activities of three emergent macrophytes on several monospecific cyanobacterial species and natural phytoplankton assemblages. Pol. J. Environ. Stud. 2015, 24, 397–402. [Google Scholar] [CrossRef]
- Reynolds, S.A.; Aldridge, D.C. Embracing the Allelopathic Potential of Invasive Aquatic Plants to Manipulate Freshwater Ecosystems. Front. Environ. Sci. 2021, 8, 2296X–2665X. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, L.; Hilt, S.; Xu, R.; Wang, B.; Li, C.; Chang, X. Root exudated algicide of Eichhornia crassipes enhances allelopathic effects of cyanobacteria Microcystis aeruginosa on green algae. Hydrobiologia 2018, 823, 67–77. [Google Scholar] [CrossRef]
- Cheng, G.G.; Liu, Y.P.; Gu, J.; Qian, S.Y.; Yang, H.J.; Na, Z.Y.; Luo, X.D. Phytochemicals and Allelopathy of Induced Water Hyacinth against Microcystis aeruginosa. J. Nat. Prod. 2021, 84, 1772–1779. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, G.; Cui, N.; Pan, Q.; Song, X.; Zou, G. Allelopathic Effects on Microcystis aeruginosa and Allelochemical Identification in the Cuture Solutions of Typical Artificial Floating-Bed Plants. Bull. Environ. Contam. Toxicol. 2019, 102, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Zou, G.; Okuda, T.; Tsai, T.Y.; Song, X.; Nishijima, W.; Okada, M. Anti-cyanobacterial allelopathic effects of plants used for artificial floating islands. Allelopath. J. 2010, 26, 113–121. Available online: https://api.semanticscholar.org/CorpusID:88454479 (accessed on 4 October 2024).
- Van Nguyen, Q.; Tran, T.H.; Pham, T.N.; Van, T.D. Inhibitory Effects of Bidens pilosa Plant Extracts on the Growth of the Bloom-Forming Alga Microcystis aeruginosa. Water Air Soil. Pollut. 2019, 230, 24. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, R.; Su, H.; Chen, H.; Luo, M.; Yang, L.; Zhang, M. The functional identification of glycine-rich TtASR from Tetragonia tetragonoides (Pall.) Kuntze involving in plant abiotic stress tolerance. Plant Physiol. Biochem. 2019, 143, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Nene, T.; Yadav, M.; Yadav, H.S. Plant catalase in silico characterization and phylogenetic analysis with structural modeling. J. Genet. Eng. Biotechnol. 2022, 20, 125. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Wickremasinghe, D.; Peiris, H.; Chandrasena, L.G.; Senaratne, V.; Perera, R. Case control feasibility study assessing the association between severity of coronary artery disease with Glutathione Peroxidase-1 (GPX-1) and GPX-1 polymorphism (Pro198Leu). BMC Cardiovasc. Disord. 2016, 16, 111. [Google Scholar] [CrossRef]
- Jacobson, G.A.; Ives, S.J.; Narkowicz, C.; Jones, G. Plasma glutathione peroxidase (GSH-Px) concentration is elevated in rheumatoid arthritis: A case-control study. Clin. Rheumatol. 2012, 31, 1543–1547. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon Off. J. Int. Soc. Toxinol. 2004, 43, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, J.; Zhang, J.; Yang, Z. Time-Dependent Oxidative Stress Responses of Submerged Macrophyte Vallisneria natans Seedlings Exposed to Ammonia in Combination with Microcystin Under Laboratory Conditions. Bull. Environ. Contam. Toxicol. 2012, 89, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Aulhorn, M.; Grimm, B. Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol. 2007, 175, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, C. Effects of microcystin-LR on the tissue growth and physiological responses of the aquatic plant Iris pseudacorus L. Aquat. Toxicol. 2018, 200, 197–205. [Google Scholar] [CrossRef]
- Jones, G.J.; Orr, P.T. Release and degradation of microcystin following algicide treatment of a microcystis aeruginosa bloom in a recreational lake, as determined by hplc and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Beattie, K.A.; Krause, E.; Steinberg, C.E.; Codd, G.A. Uptake, effects, and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (cav.) trin. ex steud. Environ. Toxicol. Chem. 2001, 20, 846–852. [Google Scholar] [CrossRef]
- Saqrane, S.; Ghazali, I.E.; Ouahid, Y.; Hassni, M.E.; Hadrami, I.E.; Bouarab, L.; del Campo, F.F.; Oudra, B.; Vasconcelos, V. Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: Microcystin accumulation, detoxication and oxidative stress induction. Aquat. Toxicol. 2007, 83, 284–294. [Google Scholar] [CrossRef]
- Bashenkhaeva, M.V.; Galachyants, Y.P.; Khanaev, I.V.; Sakirko, M.V.; Petrova, D.P.; Likhoshway, Y.V.; Zakharova, Y.R. Comparative analysis of free-living and particle-associated bacterial communities of Lake Baikal during the ice-covered period. J. Great Lakes Res. 2020, 46, 508–518. [Google Scholar] [CrossRef]
- Liu, B.; Song, C.; Gao, Q.; Liu, B.; Zhou, Q.; Sun, C.; Zhang, H.; Liu, M.; Tadese, D.A. Maternal and environmental microbes dominate offspring microbial colonization in the giant freshwater prawn Macrobrachium rosenbergii. Sci. Total Environ. 2021, 790, 148062. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Beskrovnaya, P.; Fakih, D.; Morneau, I.; Hashimi, A.; Bello, D.G.; Xing, S.; Nanci, A.; Huan, T.; Tocheva, E.I. No Endospore Formation Confirmed in Members of the Phylum Proteobacteria. Appl. Environ. Microbiol. 2021, 87, e02312-20. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.X.; Zhou, Y.X.; Vadivelu, V.M. Enhanced volatile fatty acid production and microbial population analysis in anaerobic treatment of high strength wastewater. J. Water Process Eng. 2020, 33, 101058. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Han, L.; Xia, Y.; Xie, J. A virus induces alterations in root morphology while exerting minimal effects on the rhizosphere and endosphere microorganisms in rice. FEMS Microbiol. Ecol. 2023, 99, 11–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ge, Z.; Ma, Z.; Huang, D.; Zhang, J. Seasonal changes driving shifts of aquatic rhizosphere microbial community structure and the functional properties. J. Environ. Manag. 2022, 322, 116124. [Google Scholar] [CrossRef]
- Yang, C.; Hou, X.; Wu, D.; Chang, W.; Zhang, X.; Dai, X.; Du, H.; Zhang, X.; Igarashi, Y.; Luo, F. The characteristics and algicidal mechanisms of cyanobactericidal bacteria, a review. World J. Microbiol. Biotechnol. 2020, 36, 188. [Google Scholar] [CrossRef]
- Lv, Y.; Wan, C.; Lee, D.J.; Liu, X.; Tay, J.H. Microbial communities of aerobic granules: Granulation mechanisms. Bioresour. Technol. 2014, 169, 344–351. [Google Scholar] [CrossRef]
- Lezcano, M.Á.; Velázquez, D.; Quesada, A.; El-Shehawy, R. Diversity and temporal shifts of the bacterial community associated with a toxic cyanobacterial bloom: An interplay between microcystin producers and degraders. Water Res. 2017, 125, 52–61. [Google Scholar] [CrossRef]
- Le, V.V.; Ko, S.; Kang, M.; Shin, Y.; Lim, B.; Kang, Y.; Oh, H.; Ahn, C. Periphyton reduces cyanobacterial blooms by promoting potentially cyanobactericidal bacteria. J. Appl. Phycol. 2023, 35, 1285–1299. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, H.; Li, L.; Gao, X.; Li, M.; Dong, J.; Zhang, M.; Zhang, J.; Li, X.; Lu, Z.; et al. Higher resistance of a microcystin (MC)-producing cyanobacterium, Microcystis, to the submerged macrophyte Myriophyllum spicatum. Environ. Sci. Pollut. Res. Int. 2023, 30, 63941–63952. [Google Scholar] [CrossRef]
- Mugani, R.; El Khalloufi, F.; Redouane, E.M.; Haida, M.; Aba, R.P.; Essadki, Y.; El Amrani Zerrifi, S.; Hejjaj, A.; Ouazzani, N.; Campos, A.; et al. Unlocking the potential of bacterioplankton-mediated microcystin degradation and removal: A bibliometric analysis of sustainable water treatment strategies. Water Res. 2024, 255, 121497. [Google Scholar] [CrossRef]
- Scherer, P.I.; Millard, A.D.; Miller, A.; Schoen, R.; Raeder, U.; Geist, J.; Zwirglmaier, K. Temporal Dynamics of the Microbial Community Composition with a Focus on Toxic Cyanobacteria and Toxin Presence during Harmful Algal Blooms in Two South German Lakes. Front. Microbiol. 2017, 8, 2387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zuo, J.; Song, L.; Gan, N. Microcystin-degrading bacteria affect mcyD expression and microcystin synthesis in Microcystis spp. J. Environ. Sci. 2016, 41, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Blakeley, R.L.; Riddles, P.; Jones, G.J. Biodegradation of the cyanobacterial toxin microcystin LR in natural water and biologically active slow sand filters. Water Res. 2006, 40, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Lu, X.W.; Li, X.N.; Pu, Y. Biological degradation of algae and microcystins by microbial enrichment on artificial media. Ecol. Eng. 2009, 35, 1584–1588. [Google Scholar] [CrossRef]
- Zeng, G.; Gao, P.; Wang, J.; Zhang, J.; Zhang, M.; Sun, D. Algicidal Molecular Mechanism and Toxicological Degradation of Microcystis aeruginosa by White-Rot Fungi. Toxins 2020, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Salter, C.; VanMensel, D.; Reid, T.; Birbeck, J.; Westrick, J.; Mundle, S.O.C.; Weisener, C.G. Investigating the microbial dynamics of microcystin-LR degradation in Lake Erie sand. Chemosphere 2021, 272, 129873. [Google Scholar] [CrossRef]
- Jones, G.J.; Bourne, D.G.; Blakeley, R.L.; Doelle, H. Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat. Toxins 1994, 2, 228–235. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Xu, Q.; Liu, Z.; Teng, J.; Yan, H.; Liu, X. Microcystins in Water: Detection, Microbial Degradation Strategies, and Mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 13175. [Google Scholar] [CrossRef]
- Dziga, D.; Kokociński, M.; Barylski, J.; Nowicki, G.; Maksylewicz, A.; Antosiak, A.; Banaś, A.K.; Strzałka, W. Correlation between specific groups of heterotrophic bacteria and microcystin biodegradation in freshwater bodies of central Europe. FEMS Microbiol. Ecol. 2019, 95, fiz162. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.A. Mini Review on Microcystins and Bacterial Degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Sha, Y.; Tang, Y.; Li, L.; Wang, F.; Dong, J.; Li, X.; Gao, Y.; Gao, X.; Yuan, H.; et al. Laboratory-Simulated Inhibitory Effects of the Floating-Bed Plants on Microcystis aeruginosa and Their Microbial Communities’ Responses to Microcystins. Microorganisms 2024, 12, 2035. https://doi.org/10.3390/microorganisms12102035
Zhang S, Sha Y, Tang Y, Li L, Wang F, Dong J, Li X, Gao Y, Gao X, Yuan H, et al. Laboratory-Simulated Inhibitory Effects of the Floating-Bed Plants on Microcystis aeruginosa and Their Microbial Communities’ Responses to Microcystins. Microorganisms. 2024; 12(10):2035. https://doi.org/10.3390/microorganisms12102035
Chicago/Turabian StyleZhang, Shuwen, Yuanpu Sha, Yuanyuan Tang, Longjie Li, Feihu Wang, Jing Dong, Xuejun Li, Yunni Gao, Xiaofei Gao, Huatao Yuan, and et al. 2024. "Laboratory-Simulated Inhibitory Effects of the Floating-Bed Plants on Microcystis aeruginosa and Their Microbial Communities’ Responses to Microcystins" Microorganisms 12, no. 10: 2035. https://doi.org/10.3390/microorganisms12102035





