Regulation Effect of Toxocara canis and Anthelmintics on Intestinal Microbiota Diversity and Composition in Dog
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Experiment Grouping and Sampling
2.3. 16S rRNA High-Throughput Sequencing and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
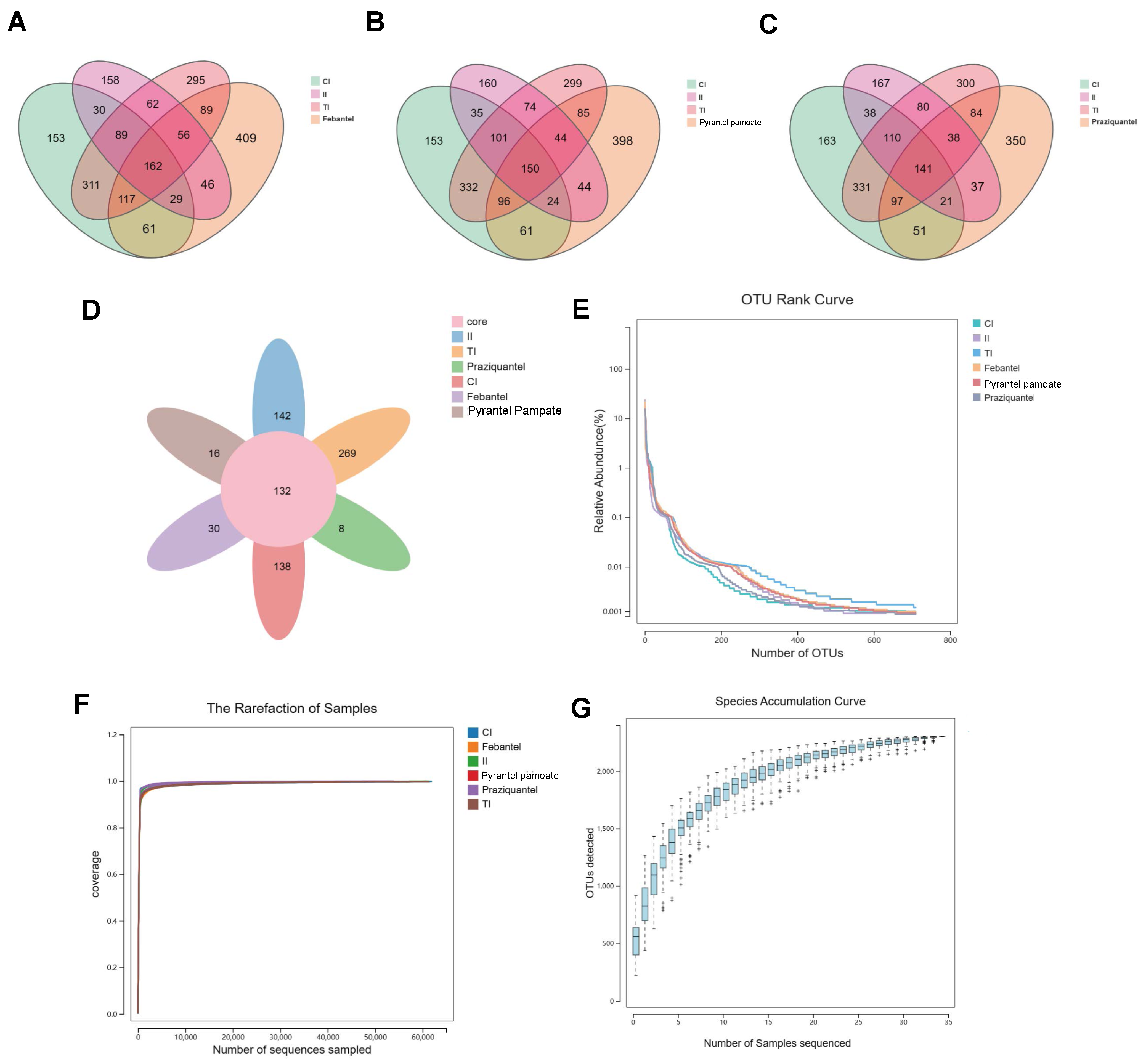
3.1. 16S rRNA High-Throughput Sequencing Result
3.2. Diversity Analysis
3.2.1. Alpha Diversity Analysis
3.2.2. Beta Diversity Analysis
3.2.3. LEfSe Analysis
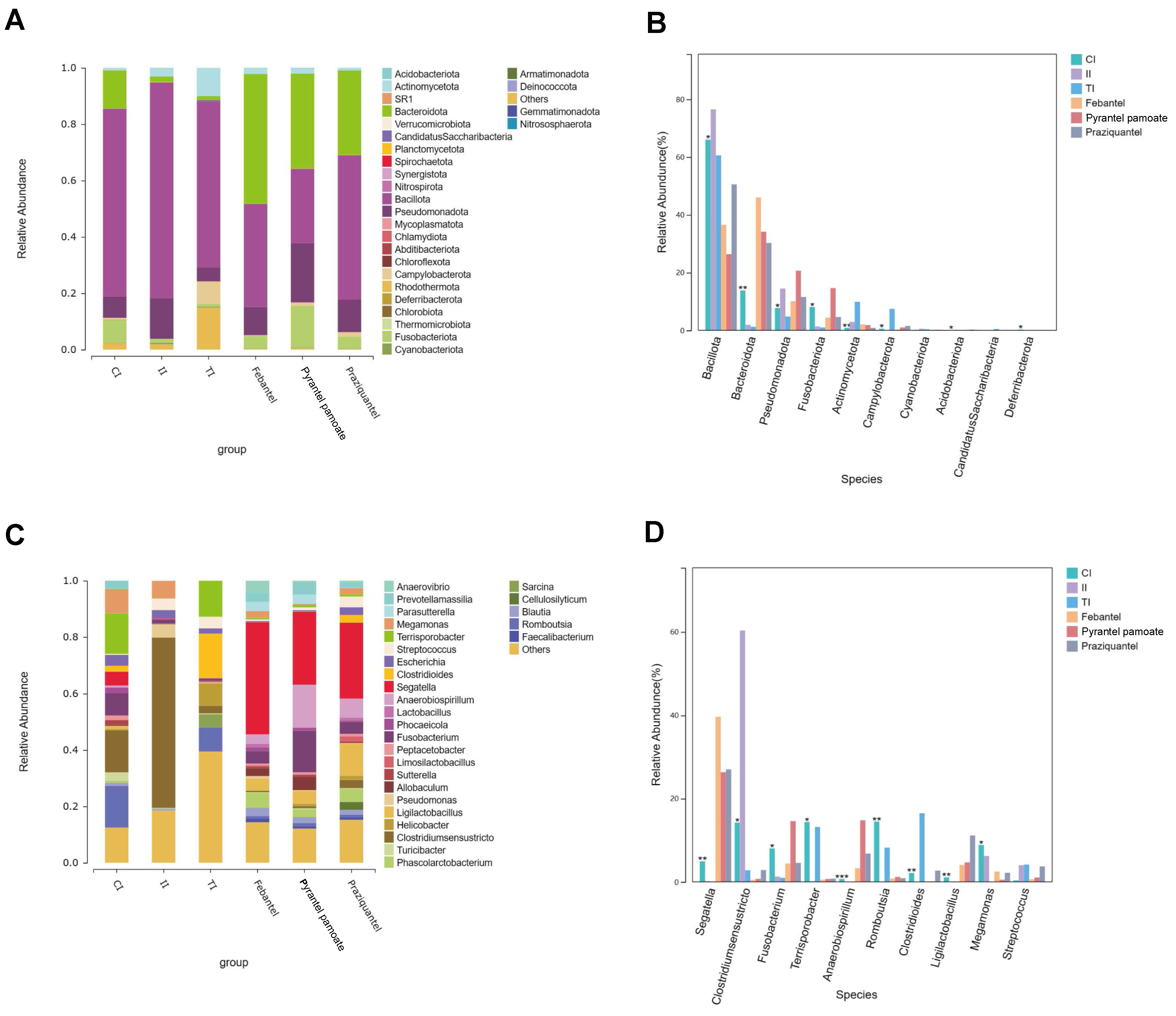
3.3. Species Composition and Difference Analysis
3.3.1. Species Composition and Difference Analysis on Phylum Level
3.3.2. Species Composition and Difference Analysis on Genus Level
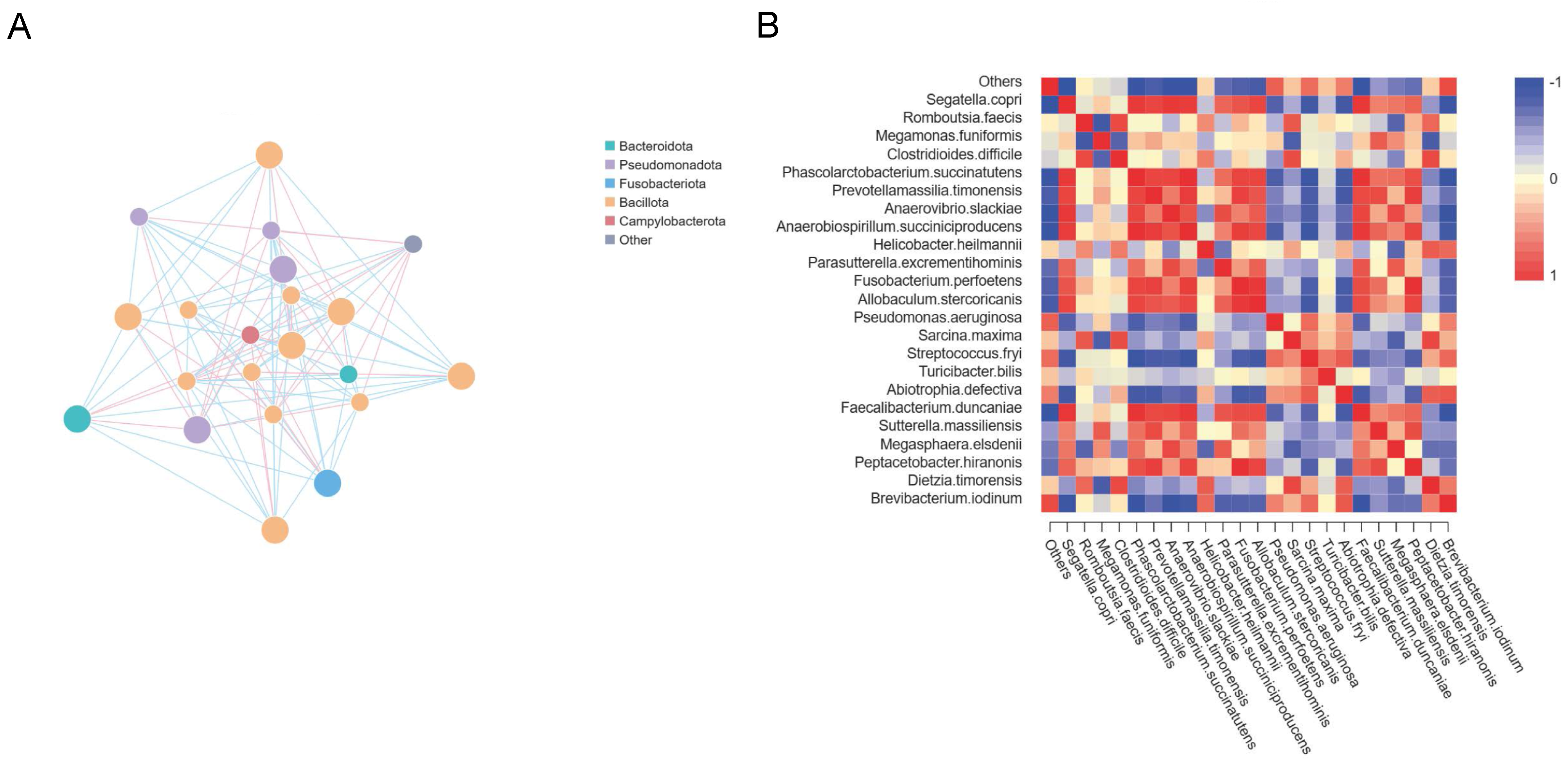
3.4. Interconnections between Major Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strube, C.; Heuer, L.; Janecek, E. Toxocara spp. infections in paratenic hosts. Vet. Parasitol. 2013, 193, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D. Toxocariasis: Clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin. Microbiol. Rev. 2003, 16, 265–272. [Google Scholar] [CrossRef]
- Smith, H.; Holland, C.; Taylor, M.; Magnaval, J.; Schantz, P.; Maizels, R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009, 25, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R.; Jones, S.W. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet. Clin. Small Anim. Pract. 2018, 48, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Neglected Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Hooda, S.; Minamoto, Y.; Suchodolski, J.S.; Swanson, K.S. Current state of knowledge: The canine gastrointestinal microbiome. Anim. Health Res. Rev. 2012, 13, 78–88. [Google Scholar] [CrossRef]
- Morton, E.R.; Lynch, J.; Froment, A.; Lafosse, S.; Heyer, E.; Przeworski, M.; Blekhman, R.; Segurel, L. Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence. PLoS Genet. 2015, 11, e1005658. [Google Scholar] [CrossRef]
- Yang, C.A.; Liang, C.; Lin, C.L.; Hsiao, C.T.; Peng, C.T.; Lin, H.C.; Chang, J.G. Impact of Enterobius vermicularis infection and mebendazole treatment on intestinal microbiota and host immune response. PLoS Neglected Trop. Dis. 2017, 11, e0005963. [Google Scholar] [CrossRef]
- Cooper, P.; Walker, A.W.; Reyes, J.; Chico, M.; Salter, S.J.; Vaca, M.; Parkhill, J. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS ONE 2013, 8, e76573. [Google Scholar] [CrossRef] [PubMed]
- Cantacessi, C.; Giacomin, P.; Croese, J.; Zakrzewski, M.; Sotillo, J.; McCann, L.; Nolan, M.J.; Mitreva, M.; Krause, L.; Loukas, A. Impact of experimental hookworm infection on the human gut microbiota. J. Infect. Dis. 2014, 210, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Finlay, B.B.; Maizels, R.M. Commensal-pathogen interactions in the intestinal tract: Lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014, 5, 522–532. [Google Scholar] [CrossRef]
- Bautista-Garfias, C.; Ixta-Rodríguez, O.; Martínez-Gómez, F.; López, M.; Aguilar-Figueroa, B. Effect of viable or dead Lactobacillus casei organisms administered orally to mice on resistance against Trichinella spiralis infection. Parasite 2001, 8, S226–S228. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Sequeira, T.C.G.; David, É.B.; Ribeiro, C.; Guimarães, S.; Masseno, A.P.B.; Katagiri, S.; Sequeira, J.L. Effect of Bifidobacterium animalis on mice infected with Strongyloides venezuelensis. Rev. Inst. De Med. Trop. Sao Paulo 2014, 56, 105–109. [Google Scholar] [CrossRef]
- Appiah-Twum, F.; Akorli, J.; Okyere, L.; Sagoe, K.; Osabutey, D.; Cappello, M.; Wilson, M.D. The effect of single dose albendazole (400 mg) treatment on the human gut microbiome of hookworm-infected Ghanaian individuals. Sci. Rep. 2023, 13, 11302. [Google Scholar] [CrossRef]
- Liu, L.; Mahalak, K.K.; Bobokalonov, J.T.; Narrowe, A.B.; Firrman, J.; Lemons, J.M.; Bittinger, K.; Hu, W.; Jones, S.M.; Moustafa, A.M. Impact of Ivermectin on the Gut Microbial Ecosystem. Int. J. Mol. Sci. 2023, 24, 16125. [Google Scholar] [CrossRef]
- Schneeberger, P.H.; Gueuning, M.; Welsche, S.; Hürlimann, E.; Dommann, J.; Häberli, C.; Frey, J.E.; Sayasone, S.; Keiser, J. Different gut microbial communities correlate with efficacy of albendazole-ivermectin against soil-transmitted helminthiases. Nat. Commun. 2022, 13, 1063. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Šlapeta, J.; Dowd, S.E.; Alanazi, A.D.; Westman, M.E.; Brown, G.K. Differences in the faecal microbiome of non-diarrhoeic clinically healthy dogs and cats associated with Giardia duodenalis infection: Impact of hookworms and coccidia. Int. J. Parasitol. 2015, 45, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Sieng, S.; Chen, P.; Wang, N.; Xu, J.Y.; Han, Q. Toxocara canis-induced changes in host intestinal microbial communities. Parasites Vectors 2023, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Maeda, S.; Ohno, K.; Horigome, A.; Odamaki, T.; Tsujimoto, H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS ONE 2014, 9, e107909. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Alessandri, G.; Argentini, C.; Milani, C.; Turroni, F.; Cristina Ossiprandi, M.; van Sinderen, D.; Ventura, M. Catching a glimpse of the bacterial gut community of companion animals: A canine and feline perspective. Microb. Biotechnol. 2020, 13, 1708–1732. [Google Scholar] [CrossRef]
- Hayes, K.; Bancroft, A.; Goldrick, M.; Portsmouth, C.; Roberts, I.; Grencis, R. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 2010, 328, 1391–1394. [Google Scholar] [CrossRef]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: Effects reversed by pathogen clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef]
- Walshe, N.; Duggan, V.; Cabrera-Rubio, R.; Crispie, F.; Cotter, P.; Feehan, O.; Mulcahy, G. Removal of adult cyathostomins alters faecal microbiota and promotes an inflammatory phenotype in horses. Int. J. Parasitol. 2019, 49, 489–500. [Google Scholar] [CrossRef]
- Kunz, I.G.; Reed, K.J.; Metcalf, J.L.; Hassel, D.M.; Coleman, R.J.; Hess, T.M.; Coleman, S.J. Equine fecal microbiota changes associated with anthelmintic administration. J. Equine Vet. Sci. 2019, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.; Leng, J.; Ellis, R.; Swann, J.; Moore-Colyer, M.; Proudman, C. The effect of moxidectin treatment on the equine hind gut microbiome, metabonome and feed fermentation kinetics in horses with very low parasite burdens. Equine Vet. Educ. 2017, 29, 6. [Google Scholar]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- O’keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Beyhan, Y.E.; Yıldız, M.R. Microbiota and parasite relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, J.; Dong, X.; Zhao, S.; Hao, Y.; Peng, C.; Shi, H.; Zhou, Y.; Shan, L.; Sun, Q.; et al. Salivary microbial dysbiosis is associated with systemic inflammatory markers and predicted oral metabolites in non-small cell lung cancer patients. J. Cancer 2019, 10, 1651. [Google Scholar] [CrossRef]
- Li, H.; Shang, Z.; Liu, X.; Qiao, Y.; Wang, K.; Qiao, J. Clostridium butyricum alleviates enterotoxigenic Escherichia coli K88-induced oxidative damage through regulating the p62-Keap1-Nrf2 signaling pathway and remodeling the cecal microbial community. Front. Immunol. 2021, 12, 771826. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Gálvez, E.J.; Pasolli, E.; De Filippis, F.; Amend, L.; Huang, K.D.; Manghi, P.; Lesker, T.R.; Riedel, T.; Cova, L.; et al. Extension of the Segatella copri complex to 13 species with distinct large extrachromosomal elements and associations with host conditions. Cell Host Microbe 2023, 31, 1804–1819. [Google Scholar] [CrossRef]
- Allen-Vercoe, E.; Strauss, J.; Chadee, K. Fusobacterium nucleatum: An emerging gut pathogen? Gut Microbes 2011, 2, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T. Bloodstream infection with Anaerobiospirillum succiniciproducens: A potentially lethal infection. South. Med. J. 2011, 104, 205. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; O’Toole, P.W. Lactobacillus: Host–microbe relationships. In Between Pathogenicity and Commensalism; Springer: Berlin/Heidelberg, Germany, 2011; pp. 119–154. [Google Scholar]
- Conterno, L.; Fava, F.; Viola, R.; Tuohy, K.M. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011, 6, 241–260. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Sieng, S.; Chen, P.; Liang, T.; Xu, J.; Han, Q. Regulation Effect of Toxocara canis and Anthelmintics on Intestinal Microbiota Diversity and Composition in Dog. Microorganisms 2024, 12, 2037. https://doi.org/10.3390/microorganisms12102037
Wang N, Sieng S, Chen P, Liang T, Xu J, Han Q. Regulation Effect of Toxocara canis and Anthelmintics on Intestinal Microbiota Diversity and Composition in Dog. Microorganisms. 2024; 12(10):2037. https://doi.org/10.3390/microorganisms12102037
Chicago/Turabian StyleWang, Na, Soben Sieng, Ping Chen, Tian Liang, Jingyun Xu, and Qian Han. 2024. "Regulation Effect of Toxocara canis and Anthelmintics on Intestinal Microbiota Diversity and Composition in Dog" Microorganisms 12, no. 10: 2037. https://doi.org/10.3390/microorganisms12102037









