Soil Microorganism Interactions under Biological Fumigations Compared with Chemical Fumigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Field and Collection of Soil Samples
2.2. Determination of the Soil’s Physiochemical and Biological Properties
2.3. Determination of Plant Growth and Yield
2.4. Throughput Sequencing by Illumina Miseq
2.4.1. DNA Extraction
2.4.2. PCR Amplification, ITS Sequencing, and 16S rDNA Sequencing
2.5. Data Analysis
2.6. Accession Numbers
3. Results
3.1. The Soil’s Physicochemical Properties after the Eggplant Soil Fumigation Treatments
3.2. Fungal and Bacterial Diversity after the Eggplant Soil Fumigation Treatments
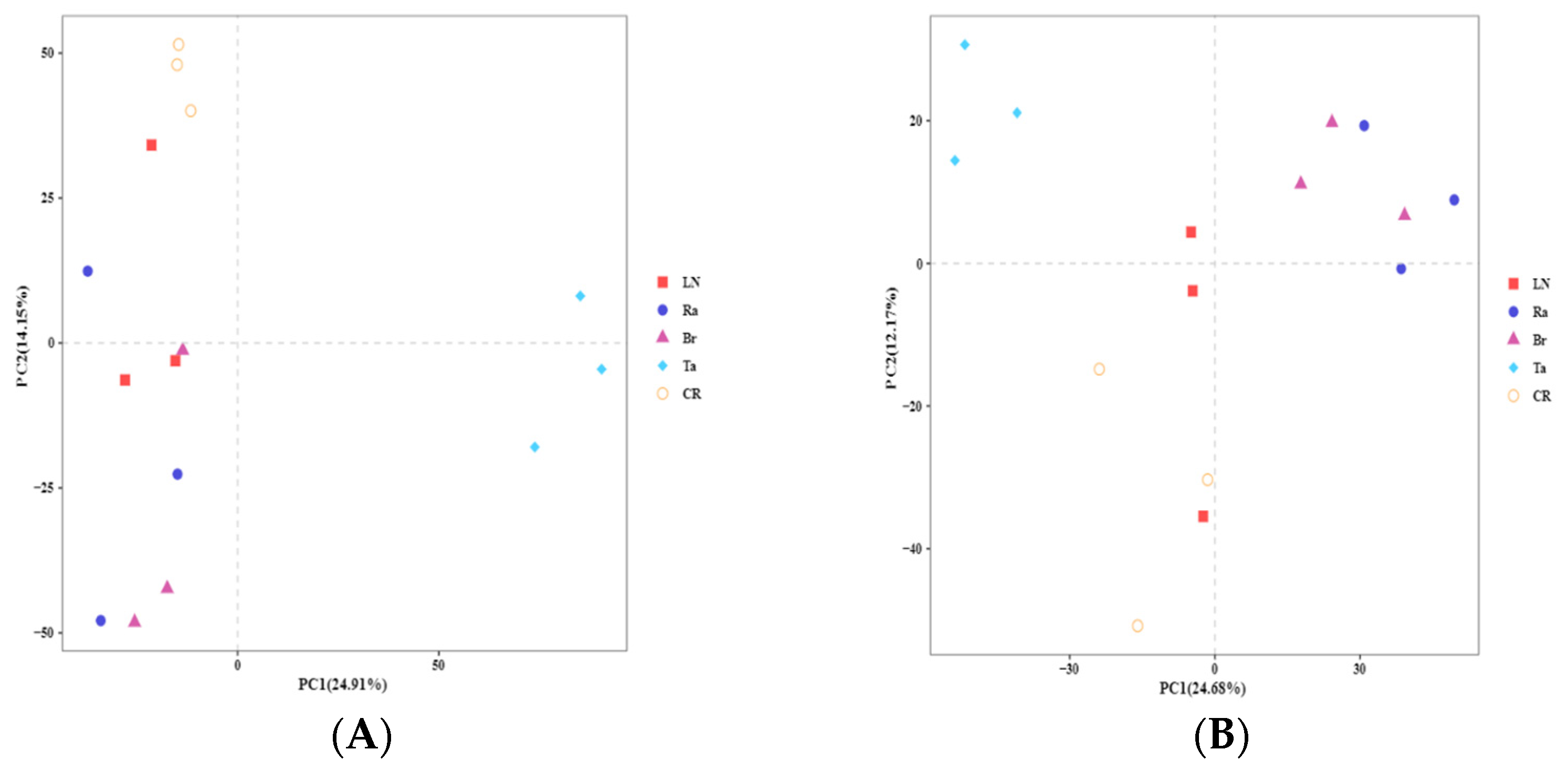
3.3. Overall Structures of Fungi and Bacteria after the Eggplant Soil Fumigation Treatments
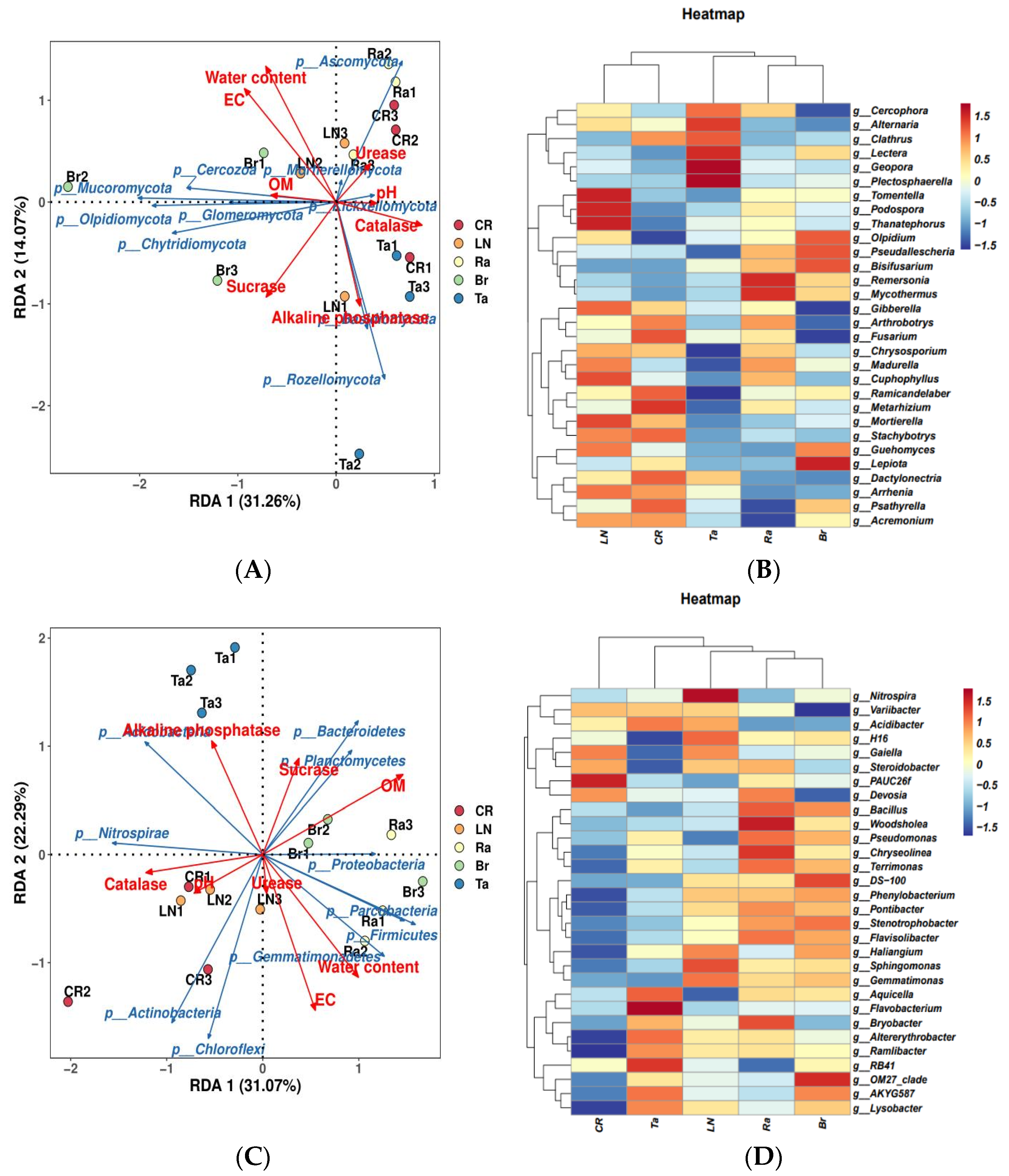
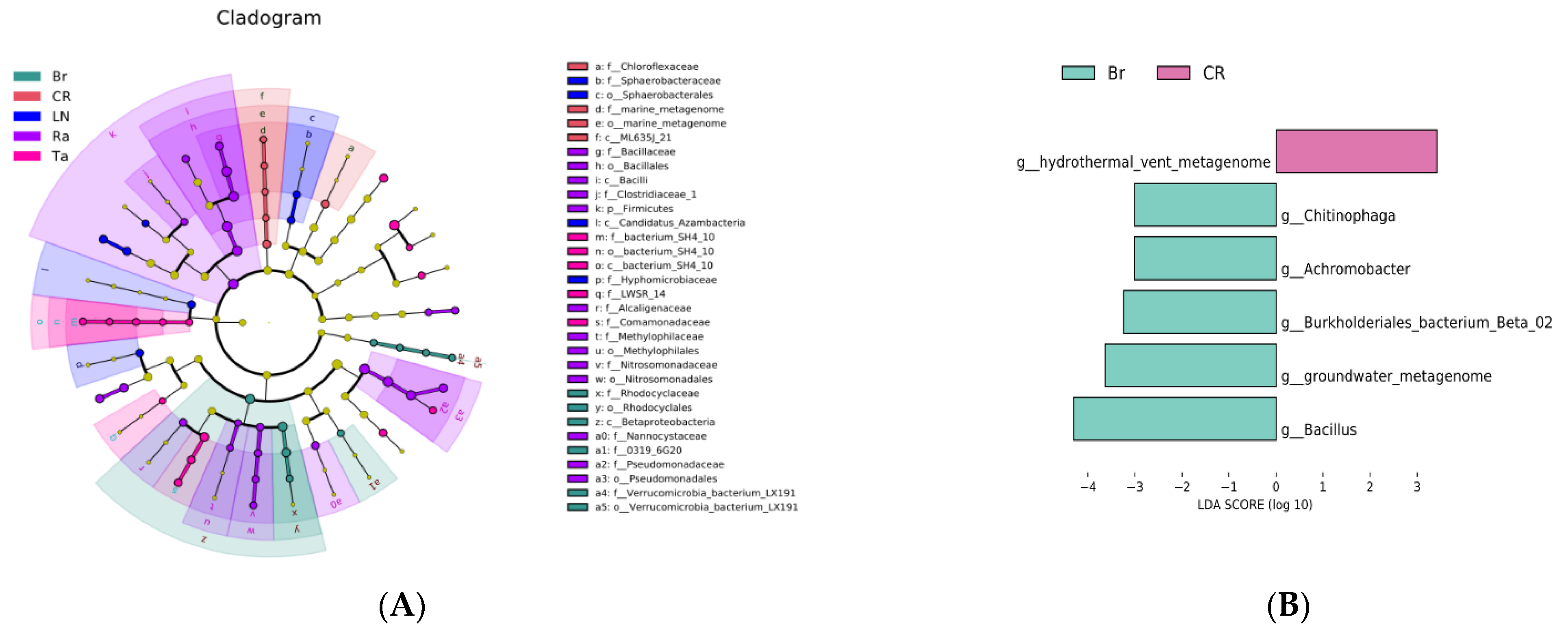
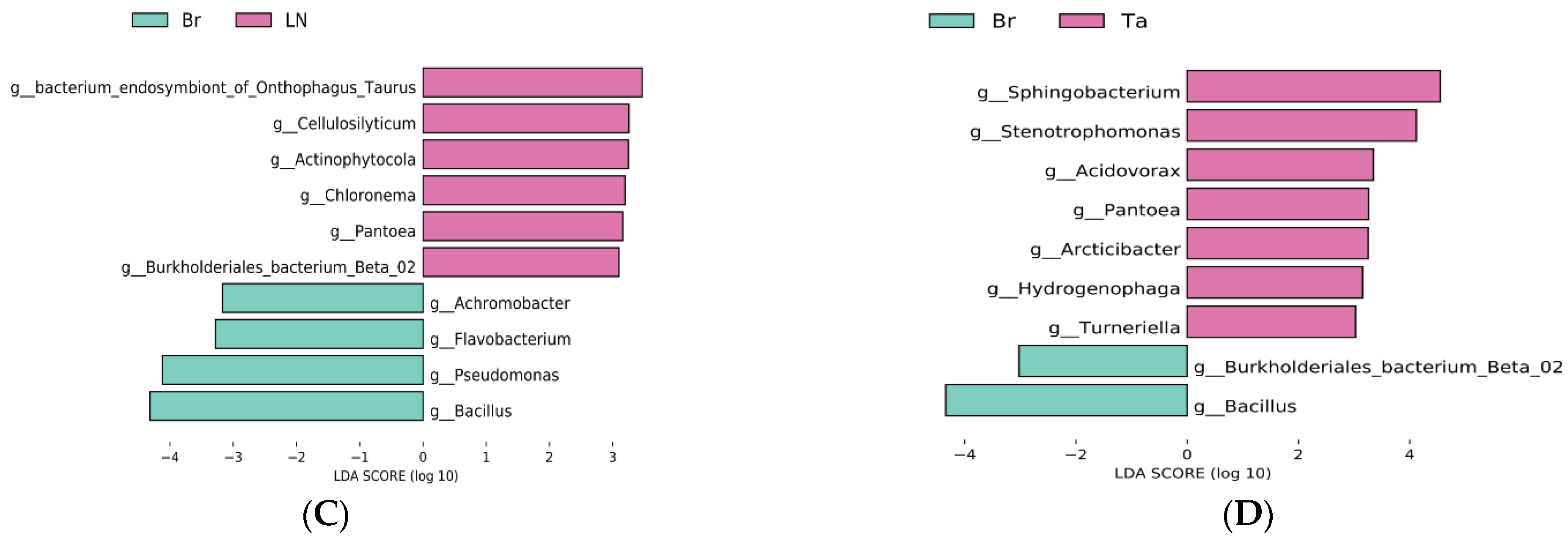
3.4. Specific Microorganism Groups after the Eggplant Soil Fumigation Treatment
3.5. Functional Prediction of Fungi after the Eggplant Soil Fumigation Treatments
3.6. Evaluation of Eggplant’s Growth Status and Yields Combined with the Soil’s Biological Characteristics during Crop Cultivation under Different Soil Fumigation Treatments
4. Discussion
4.1. Diversity and Similarity of Microorganisms’ Structures
4.2. Biological Indicators for Transformation of the Microorganic Structure under Biological Fumigation Treatments
4.3. Predicted Fungal Function and Key Microbial Groups Associated with Soil Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abou Zeid, N.M.; Noher, A.M. Efficacy of DMDS as methyl bromide alternative in controlling soil borne diseases, root-knot nematode and weeds on pepper, cucumber and tomato in Egypt. Acta Hortic. 2014, 1044, 411–414. [Google Scholar] [CrossRef]
- Tateya, A. Japan’s big effort to phase-out of methyl bromide by 2013. Acta Hortic. 2010, 883, 83–89. [Google Scholar] [CrossRef]
- Michael, J.P.; Robert, T.W. Stratospheric ozone depletion and future levels of atmospheric chlorine and bromine. Nature 1990, 344, 729–734. [Google Scholar]
- Zhang, L.; Shi, G.; Shi, G.; Yu, Y.; Li, M.; Su, G.; Jia, X. Soil fumigation-microbial fungicide combination to alleviate the continuous crop disorder of Lanzhou lily (Lilium davidii var. unicolor). Desert China 2020, 5, 169–179. (In Chinese) [Google Scholar]
- Ugolini, L.; Pagnotta, E.; Matteo, R.; Malaguti, L.; Di Francesco, A.; Lazzeri, L. Brassica meal-derived allyl-isothiocyanate postharvest application: Influence on strawberry nutraceutical and biochemical parameters. J. Sci. Food Agric. 2019, 9, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; He, Y.; Shen, D.; Dou, D.; Tian, Y. Inhibitory effect of mustard biofumigation on tobacco epidemic mould. Chin. J. Ecol. Agric. 2023, 4, 567–576. (In Chinese) [Google Scholar]
- Wang, D.; Rosen, C.; Kinkel, L.; Cao, A.; Tharayil, N.; Gerik, J. Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 2009, 324, 185–197. [Google Scholar] [CrossRef]
- Matthiessen, N.J.; Kirkegaard, A.J. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. Crit. Rev. Plant Sci. 2006, 3, 235–265. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Morra, J.M.; Eberlein, V.C.; Brown, D.P.; Borek, V. Allelochemicals released in soil following incorporation of rapeseed (Brassica napus) green manures. J. Agric. Food Chem. 1999, 9, 3837–3842. [Google Scholar] [CrossRef]
- Mohammad, M.; Ghasemi, A.P.; Khyrollah, B.; Nasrin, F. Chemical Compositions of Essential Oil from the Aerial Parts of Tagetes patula L. and Tagetes erecta L. Cultivated in Northeastern Iran. J. Essent. Oil Bear. Plants 2021, 5, 990–997. [Google Scholar]
- Camacho-Campos, C.; Pérez-Hernández, Y.; Valdivia-Ávila, A.; Ramírez-Pérez, H.L.; Gómez-Brisuela, L. Propiedades fitoquímicasy antibacterianas de extractosde Tagetes erecta L. (Asteraceae). Rev. Cuba. Química 2019, 1, 53–64. [Google Scholar]
- Fan, Z.; Guo, C.; Wang, J. Study on the bacteriostatic active components and mechanism of action of marigold root extract on watermelon wilt. J. Phytopathol. 2010, 2, 195–201. (In Chinese) [Google Scholar]
- Gómez-Rodríguez, O.; Zavaleta-Mejía, E.; González-Hernández, V.A.; Livera-Muñoz, M.; Cárdenas-Soriano, E. Allelopathy and microclimatic modification of intercropping with marigold on tomato early blight disease development. Field Crops Res. 2003, 1, 27–34. [Google Scholar] [CrossRef]
- Hunt, H.W.W.D.H. Modelling the effects of loss of soil biodiversity on ecosystem function. Glob. Change Biol. 2002, 1, 33–50. [Google Scholar] [CrossRef]
- Cameron, W.; Franz, S.B.; Franco, W.; van der Marcel, G.A.H. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 14, 5266–5270. [Google Scholar]
- Li, Y.; Feng, J.; Wang, L.; Fu, Y.; Li, X. Role of marigold-tobacco rotation in regulating soil bacterial community structure in tobacco plantation. China Tob. Sci. 2021, 1, 14–19. (In Chinese) [Google Scholar]
- Chang, F.; Zhang, G.; Zhang, L.; Lv, B.; Liu, Z.; Fan, Q.; Yao, Z. Effects of biofumigation with microbial agents on fungal community structure in watermelon continuous cropping soil. Chin. J. Ecol. Agric. 2022, 2, 248–257. (In Chinese) [Google Scholar]
- Liu, S.; Hu, X.; Wang, Y.; Li, C.; Tao, C.; Li, R.; Shen, Q. Effects of lime-ammonium carbon fumigation combined with bio-organic fertilizer application on banana soil fungal communities. J. Appl. Environ. Biol. 2021, 5, 1326–1333. (In Chinese) [Google Scholar]
- Bunlong, Y.; Heike, N.; Andreas, W.; Samuel, J.; JSøren, S.; Traud, W.; Kornelia, S. Effects of Soil Pre-Treatment with Basamid® Granules, Brassica juncea, Raphanus sativus, and Tagetes patula on Bacterial and Fungal Communities at Two Apple Replant Disease Sites. Front. Microbiol. 2017, 8, 81604. [Google Scholar]
- Shi, G.; Jia, X.; Shi, G.; Lv, H.; Yang, H.; Zhang, L. Mitigation effects of different soil disinfectants on continuous crop failure in eggplant (Solanum melongena L.) facilities. Genom. Appl. Biol. 2021, Z3, 3216–3221. (In Chinese) [Google Scholar]
- Jia, X.; Shi, G.; Lv, H.; Li, Y.; Huang, W.; Zhang, L. Study on soil biochemical effects of soil fumigation-microbial organic fertilizer combination to alleviate continuous crop disorder in facility aubergine. J. Gansu Agric. Univ. 2019, 03, 84–92. (In Chinese) [Google Scholar]
- Shi, G.; Sun, H.; Alejandro, C.; Jia, X.; Yang, H.; Su, G. Soil Fungal Diversity Loss and Appearance of Specific Fungal Pathogenic Communities Associated with the Consecutive Replant Problem (CRP) in Lily. Front. Microbiol. 2020, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, H.; Sun, H.; Dawuda, M.; Li, M.; Shi, G. Soil intrinsic properties changes and yield loss of Lanzhou lily (Lilium davidii var. Unicolor) under continuous cropping in the arid area of western China. Pak. J. Bot. 2023, 55, 2091–2099. [Google Scholar]
- Yu, Y.; Zhang, L.; Li, Y.; Hou, L.; Yang, H.; Shi, G. Silicon Fertilizer and Microbial Agents Changed the Bacterial Community in the Consecutive Replant Soil of Lilies. Agronomy 2022, 7, 1530. [Google Scholar] [CrossRef]
- Reazin, C.; Morris, S.; Smith, J.E.; Cowan, A.D.; Jumpponen, A. Fires of differing intensities rapidly select distinct soil fungal communities in a Northwest US ponderosa pine forest ecosystem. For. Ecol. Manag. 2016, 377, 118–127. [Google Scholar] [CrossRef]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS ONE 2012, 11, e48289. [Google Scholar] [CrossRef]
- Miller, G.E.; Engen, P.A.; Gillevet, P.M.; Shaikh, M.; Sikaroodi, M.; Forsyth, C.B.; Mutlu, E.; Keshavarzian, A. Lower Neighborhood Socioeconomic Status Associated with Reduced Diversity of the Colonic Microbiota in Healthy Adults. PLoS ONE 2016, 2, e0148952. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.; He, Y.; Wu, J.; Jiang, Y.; Tam Nora, F.; Zhou, H. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 23, 8264–8271. [Google Scholar] [CrossRef]
- Nicola, S.; Jacques, I.; Levi, W.; Dirk, G.; Larisa, M.; SWendy, G.; Curtis, H. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 6, R60. [Google Scholar]
- Roy, S.; Roy, M.M.; Jaiswal, K.A.; Baitha, A. Soil Arthropods in Maintaining Soil Health: Thrust Areas for Sugarcane Production Systems. Sugar Tech 2018, 4, 376–391. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqi, H.; Bano, S.; Naz, A.; Lubna Mazhar, K.; Nasim, S.; Riaz, T.; Kamal, S.; Ahmad, A.; Khan, A.S. Antibacterial and Antifungal Activities of Different Parts of Tagetes patula.: Preparation of Patuletin Derivatives. Pharm. Biol. 2008, 5, 309–320. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ren, X.; Chen, B.; Zhang, Y.; Shen, C.; Wang, F.; Wu, D. Long-Term Greenhouse Cucumber Production Alters Soil Bacterial Community Structure. J. Soil Sci. Plant Nutr. 2020, 8, 306–321. [Google Scholar] [CrossRef]
- Li, T.; Liu, T.; Zheng, C.; Kang, C.; Yang, Z.; Yao, X.; Song, F.; Zhang, R.; Wang, X.; Xu, N.; et al. Changes in soil bacterial community structure as a result of incorporation of Brassica plants compared with continuous planting eggplant and chemical disinfection in greenhouses. PLoS ONE 2017, 3, e0173923. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Wang, Y.; Huang, Y.; Fan, F.; Liu, S. Soil properties and microbial diversity of melon planted in different years of continuous cropping. Microbiol. Bull. 2022, 01, 101–114. (In Chinese) [Google Scholar]
- Wang, Y.; Li, T.; Li, C.; Song, F. Differences in Microbial Community and Metabolites in Litter Layer of Plantation and Original Korean Pine Forests in North Temperate Zone. Microorganisms 2020, 12, 2023. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ma, J.; Yang, Y.; Wang, Y.; Fu, C. Flooding Irrigation Weakens the Molecular Ecological Network Complexity of Soil Microbes during the Process of Dryland-to-Paddy Conversion. Int. J. Environ. Res. Public Health 2020, 2, 561. [Google Scholar] [CrossRef]
- Geng, N.; Kang, X.; Yan, X.; Yin, N.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Biochar mitigation of soil acidification and carbon sequestration is influenced by materials and temperature. Ecotoxicol. Environ. Saf. 2022, 232, 113241. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and Organic Fertilizers Distinctly Affect Fungal Communities in the Crop Rhizosphere. J. Fungi 2022, 3, 251. [Google Scholar] [CrossRef]
- D’Ann, R. Fungal transmission of plant viruses. Curr. Protoc. Microbiol. 2009, 1, Unit16B.4. [Google Scholar]
- Lay, C.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenicity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 9, 837–846. [Google Scholar] [CrossRef]
- Tao, G.; Liu, Y.; Hyde, D.K.; Liu, X.; Yu, Z. Whole rDNA analysis reveals novel and endophytic fungi in Bletilla ochracea (Orchidaceae). Fungal Divers. 2008, 33, 101–122. [Google Scholar]
- Jia, M.; Chen, L.; Xin, H.; Zheng, C.; Rahman, K.; Han, T.; Qin, L. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Du, W.; Yao, Z.; Li, J.; Sun, C.; Xia, J.; Wang, B.; Shi, D.; Ren, L. Diversity and antimicrobial activity of endophytic fungi isolated from Securinega suffruticosa in the Yellow River Delta. PLoS ONE 2020, 3, e0229589. [Google Scholar] [CrossRef]
- Yao, H.; Sun, X.; He, C.; Maitra, P.; Li, X.; Guo, L. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 2019, 1, 57. [Google Scholar] [CrossRef]
- Daniele, C.; Rolf, M.; Julia, W.; Danielle, V.; KarlDieter, M.; Bärbel, H.; Claudia, W. Molecular identification of Nucleophaga terricolae sp. nov. (Rozellomycota), and new insights on the origin of the Microsporidia. Parasitol. Res. 2016, 8, 3003–3011. [Google Scholar]
- Almeida, B.J.R.D.; Joannis, G.J.P.; Sturion, A.L.; Batista, D.P.; Martins, A.C.; Cunha, M.M.B.; Martins, M.H.V. How Deep Can the Endophytic Mycobiome Go? A Case Study on Six Woody Species from the Brazilian Cerrado. J. Fungi 2023, 9, 508. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dörfelt, H.; Gube, M.; Jackson, J.D.; Reitner, J.; Seyfullah, J.L.; et al. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenetics Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef]
- Fu, D.; Chen, Y.; Guo, Q.; Mao, L.; Fei, J.; Qiu, J. Progress in the study of metabolites of Seatophora and its sexual type (Ascomycota). J. Mycol. 2018, 05, 541–554. (In Chinese) [Google Scholar]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, M.J.; Wang, J.; Eldridge, J.D.; Bardgett, D.R.; Maestre, T.F.; Singh, K.B. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Thompson, M.S.; Tan, P.Y.; Neate, M.S.; Grams, R.M.; Shivas, R.G.; Lindbeck, K.; Aitken, B.A.E. Diaporthe novem isolated from sunflower (Helianthus annuus) and other crop and weed hosts in Australia. Eur. J. Plant Pathol. 2018, 3, 823–831. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Nicola, D.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant Nutr. 2013, 3, 730–742. [Google Scholar] [CrossRef]



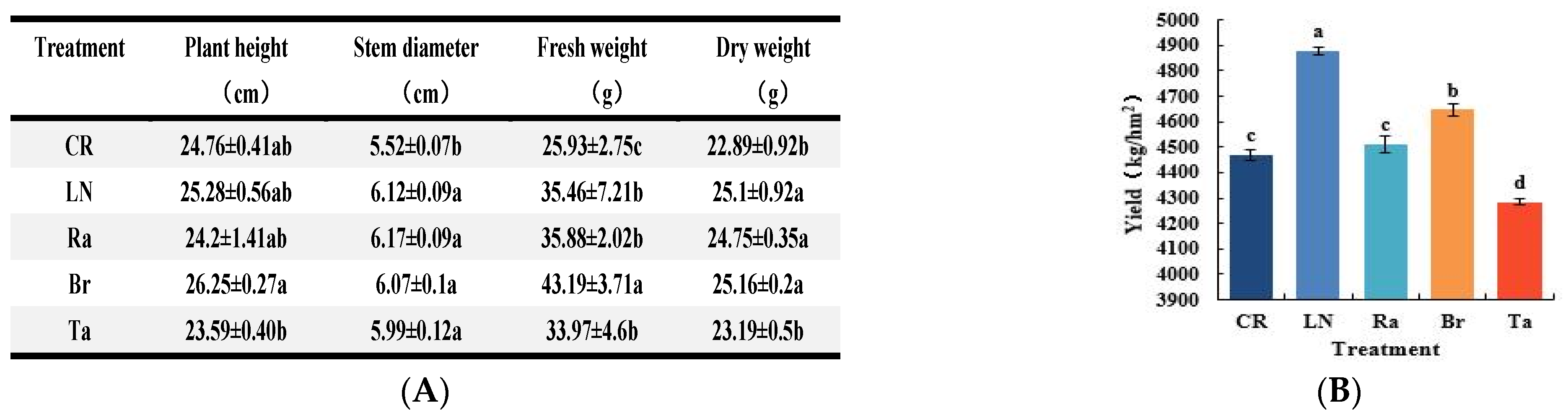
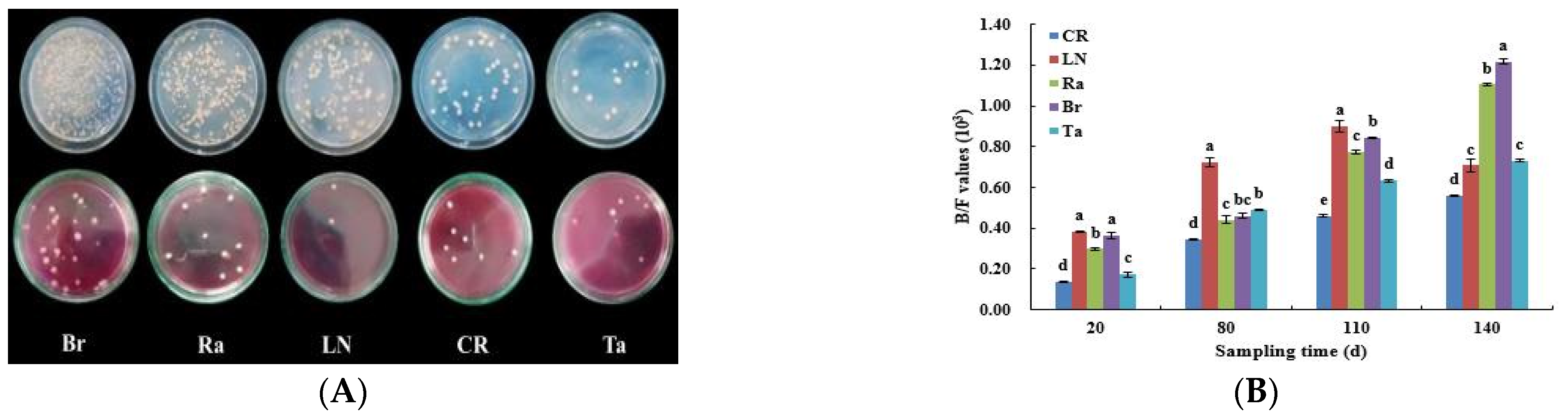
| Treatment | OM (g/kg) | EC (ms/cm) | WC (%) | pH | Urease (mg/g) | Alkaline Phosphatase (mg/g) | Sucrase (mg/g) | Catalase (mg/g) |
|---|---|---|---|---|---|---|---|---|
| CR | 12.34 ± 0.20 c | 515 ± 6.06 b | 11.9 ± 0.0002 ab | 7.52 ± 0.02 a | 0.98 ± 0.00 a | 2.23 ± 0.06 b | 1.47 ± 0.03 b | 1.74 ± 0.01 a |
| LN | 11.31 ± 0.76 c | 495 ± 3.21 bc | 12.2 ± 0.0036 a | 5.86 ± 0.02 c | 1.06 ± 0.03 a | 2.49 ± 0.16 a | 1.67 ± 0.10 a | 1.49 ± 0.20 ab |
| Ra | 18.42 ± 0.51 ab | 483 ± 3.84 c | 12.7 ± 0.0029 a | 7.55 ± 0.01 a | 1.05 ± 0.02 a | 2.29 ± 0.21 b | 1.59 ± 0.05 ab | 1.25 ± 0.04 b |
| Br | 20.27 ± 0.65 a | 556 ± 6.35 a | 12.4 ± 0.0030 a | 7.43 ± 0.01 b | 0.95 ± 0.00 a | 2.28 ± 0.09 b | 1.69 ± 0.01 a | 1.26 ± 0.05 b |
| Ta | 17.07 ± 1.16 b | 345 ± 5.49 d | 11.2 ± 0.0019 b | 7.54 ± 0.00 a | 0.98 ± 0.16 a | 2.48 ± 0.01 a | 1.68 ± 0.00 a | 1.54 ± 0.02 ab |
| Treatment | CR | LN | Ra | Br | Ta |
|---|---|---|---|---|---|
| Fungi | |||||
| p__Ascomycota | 54.87% ab | 47.75% b | 66.99% a | 49.42% b | 49.76% b |
| p__Mortierellomycota | 15.61% ab | 19.18% a | 10.19% b | 11.69% ab | 11.17% b |
| p__Rozellomycota | 0.23% b | 0.53% b | 0.61% b | 0.65% b | 13.20% a |
| p__Olpidiomycota | 0.07% b | 1.80% b | 1.04% b | 9.16% a | 1.14% b |
| Bacteria | |||||
| p__Acidobacteria | 29.69% ab | 29.5% ab | 23.72% b | 27.6% ab | 35.33% a |
| p__Chloroflexi | 15.15% a | 13.21% ab | 12.75% ab | 12.14% bc | 10.36% c |
| p__Gemmatimonadetes | 7.25% b | 7.87% ab | 8.52% ab | 8.92% a | 6.22% c |
| p__Actinobacteria | 7.41% a | 6.92% ab | 5.71% bc | 4.94% c | 4.67% c |
| p__Planctomycetes | 1.45% b | 2.14% ab | 2.37% a | 2.56% a | 2.32% a |
| p__Nitrospirae | 2.22% a | 2.09% ab | 1.57% c | 1.7% b | 2.07% ab |
| p__Firmicutes | 0.99% bc | 0.7% c | 2.71% a | 1.9% ab | 0.46% c |
| p__Parcubacteria | 0.81% bc | 1.31% ab | 1.6% a | 1.72% a | 0.7% c |
| p__Saccharibacteria | 0.9% b | 0.77% b | 1.07% ab | 1.18% ab | 1.35% a |
| p__Latescibacteria | 0.85% ab | 1.15% ab | 0.77% b | 1.07% ab | 1.34% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Man, H.; Han, J.; Jia, X.; Wang, L.; Yang, H.; Shi, G. Soil Microorganism Interactions under Biological Fumigations Compared with Chemical Fumigation. Microorganisms 2024, 12, 2044. https://doi.org/10.3390/microorganisms12102044
Li H, Man H, Han J, Jia X, Wang L, Yang H, Shi G. Soil Microorganism Interactions under Biological Fumigations Compared with Chemical Fumigation. Microorganisms. 2024; 12(10):2044. https://doi.org/10.3390/microorganisms12102044
Chicago/Turabian StyleLi, Hui, Huali Man, Jia Han, Xixia Jia, Li Wang, Hongyu Yang, and Guiying Shi. 2024. "Soil Microorganism Interactions under Biological Fumigations Compared with Chemical Fumigation" Microorganisms 12, no. 10: 2044. https://doi.org/10.3390/microorganisms12102044





