The pH and Sucrose Influence Rhamnolipid Action Toward Planktonic and Biofilms of Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhamnolipids (RL)
2.2. Microorganism and Inoculum Preparation
2.3. Determination of Minimum Inhibitory (MIC) and Minimum Bactericidal (MBC) Concentrations
2.4. Bacterial Growth Curve
2.5. Antimicrobial Activity in Bacterial Biofilms
2.6. Determination of Minimal Biofilm Inhibitory and Minimal Biofilm Eradication Concentrations (MBIC) and (MBEC)
2.7. Critical Micellar Concentration (CMC)
2.8. Assessment of Molecular Aggregates of RL
2.8.1. Fluorescence Microscopy
2.8.2. Cryo-TEM
2.9. Dynamic Light Scattering (DLS)
2.10. Statistical Analysis
3. Results and Discussion
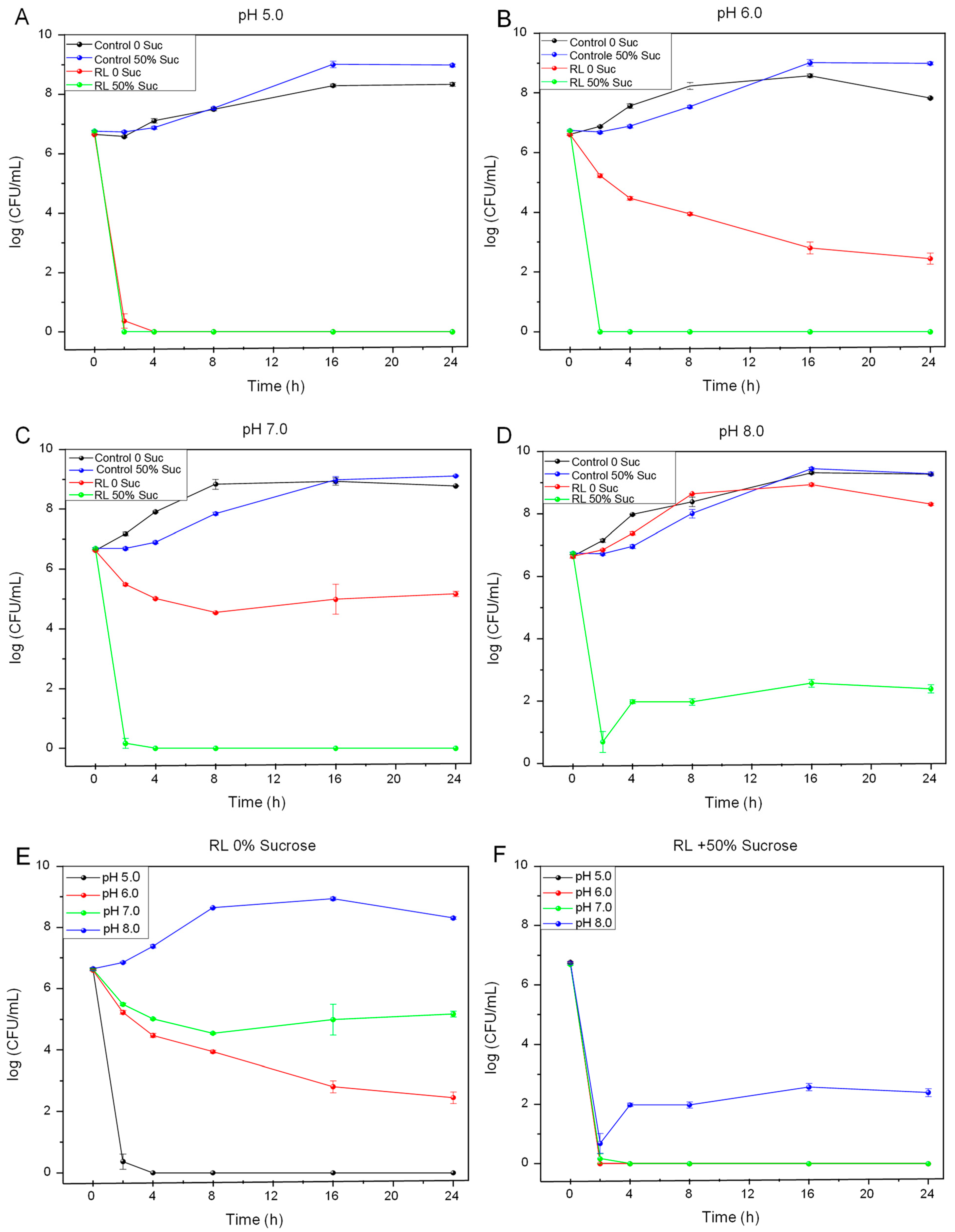
3.1. Antimicrobial Activity in Planktonic Cells
3.2. Antimicrobial Activity in Biofilms
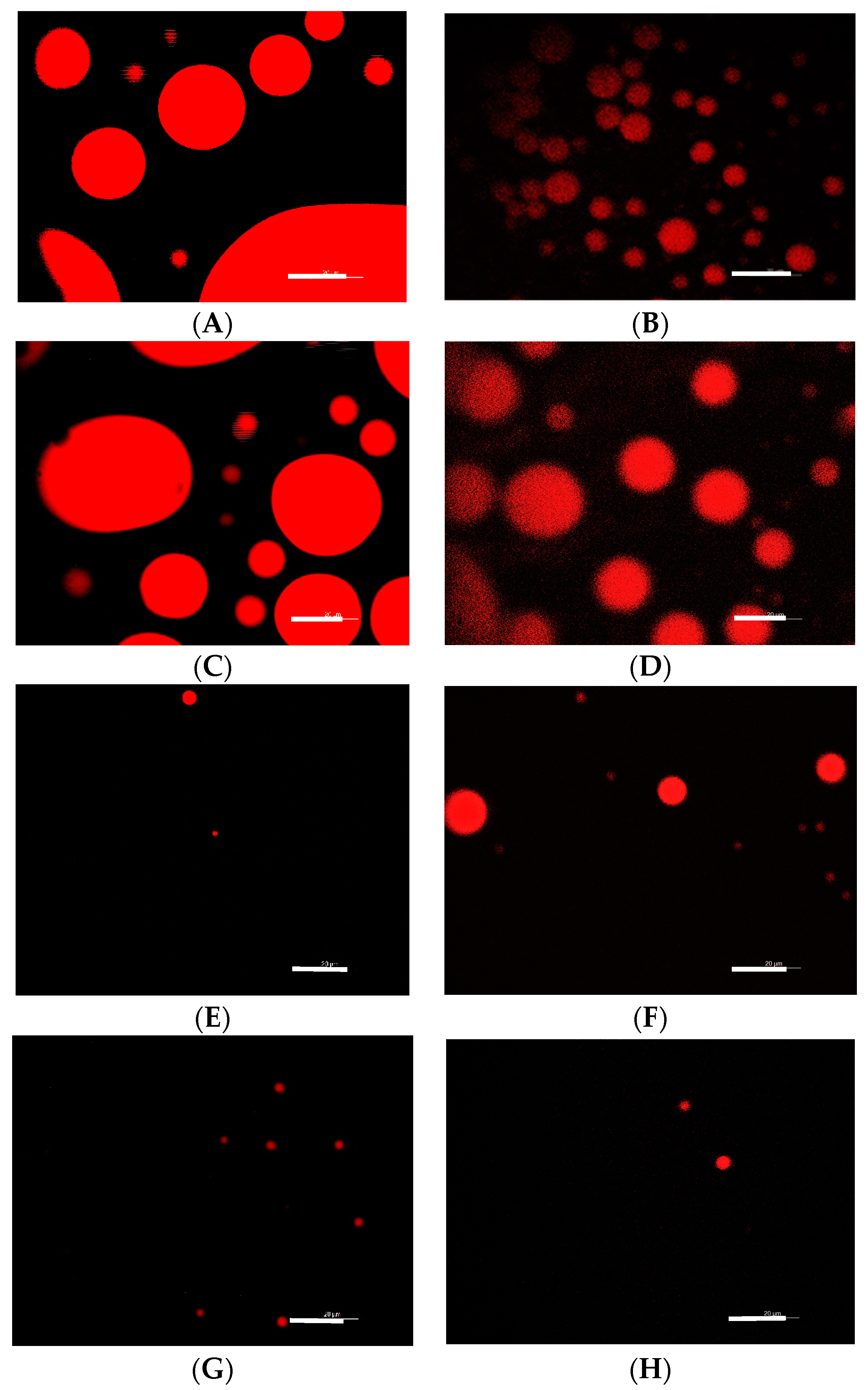
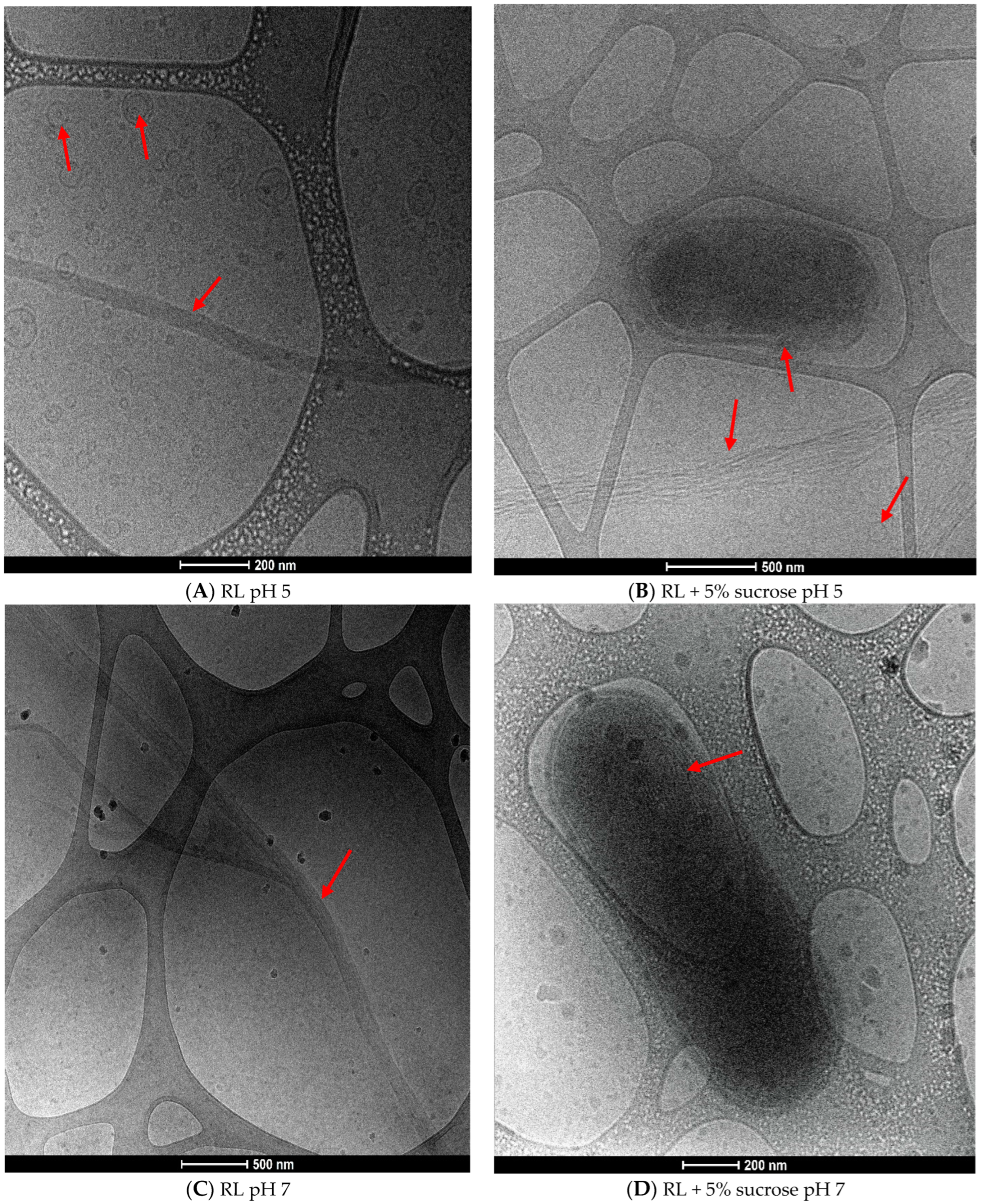
3.3. Impact of pH and Sucrose on Aggregation Behavior of RL
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Listeriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 16 August 2024).
- CDC. Listeria Infection (Listeriosis). Available online: https://www.cdc.gov/listeria/about/index.html (accessed on 17 September 2024).
- CDC. Listeria Outbreak Linked to Meats Sliced at Delis. Available online: https://www.cdc.gov/listeria/outbreaks/delimeats-7-24.html?CDC_AAref_Val=https://www.cdc.gov/listeria/outbreaks/delimeats-7-24/index.html (accessed on 9 September 2024).
- ECDC. Listeriosis—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2022 (accessed on 20 August 2024).
- ECDC. Rapid Outbreak Assessment: Prolonged Multi-Country Outbreak of Listeria monocytogenes ST1607 Linked to Smoked Salmon Products. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-outbreak-assessment-prolonged-multi-country-outbreak-listeria-monocytogenes (accessed on 11 September 2024).
- Costa, P.V.; Nascimento, J.S.; Costa, L.E.O.; Ferreira, P.B.M.; Brandão, M.L.L. Listeria monocytogenes: Challenges of microbiological control of food in Brazil. Food Sci. Technol. 2022, 42, 08322. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.V.; Abreu, R.M.; Santo-Buelga, C.R.; Ferreira, C.F.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Chiamaka, L.M.; Otobong, D.A.; Chunfang, Z.; Mengzhen, H.; Nsemeke, L.; He, Z.; Sherifah, M.W. Integration of green economy concepts for sustainable biosurfactant production—A review. Bioresour. Technol. 2022, 364, 128021. [Google Scholar] [CrossRef]
- Dias, M.A.M.; Nitschke, M. Bacterial-derived surfactants: An update on general aspects and forthcoming applications. Braz. J. Microbiol. 2023, 54, 103–123. [Google Scholar] [CrossRef]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/biosurfactant-market-163644922.html (accessed on 18 November 2023).
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the world of rhamnolipids: A critical review of their production, interfacial properties, and potential application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Adejumo, I.O.; Afolabi, I.S.; Adetunji, J.B.; Ajisejiri, E.S. Prolonging the shelf life of ‘Agege Sweet’ orange with chitosan–rhamnolipid coating. Hortic. Environ. Biotechnol. 2018, 59, 687–697. [Google Scholar] [CrossRef]
- Ferreira, J.D.F.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Niaz, T.; Imran, M. Diffusion kinetics of nisin from composite coatings reinforced with nano-rhamnosomes. J. Food Eng. 2021, 288, 110143. [Google Scholar] [CrossRef]
- Silva, S.S.; Carvalho, J.W.P.; Aires, C.P.; Nitschke, M. Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J. Dairy Sci. 2017, 100, 7864–7873. [Google Scholar] [CrossRef]
- Magalhães, L.; Nitschke, M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control. 2013, 29, 138–142. [Google Scholar] [CrossRef]
- Mishra, A.; Tiwari, P.; Pandey, L.M. Surface, interfacial and thermodynamic aspects of the Rhamnolipid-salt systems. J. Mol. Liq. 2023, 384, 122245. [Google Scholar] [CrossRef]
- Passos, T.F.; Nitschke, M. The combined effect of pH and NaCl on the susceptibility of Listeria monocytogenes to rhamnolipids. Food Res. Int. 2024, 192, 114744. [Google Scholar] [CrossRef]
- Lenaerts, L.; Passos, T.F.; Gayán, E.; Michiels, C.W.; Nitschke, M. Hurdle technology approach to control Listeria monocytogenes using rhamnolipid biosurfactant. Foods 2023, 12, 570. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI document M07-A10; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Passos, T.F.; Souza, M.T.; Zanotto, E.D.; de Souza, C.W.O. Bactericidal activity and biofilm inhibition of F18 bioactive glass against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 118, 111475. [Google Scholar] [CrossRef]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef]
- Rodrigues, A.I.; Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Sodium chloride effect on the aggregation behaviour of rhamnolipids and their antifungal activity. Sci. Rep. 2017, 7, 12907. [Google Scholar] [CrossRef]
- Ishigami, Y.; Gama, Y.; Nagaroha, H. The pH sensitive conversion of molecular aggregates of rhamnolipid biosurfactant. Chem. Lett. 1987, 5, 763–766. [Google Scholar] [CrossRef]
- Abbasi, H.; Noghabi, K.A.; Hamedi, M.M.; Zahiri, H.S.; Moosavi-Movahedi, A.A.; Amanlou, M.; Teruel, J.A.; Ortiz, A. Physicochemical characterization of a monorhamnolipid secreted by Pseudomonas aeruginosa MA01 in aqueous media. An experimental and molecular dynamics study. Colloids Surf. B 2013, 101, 256–265. [Google Scholar] [CrossRef]
- Kadakia, P.; Valentin, J.D.P.; Hong, L.; Watts, S.; Hameed, O.A.; Walch, M.; Salentinig, S. Biocompatible rhamnolipid self-assemblies with pH-responsive antimicrobial activity. Adv. Healthc. Mater. 2024, 13, 2302596. [Google Scholar] [CrossRef]
- Chauhan, S.; Jyoti, J.; Pathania, L.; Umar, A.; Chauhan, M.S. Carbohydrate-surfactant interactions in aqueous and mixed organic solvents at various temperatures: Volumetric, compressibility and acoustical studies. J. Mol. Liq. 2016, 218, 637–648. [Google Scholar] [CrossRef]
- Bremer, E.; Krämer, R. Responses of microorganisms to osmotic stress. Ann. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- McLandsborough, L.; Rodriguez, A.; Pérez-Conesa, D.; Weiss, J. Biofilms: At the interface between biophysics and microbiology. Food Biophys. 2006, 1, 94–114. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Wang, Y. Selective antimicrobial activities and action mechanism of micelles self-assembled by cationic oligomeric surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta–Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalid, Z.M.; Khan, M.S.; Banat, I.M.; Rehman, A.; Naeem, A.; Saddique, M.T. Surface properties and sub-surface aggregate assimilation of rhamnolipid surfactants in different aqueous systems. Biotechnol. Lett. 2010, 32, 811–816. [Google Scholar] [CrossRef]
- Mizzi, L.; Maniscalco, D.; Gaspari, S.; Chatzitzika, C.; Gatt, R.; Valdramidis, V.P. Assessing the individual microbial inhibitory capacity of different sugars against pathogens commonly found in food systems. Lett. Appl. Microbiol. 2020, 71, 251–258. [Google Scholar] [CrossRef]
- Argudo, A.; Zhou, L.; Rousseau, D. Sugar-sugar interactions in oil suspensions containing surfactants and effects on macroscopic phenomena. Food Res. Int. 2022, 157, 111217. [Google Scholar] [CrossRef]
- Dhasaiyan, P.; Le Griel, P.; Roelants, S.; Redant, E.; van Bogaert, I.N.; Prevost, S.; Prasad, B.L.; Baccile, N. Micelles versus Ribbons: How congeners drive the self-assembly of acidic sophorolipid biosurfactants. ChemPhysChem 2017, 18, 643–652. [Google Scholar] [CrossRef]
- Baccile, N.; Seyrig, C.; Poirier, A.; Alonso-de Castro, S.; Roelants, S.; Abel, S. Self-assembly, interfacial properties, interactions with macromolecules and molecular modelling and simulation of microbial bio-based amphiphiles (biosurfactants). A tutorial review. Green Chem. 2021, 23, 3842–3944. [Google Scholar] [CrossRef]
- Ortiz, A.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, Á.; Aranda, F.J. Effects of dirhamnolipid on the structural properties of phosphatidylcholine membranes. Int. J. Pharm. 2006, 325, 99–107. [Google Scholar] [CrossRef]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N.; Karpenko, E.; Shulga, A. Rhamnolipid—Biosurfactant Permeabilizing Effects on Gram-Positive and Gram-Negative Bacterial Strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef]

| pH | % Sucrose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 5% | 10% | 25% | 50% | ||||||
| MIC * | MBC ** | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 5.0 | 19.5 | 78.1 | 19.5 | 78.1 | 19.5 | 78.1 | 19.5 | 312.5 | 19.5 | 625.0 |
| 6.0 | 39.0 | >2500 | 39.0 | >2500 | 39.0 | >2500 | 39.0 | 1250.0 | 39.0 | 625.0 |
| 7.0 | 625.0 | >2500 | 312.5 | >2500 | 156.2 | >2500 | 156.2 | >2500 | 39.0 | 625.0 |
| 8.0 | >2500 | >2500 | >2500 | >2500 | >2500 | >2500 | >2500 | >2500 | 78.1 | 1250.0 |
| pH | Treatment | MBIC * (mg/L) | MBEC ** (mg/L) |
|---|---|---|---|
| 5.0 | RL | 19.5 | 78.1 |
| RL + 50% sucrose | 19.5 | 19.5 | |
| 7.0 | RL | >2500 | >2500 |
| RL + 50% sucrose | 39.0 | 156.2 |
| pH | % Sucrose | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 25 | 50 | |
| 5.0 | 19.6 | 12.4 | 24.8 | 39.7 | 25.6 |
| 6.0 | 93.2 | 39.7 | 41.2 | 82.9 | 112.6 |
| 7.0 | 94.2 | 41.9 | 96.6 | 87.7 | 117.1 |
| 8.0 | 121.4 | 119.7 | 117.3 | 94.5 | 119.1 |
| % Sucrose | pH | |||
|---|---|---|---|---|
| 5.0 | 6.0 | 7.0 | 8.0 | |
| 0 | 0.421 ± 0.01 | 0.316 ± 0.01 | 0.428 ± 0.05 | 0.427 ± 0.01 |
| 50 | 0.533 ± 0.02 | 0.604 ± 0.01 | 0.573 ± 0.03 | 0.991 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passos, T.F.; Nitschke, M. The pH and Sucrose Influence Rhamnolipid Action Toward Planktonic and Biofilms of Listeria monocytogenes. Microorganisms 2024, 12, 2078. https://doi.org/10.3390/microorganisms12102078
Passos TF, Nitschke M. The pH and Sucrose Influence Rhamnolipid Action Toward Planktonic and Biofilms of Listeria monocytogenes. Microorganisms. 2024; 12(10):2078. https://doi.org/10.3390/microorganisms12102078
Chicago/Turabian StylePassos, Tathiane Ferroni, and Marcia Nitschke. 2024. "The pH and Sucrose Influence Rhamnolipid Action Toward Planktonic and Biofilms of Listeria monocytogenes" Microorganisms 12, no. 10: 2078. https://doi.org/10.3390/microorganisms12102078
APA StylePassos, T. F., & Nitschke, M. (2024). The pH and Sucrose Influence Rhamnolipid Action Toward Planktonic and Biofilms of Listeria monocytogenes. Microorganisms, 12(10), 2078. https://doi.org/10.3390/microorganisms12102078







