Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals
Abstract
1. Introduction
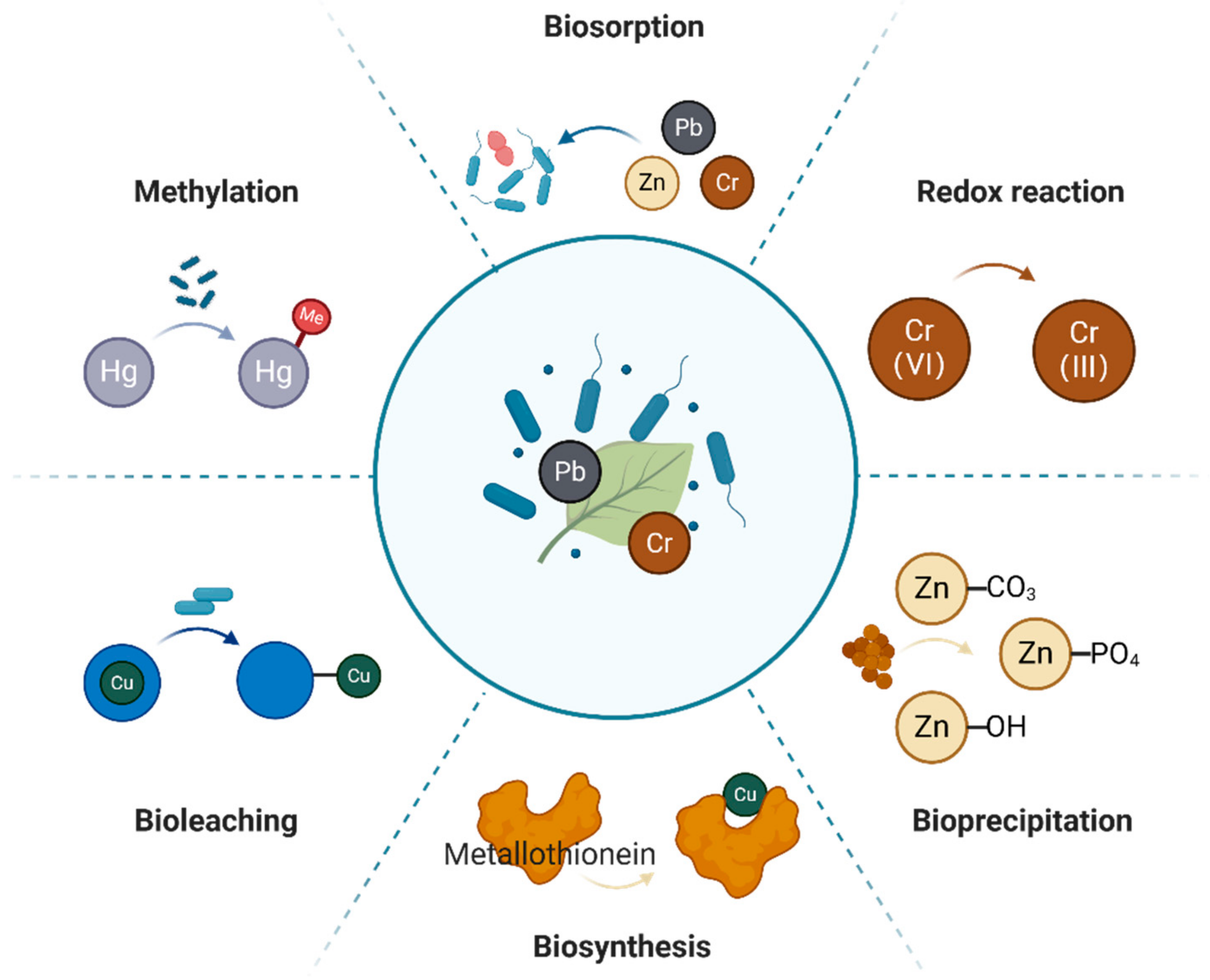
2. Mechanism of the Resistance of Endophytic Bacteria to Heavy Metals
- (1)
- Biosorption. Biosorption is a process in which the functional groups on the cell wall of endophytes bind metal ions to their cell surface, and the cell surface accumulates heavy metals through adsorption, thereby reducing the toxicity of heavy metals to plants. This method can rapidly transfer heavy metal ions into the cells to limit their movement, thereby reducing their toxicity [18]. The bacterial cell wall usually has a negative charge, which enables it to easily react with metals in the outer membrane of the cell, and it is highly effective at forming complexes with dissolved heavy metal ions [19,20]. Raheem et al. [21] found Bacillus amyloliquefaciens RWL-1 could adsorb copper (Cu) ions on RWL-1 cell surfaces, which enhanced the seedling biomass of rice (Oryza sativa) and increased its levels of antioxidants.
- (2)
- Methylation. Microbial methylation in vivo can effectively reduce the toxic effect of heavy metal ions on organisms. This is particularly true for the methylation of lead (Pb) and mercury (Hg) ions. When the methyl group has a stronger affinity with the divalent mercury ion, it forms methylmercury (MeHg), which weakens the toxicity of Hg. Sulfate-reducing bacteria can catalyze the methylation of Hg. Graham et al. [22] found that some species of Desulfovibrio could produce MeHg, and they identified four new Hg methylators (Desulfovibrio aespoeensis, D. alkalitolerans, D. psychrotolerans, and D. sulfodismutans). Smith et al. [23] showed that D. desulfuricans ND132 could methylate Hg, and Cys73 and Cys93 are the essential amino acids for methylation.
- (3)
- Redox strategies. Heavy metals with different valences, such as arsenic (As), chromium (Cr), and cobalt (Co), are usually found in different valence states in the natural environment. Endophytic bacteria can change the valence of heavy metals through intracellular redox reactions, thereby reducing their toxicity [24]. Xu et al. [25] found that As(III) was oxidized to As(V) after it became attached to the endophytic bacterium W1-2B, which significantly enhanced the strain’s resistance to As(V) and reduced its toxicity.
- (4)
- Bioleaching. Bioleaching is a technology that utilizes microorganisms to recover metals from minerals through the oxidation and dissolution of metal ions [26]. Zhu et al. [27] used a method that combined autotrophic and heterotrophic bacteria to leach the heavy metals zinc (Zn), manganese (Mn), Cu, and cadmium (Cd) from sludge. The auto- and heterotrophic bacteria effectively improved the efficiency of bioleaching, and the main fractions of the heavy metals (Zn, Mn, Cu, and Cd) in the sediments were Fe-Mn oxide, an organic-associated form, and a residual form after bioleaching. The biotoxicity of the sediments decreased greatly.
- (5)
- Bioprecipitation. Plant-growth-promoting rhizosphere bacteria (PGPR) secrete extracellular polymers and other compounds, which directly chelate heavy metals to precipitate or complex extracellular molecules to reduce the toxicity of heavy metals [28].
- (6)
- Biosynthesis. Endophytic bacteria can convert heavy metals into less toxic forms by synthesizing proteins, such as metallothioneins (MTs), glutathione (GSH), and plant lectins (PCs), that are stable to heat and bind to heavy metal ions [29].
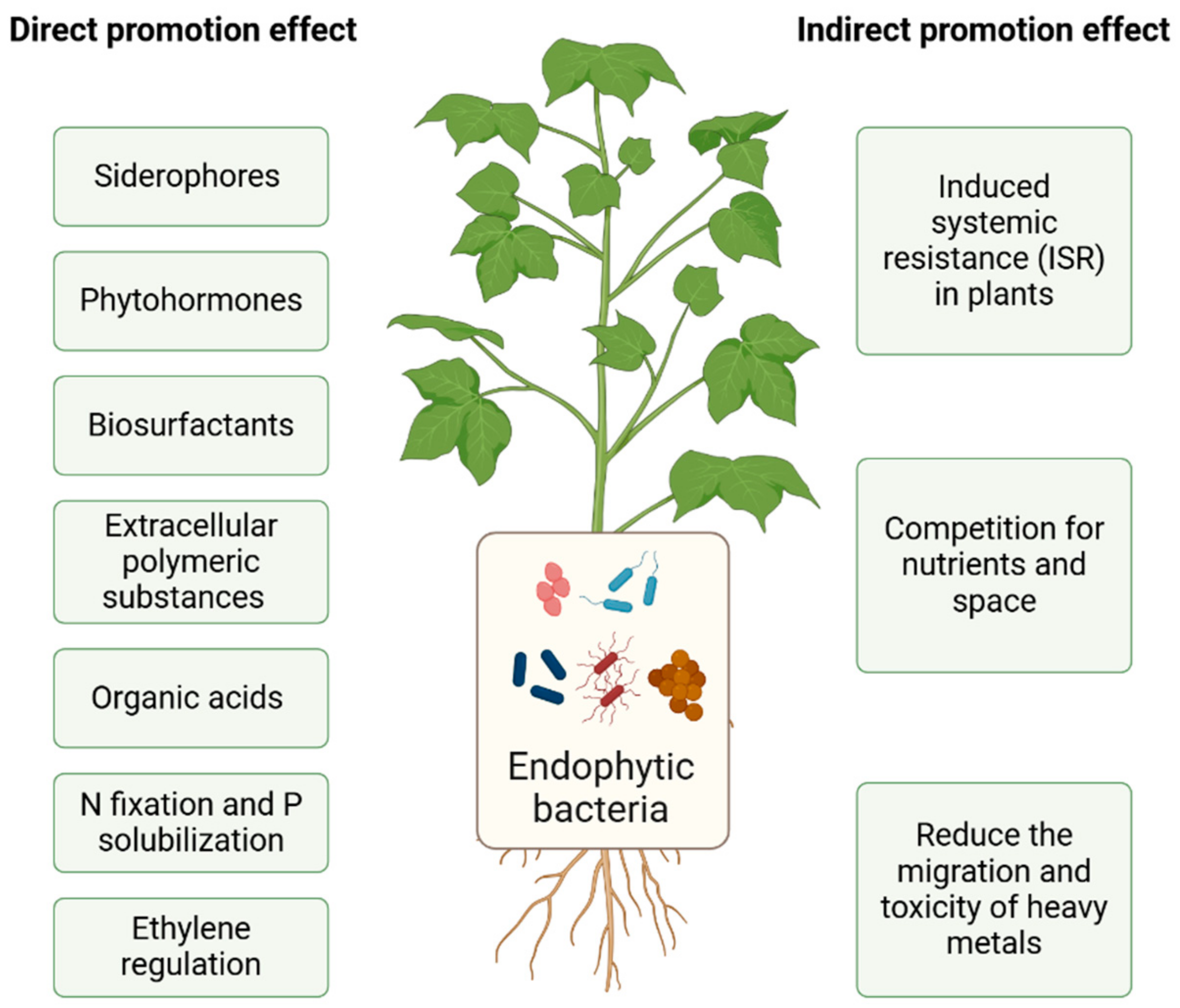
3. Mechanisms Used by Endophytic Bacteria to Enhance the Phytoremediation of Heavy Metals
3.1. Direct Promotion Effect
- (1)
- Secretion of siderophores. Iron in the soil primarily exists in the form of highly insoluble trivalent iron (Fe3+) oxides, hydroxides, phosphates, and carbonates, which generally cannot meet the needs of soil microorganisms to reproduce and plants to grow and develop [32]. Siderophores are a class of small molecular compounds that are produced by microorganisms inside the cell and secreted to its exterior. Rhizosphere bacteria produce siderophores in the absence of iron ions in the plant rhizosphere. Siderophores can form complexes with heavy metals, and this process plays an important role in the phytoremediation of heavy metals. Some endophytic bacteria, such as Pseudomonas, Serratia, and Streptomyces, can secrete siderophores. Chen et al. [33] isolated the endophytic bacteria Serratia nematodiphila LRE07, Enterobacter sp. LRE04, Enterobacter aerogenes LRE17, and Acinetobacter sp. LSE06 from Solanum nigrum. These endophytes can all secrete siderophores, promote plant growth, and improve the ability of plants to remediate Cd pollution in the soil.
- (2)
- N fixation and P solubilization. Nitrogen is an essential nutrient for plant growth. Endophytic azotobacter can directly supply fixed N to plants for absorption and assimilation and promote the growth of their roots [34]. Phosphorus is another essential element that is required for life. It is primarily found in the form of insoluble P in the soil, which is difficult for plants to absorb and utilize. Some endophytic bacteria, such as Micrococcus luteus and Enterobacter cloacae, are very effective at solubilizing it, which enables the plants to take up this form of P [12].
- (3)
- Secretion of organic acids. Organic acids are acidic organic compounds, which can activate heavy metal ions, change the form of heavy metals, and enhance mineral nutrients. Bacteria in the rhizosphere secrete these compounds to increase the uptake of plant nutrients by inducing root exudates [35].
- (4)
- Secretion of biosurfactants. Biosurfactants are biodegradable low-molecular-weight organic compounds secreted by microorganisms. They include glycolipids, neutral lipid derivatives, and lipoproteins. When the concentration of surfactant exceeds the critical micelle concentration, micelles form in the solution. When heavy metals bind to these micelles, they are transferred to the liquid phase of the soil, which increases their solubility and bioavailability in the soil, thereby favoring phytostabilization [36]. Pseudomonas aeruginosa, Burkholderia sp., Paenibacillus sp., and Citrobacter freundii can secrete biosurfactants. They contain a variety of groups with heavy metal complexation and coordination abilities (such as sulfhydryl, carboxyl, hydroxyl, amino, and phosphate groups and bicarbonates), among others, which can be combined with heavy metal ions to form soluble metal–organic complexes; these complexes are more easily absorbed by plants [37,38].
- (5)
- Secretion of EPSs. EPSs are a type of complex polymer that attaches to the surface of bacteria or surrounding microbes. The EPS complexes bind heavy metal ions to form a complex, which reduces the migration of heavy metal ions and therefore reduces their toxicity to plants. Schue et al. [39] showed that Rhizobium alamii, an exopolysaccharide-producing bacterium, formed a biofilm on plant roots, which protected the cells of sunflower (Helianthus annuus) by preventing Cd from entering them.
- (6)
- Secretion of phytohormones. Phytohormones are compounds that promote the growth, division, and differentiation of plant tissues. Based on their source, these hormones can be divided into endogenous hormones secreted by plants in vitro and exogenous hormones, which primarily include indole-3-acetic acid (IAA), cytokinin (CTK), and gibberellin (GA), among others [40]. Chen et al. [41] inoculated Lolium perenne under Cu stress with Rhizobium sp. W33, and this strain increased the nutrient and biomass of L. perenne and promoted its growth. He et al. [42] found that the endophytic bacteria AR1, AY1, and BG4 isolated from S. nigrum can all produce IAA. Moreover, the inoculation of these strains onto rape (Brassica napus) contaminated with Cd could promote its growth.
- (7)
- Regulation of ethylene. Some endophytes have ACC deaminase activity, and the precursor ACC of ethylene biosynthesis will be broken down into α-butanoic acid and ammonia molecules, thereby reducing the level of ethylene in plants [43]. Plants can use ammonia molecules as a source of nitrogen, which relieves the pressure caused by adverse conditions of plant growth and development and increases the plant’s absorption of heavy metal ions. The gene acdS, which encodes 1-aminocyclopropane-1-carboxylate or ACC-deaminase, is associated with an increase in plant biomass and stress tolerance. The expression of acdS decreases the levels of ethylene induced by stress, and the enzyme is abundant in rhizosphere colonizers. Paraburkholderia dioscoreae Msb3 that produces ACC-deaminase colonized the leaf sphere of tomato and promoted their growth compared with the controls [44]. Souza et al. [45] found that Paenibacillus sp. FeS53 possessed ACC-deaminase activity and promoted the dry shoot biomass of rice and its uptake of nutrients in the presence of excess Fe.
3.2. Indirect Promotion Effect
- (1)
- Induced systemic resistance (ISR) in plants. Under heavy metal stress, plants will accumulate a large amount of reactive oxygen species (ROS), and the cells will release excessive numbers of free radicals of oxygen, which will destroy the integrity of cell membranes. Endophytic bacteria can induce, activate, and enhance the activities of plant antioxidant enzymes [17], such as peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and glutathione peroxidase (GPX) and increase the content of proline, which increases the resistance of plants to heavy metals [32]. The ethylene response factor (ERF) protein plays an important role in the responses to plant stress and developmental regulation. It was reported that JERF3 activated the expression of oxidative stress response genes in transgenic tobacco and enhanced its tolerance to adverse environments [47].
- (2)
- Competition for nutrients and space. The plant endophytes stimulate the plants to secrete specific enzymes, such as hydrolases, along with plant hormones and antibiotics, which regulate the plant rhizosphere microbial community structure, reduce the absorption of heavy metals by plants, and increase plant biomass. The change in the morphology of plant roots caused by endophytes can indirectly promote the growth of plants in environments that are highly polluted with heavy metals [48].
- (3)
- Change the valence state of heavy metals to reduce their ability to migrate and their toxicity. Endophytic bacteria can change the valence state of heavy metals through their mechanisms that limit the metabolism of heavy metals. Insoluble heavy metal ions are transformed into easily soluble ones, which can change them from more toxic to less toxic ionic forms [49]. Endophytic bacteria can bind heavy metal ions through adsorption, exchange, and chelation, which can effectively weaken the migration of heavy metal ions and thus reduce their toxicity to plants [17]. Serratia marcescens BacI56 and Pseudomonas sp. BacI38 increased the volatilization of Hg in plants by 47.16% and 62.42%, respectively [50].
4. Approaches of the Phytoremediation of Heavy Metals Involving Endophytic Bacteria
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Bai, Z.X.; Bo, W.H.W.; Lin, J.; Yang, J.J.; Chen, T. Analysis and evaluation of heavy metal pollution in farmland soil in China: A metal analysis. Environ. Sci. 2024, 45, 2913–2925. [Google Scholar]
- Saiful, I.M.; Ram, P.; Mohammad, A.H.; Fazlul, H.M.; Shahin, H.M.; Nazirul, I.S.M. Assessment of heavy metals in foods around the industrial areas: Health hazard inference in bangladesh. Geocarto Int. 2020, 35, 280–289. [Google Scholar]
- Peng, J.Y.; Zhang, S.; Han, Y.Y.; Bate, B.; Ke, H.; Chen, Y.M. Soil heavy metal pollution of industrial legacies in China and health risk assessment. Sci. Total Environ. 2022, 816, 151632. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.H.; Ali, M.U.; Xiao, P.W.; Zhao, P.; Wang, H.Y.; Bibi, S. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 2024, 274, 116189. [Google Scholar] [CrossRef]
- Nie, J.; Wang, M.Q.; Han, J.L.; Li, J.S. Synergistic remediation strategies for soil contaminated with compound heavy metals and organic pollutants. J. Environ. Chem. Eng. 2024, 12, 113145. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna.; Lee, I.J.; Kim, K.M. Metal resistant endophytic bacteria reduce cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef]
- Singh, S.B.; Twinkle, C.; Singh, M.B.; Dharmender, K.; Ravinder, K.; Kumar, S.P.; Singh, D.J. Microbe-plant interactions targeting metal stress: New dimensions for bioremediation applications. Xenobiotics 2023, 13, 252–269. [Google Scholar]
- He, C.; Wang, W.Q.; Hou, J.T. Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development. BMC Plant Biol. 2020, 20, 325. [Google Scholar] [CrossRef]
- Yung, L.; Blaudez, D.; Maurice, N.; Barre, A.A.; Sirguey, C. Dark septate endophytes isolated from non-hyperaccumulator plants can increase phytoextraction of Cd and Zn by the hyperaccumulator Noccaea caerulescens. Environ. Sci. Pollut. Res. 2021, 28, 16544–16557. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.F.; Huang, L.K.; Xu, S.A.; Fu, Y.Y.; Hou, D.D.; Feng, Y.; Yang, X. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol. Environ. Saf. 2022, 237, 113541. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838 Pt 4, 156435. [Google Scholar] [CrossRef] [PubMed]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Almokadem, A.Z. Alleviation of cadmium and nickel toxicity and phyto-stimulation of tomato plant l. by endophytic Micrococcus luteus and Enterobacter cloacae. Plants 2022, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Liu, X.Y.; Zhao, X.D.; Ma, H.Z.; Liang, H.C. Research progress on community composition and function of plant endophytic bacteria. Life Sci. 2023, 35, 132–139. [Google Scholar]
- Chi, Y.W.; Ma, X.Z.; Zhang, X.; Wang, R.Y.; Zhang, D.W.; Chu, S.H.; Zhao, T.; Zhou, P.; Zhang, D. Plant growth promoting endophyte modulates soil ecological characteristics during the enhancement process of cadmium phytoremediation. J. Environ. Manag. 2024, 369, 122206. [Google Scholar] [CrossRef]
- Chodak, M.; Gołębiewski, M.; Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Shi, X.J.; Chen, X.P.; Li, Z.L. Mechanism of action and application of plant growth promoting bacteria in heavy metal bioremediation. Environ. Sci. 2022, 43, 4911–4922. [Google Scholar]
- Li, J.K.; Yu, H.; Zeng, W.M.; Wu, X.L.; Yu, R.L. Research progress of rhizosphere growth promoting bacteria to strengthen phytoremediation of heavy metal contaminated soil. Life Sci. 2017, 29, 434–442. [Google Scholar]
- Kimiko, T.; Yuichiro, K.; Yumiko, S.; Shintani, S.K.; Masashi, Y.; Kuramitsu, H.K.; Ishihara, K.K. Characterization of a potential ABC-type bacteriocin exporter protein from Treponema denticola. BMC Oral Health 2016, 17, 18. [Google Scholar]
- Chen, M.; Zhao, Y.H. Experimental study on adsorption of heavy metal ions by microorganisms. J. South. Inst. Metall. 2001, 22, 168–173. [Google Scholar]
- Wang, F.; Cao, F.; Zhu, C.B.; Chu, F.H.; Li, F.S.; Feng, F. Research progress of bioadsorbents in the treatment of wastewater containing heavy metal ions. New Chem. Mater. 2024, 52, 43–46. [Google Scholar]
- Raheem, S.; Saqib, B.; Muhammad, I.; Latif, K.A.; Ahmed, A.A.; Abdullah, A.H.; Hanan, H.; Suriya, R.; Jung, L. Amelioration of heavy metal stress by endophytic Bacillus amyloliquefaciens RWL-1 in rice by regulating metabolic changes: Potential for bacterial bioremediation. Biochem. J. 2019, 476, 3385–3400. [Google Scholar]
- Graham, A.M.; Bullock, A.L.; Maizel, A.C.; Elias, D.A.; Gilmour, C.C. Detailed assessment of the kinetics of Hg-cell association, Hg methylation, and methylmercury degradation in several Desulfovibrio species. Appl. Environ. Microbiol. 2012, 78, 7337–7346. [Google Scholar] [CrossRef]
- Smith, S.D.; Bridou, R.; Johs, A.; Parks, J.M.; Elias, D.A.; Hurt, R.A.; Brown, S.D.; Podar, M.; Wall, J.D. Site-directed mutagenesis of HgcA and HgcB reveals amino acid residues important for mercury methylation. Appl. Environ. Microbiol. 2015, 81, 3205–3217. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Xu, J.Y.; Han, Y.H.; Chen, Y.S.; Zhu, L.J.; Ma, L.Q. Arsenic transformation and plant growth promotion characteristics of As-resistant endophytic bacteria from As-hyperaccumulator Pteris vittate. Chemosphere 2016, 144, 1233–1240. [Google Scholar] [CrossRef]
- Diao, M.X.; Taran, E.; Mahler, S.; Nguyen, A.V. A concise review of nanoscopic aspects of bioleaching bacteria-mineral interactions. Adv. Colloid Interface Sci. 2014, 212, 45–63. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhang, J.X.; Li, Q.; Han, T.; Hu, Y.H. Bioleaching of heavy metals from contaminated alkaline sediment by auto- and heterotrophic bacteria in stirred tank reactor. Trans. Nonferrous Met. Soc. China 2014, 24, 2969–2975. [Google Scholar] [CrossRef]
- Li, Y.S.; Feng, C.L.; Wu, X.F.; Shi, F. Research progress of microbial functions in phytoremediation of heavy metal contaminated soils. Acta Ecol. Sin. 2015, 35, 6881–6890. [Google Scholar]
- Glick, R.B. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Y.M.; Teng, Y.; Li, X.H. Research progress on phytoremediation of heavy metal contaminated soil enhanced by endophytic bacteria. Acta Ecol. Sin. 2013, 50, 195–202. [Google Scholar]
- Zhang, W.C.; Li, J.; Wang, Z.Y.; Yang, H.; Liu, Q.H.; Li, Y.; Yan, W.H. Endophytes-plant joint repair contaminated soil is reviewed. J. Agric. Resour. Environ. 2021, 38, 355–364. [Google Scholar]
- Hardoim, R.P.; Overbeek, V.S.L.; Elsas, V.D.J. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, S.L.; Xiao, X.; Guo, H.J.; Chen, J.L.; Wan, Y.; Li, B.; Xu, T.Y.; Xi, Q.; Rao, C.; et al. Application of plant growth-promoting endophytes isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl. Soil Ecol. 2010, 46, 383–389. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Su, Z.H.; Isawa, T.; Mitssui, H.; Minamisawa, K. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [CrossRef]
- Knecht, K.; Nowack, B.; Schroth, M.H.; Suter, M.J.F.; Schulin, R. Investigation of small-scale processes in the rhizosphere of Lupinus albus using micro push-pull tests. Plant Soil 2014, 378, 309–324. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Freitas, H.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef]
- Schue, M.; Fekete, A.; Ortet, P.; Brutesco, C.; Heulin, T.; Kopplin, P.S.; Achouak, W.; Santaella, C. Modulation of metabolism and switching to biofilm prevail over exopolysaccharide production in the response of Rhizobium alamii to cadmium. PLoS ONE 2018, 6, e26771. [Google Scholar] [CrossRef]
- Kadhimi, A.A.; Alhasnawi, A.N.; Mohamad, A.; Wan, M.; Binti, R. Tissue culture and some of the factors affecting them and the micropropagation of strawberry. Life Sci. J. 2014, 11, 484–493. [Google Scholar]
- Chen, S.T.; He, L.Y.; Li, Y.; Yue, T.T.; Sheng, X.F.; Huang, Z. Effects of Rhizobium sp. W33 on copper absorption and rhizosphere secretions in different plants. J. Environ. Sci. 2014, 34, 2077–2084. [Google Scholar]
- He, L.Y.; Li, Y.; Liu, T.; He, W.T.; Wang, Y.J.; Shen, X.M.; Wang, M.C.; Yu, Q.; Sheng, F.X. Screening and biological characteristics of cadmium-resistant bacteria in rhizosphere and endophyte of Solanum. J. Ecol. Rural Environ. 2011, 27, 83–88. [Google Scholar]
- Yu, Y.W.; Huang, R.F. Ethylene and plant stress resistance. China Agric. Sci. Technol. Rev. 2013, 15, 70–75. [Google Scholar]
- Herpell, J.B.; Alickovic, A.; Diallo, B.; Florian, S.; Wolfram, W. Phyllosphere symbiont promotes plant growth through ACC deaminase production. ISME J. 2023, 17, 1267–1277. [Google Scholar] [CrossRef]
- de Souza, R.; Meyer, J.; Schoenfeld, R.; da Costa, P.B.; Passaglia, M.P.L. Characterization of plant growth-promoting bacteria associated with rice cropped in iron-stressed soils. Ann. Microbiol. 2015, 65, 951–964. [Google Scholar] [CrossRef]
- Govarthanan, M.; Lee, K.J.; Cho, M.; Kim, J.S.; Kannan, S.K.; Oh, B.T. Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere 2013, 90, 2267–2272. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and barley dehydrins under cold, drought, and salinity–what can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef]
- Solís-Domínguez, A.F.; Valentín-Vargas, A.; Chorover, J.; Maier, R.M. Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci. Total Environ. 2010, 409, 1009–1016. [Google Scholar] [CrossRef]
- King, W.S.; Teck, S.L.C.; Jiwan, M.; Aziz, F.A.; Kundat, F.R.; Ahmed, O.H.; Ab Majid, N.M.; Edward, E.J.; King, W.S.; Leong, S.; et al. Antagonistic activities of endophytic bacteria against Fusarium wilt of black pepper (Piper nigrum). Int. J. Agric. Biol. 2012, 18, 36–38. [Google Scholar]
- Zhang, M.M.; Cao, L.D.; Wang, R.Q.; Shun, R.L. Research progress on the mechanism of plant endophytic bacteria in repairing soil contaminated by heavy metals. Bull. Biotechnol. 2018, 34, 42–49. [Google Scholar]
- Jesús, R.; Alejandro, H.; Adelina, G.R.; Lizzeta, A.J.; Elena, S.R.; Candy, C.; Eunice, R.J.; Stephanie, R.; Ramiro, P.J.; Roberto, M.J.; et al. Characterization of endophytic bacteria isolated from Typha latifolia and their effect in plants exposed to either Pb or Cd. Plants 2023, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lin, H.; Dong, Y.B.; Li, B.; Wang, L. Identification and characterization of plant growth-promoting endophyte RE02 from Trifolium repens L. in mining smelter. Environ. Sci. Pollut. Res. 2019, 26, 17236–17247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Chen, W.; Ruan, Y.T.; Zhang, S.Y.; Sun, H.T.; Ma, P.H.; Wei, Z.X.; Li, Y.L. Isolation and identification of Cupriavidus sp. HXC.8 and its effects on growth of Mimosa pudica contaminated with copper. J. Northwest A F Univ. (Nat. Sci. Ed.) 2023, 51, 131–140. [Google Scholar]
- Ihsan, U.; Aisha, M.; Afaq, M.A.; Aqib, I.; Alghamdi, K.M.S.; Alsolami, H.M.; Siddiqui, M.F. Heavy metal ATPase genes (HMAs) expression induced by endophytic bacteria, “AI001, and AI002” mediate cadmium translocation and phytoremediation. Environ. Pollut. 2021, 293, 118508. [Google Scholar]
- Fan, M.; Liu, Z.S.; Nan, L.J.; Wang, E.T.; Chem, W.M.; Lin, Y.B.; Wei, G.H. Isolation, characterization, and selection of heavy metal-resistant and plant growth-promoting endophytic bacteria from root nodules of Robinia pseudoacacia in a Pb/Zn mining area. Microbiol. Res. 2018, 217, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Zhou, Q.; Gao, C.; Sheng, Y.; Xiao, M.; Yun, Y.L.; Selvaraj, J.N.; Zhang, X.H.; Li, Y.D.; Yu, X.J. Endophytic bacteria for Cd remediation in rice: Unraveling the Cd tolerance mechanisms of Cupriavidus metallidurans CML2. J. Hazard. Mater. 2024, 469, 133846. [Google Scholar] [CrossRef]
- Kumar, A.; Voropaeva, O.; Maleva, M.; Panikovskaya, K.; Borisova, G.; Rajkumar, M.; Benedict, B.L. Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosphere 2021, 266, 128983. [Google Scholar] [CrossRef]
- Wu, K.R.; Luo, J.P.; Li, J.X.; An, J.X.; Yang, X.; Liang, Y.C.; Li, T.Q. Endophytic bacterium Buttiauxella sp. SasR13 improves plant growth and cadmium accumulation of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2018, 25, 21844–21854. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala, K.S.; Rajasekar, A.; Chang, Y.C. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 2016, 6, 242. [Google Scholar]
- Mello, I.S.; Targanski, S.; Pietro-Souza, W.; Fernando, F.F.S.; Ailton, J.T.; Marcos, A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020, 202, 110818. [Google Scholar] [CrossRef]
- Nguyen, T.B.K.; Phan, T.H.T.; Le, T.T.; Dang, N.T.; Nguyen, V.T.; Nguyen, L.H.T.; Nguyen, P.M. Arsenic (As)-resistant endophytic bacteria isolated from ferns growing in As-contaminated areas. Microbiology 2023, 92, 892–906. [Google Scholar] [CrossRef]
- Babu, G.A.; Kim, J.; Oh, B.T. Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J. Hazard. Mater. 2013, 250–251, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Siddique, A.; Ferdous, S.; Amin, M.A.; Qin, M.Z.; Aslam, U.; Naeem, M.; Bashir, T.; Shakoor, A. Heavy metals mitigation and growth promoting effect of endophytic Agrococcus terreus (MW 979614) in maize plants under zinc and nickel contaminated soil. Front. Microbiol. 2023, 14, 1255921. [Google Scholar] [CrossRef]
- Li, Q.Q.; Yao, S.Y.; Wen, H.; Li, W.; Jin, L.; Huang, X.X. Improving lead phytoremediation using endophytic bacteria isolated from the pioneer plant Ageratina adenophora (spreng.) from a mining area. Toxics 2024, 12, 291. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.Y.; Wu, Y.J.; Luo, S.; Chen, B.; Ma, L.Y.; Pan, F.X.; Feng, Y.; Yang, X. Promotion of the root development and Zn uptake of Sedum alfredii was achieved by an endophytic bacterium Sasm05. Ecotoxicol. Environ. Saf. 2019, 172, 97–104. [Google Scholar] [CrossRef]
- Wang, X.T.; Luo, S.; Chen, Y.H.; Zhang, R.F.; Lei, Y.; Lin, K.K.; Qiu, S.J.; Xu, H. Potential of Miscanthus floridulus associated with endophytic bacterium Bacillus cereus BL4 to remediate cadmium contaminated soil. Sci. Total Environ. 2022, 857, 159384. [Google Scholar] [CrossRef]
- Feng, W.R.; Xiao, X.; Li, J.J.; Xiao, Q.C.; Ma, L.; Gao, Q.F.; Wan, Y.K.; Huang, Y.T.; Liu, T.; Luo, X.B.; et al. Bioleaching and immobilizing of copper and zinc using endophytes coupled with biochar-hydroxyapatite: Bipolar remediation for heavy metals contaminated mining soils. Chemosphere 2023, 315, 137730. [Google Scholar] [CrossRef]
- Bacon, W.C.; White, F.J. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 2016, 68, 87–98. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Khan, L.A.; Ahmed, A.H.; Chang, K.K.; Lee, I.J. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J. Hazard. Mater. 2019, 379, 120824. [Google Scholar] [CrossRef]
- Yue, Z.H.; Chen, Y.J.; Chen, C.; Ma, K.S.; Tian, E.; Wang, Y.; Liu, H.Z.; Sun, Z.K. Endophytic Bacillus altitudinis WR10 alleviates Cu toxicity in wheat by augmenting reactive oxygen species scavenging and phenylpropanoid biosynthesis. J. Hazard. Mater. 2021, 405, 124272. [Google Scholar] [CrossRef]
- Gu, Y.F.; Wang, Y.Y.; Sun, Y.H.; Zhao, K.; Xiang, Q.J.; Yu, X.M.; Zhang, X.P.; Chen, Q. Genetic diversity and characterization of arsenic-resistant endophytic bacteria isolated from Pteris vittata, an arsenic hyperaccumulator. BMC Microbiol. 2018, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Q.; Tan, H.M.; Zhou, S.N.; Cao, L.X. Enhanced phytoremediation of toxic metals by inoculating endophytic Enterobacter sp. CBSB1 expressing bifunctional glutathione synthase. J. Hazard. Mater. 2014, 267, 17–20. [Google Scholar] [CrossRef]
- Peng, O.Y.; Wang, Y.; Peng, X.Y.; Shi, X.J.; Li, Z.L.; Ma, Y. Harnessing plant-beneficial bacterial encapsulation: A sustainable strategy for facilitating cadmium bioaccumulation in Medicago sativa. J. Hazard. Mater. 2024, 476, 135232. [Google Scholar]
- Jian, L.R.; Bai, X.L.; Zhang, H.; Song, X.Y.; Li, Z.F. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. Peer J. 2019, 7, e6875. [Google Scholar] [CrossRef]
- Shilpee, D.; Madhusmita, P.; Kumar, R.S.; Santanu, M.; Chinmay, P. Alleviating Cr (VI) stress in horse gram (Macrotyloma uniflorum Var. Madhu) by native Cr-tolerant nodule endophytes isolated from contaminated site of Sukinda. Environ. Sci. Pollut. Res. 2021, 28, 31717–31730. [Google Scholar]
- Ashraf, S.; Afzal, M.; Naveed, M.; Shahid, M.; Ahmad, Z.Z. Endophytic bacteria enhance remediation of tannery effluent in constructed wetlands vegetated with Leptochloa fusca. Int. J. Phytoremediation 2018, 20, 121–128. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, A.M.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Avelino, N.D.; Saeed, O.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, O.; Khan, A.; Nawaz, A.; Baig, K.S.; Chen, J.T. Enhancing cadmium tolerance and pea plant health through Enterobacter sp. MN17 inoculation together with biochar and gravel sand. Plants 2020, 9, 530. [Google Scholar] [CrossRef]
- Nafees, M.; Ali, S.; Naveed, M.; Muhammad, R. Efficiency of biogas slurry and Burkholderia phytofirmans PsJN to improve growth, physiology, and antioxidant activity of Brassica napus L. in chromium-contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 6387–6397. [Google Scholar] [CrossRef]
- Shahzad, A.; Aslam, U.; Ferdous, S.; Qin, M.Z.; Siddique, A.; Billah, M.; Naeem, M.; Mahmood, Z.; Kayani, S. Combined effect of endophytic Bacillus mycoides and rock phosphate on the amelioration of heavy metal stress in wheat plants. BMC Plant Biol. 2024, 24, 125. [Google Scholar] [CrossRef]
| Endophytic Bacteria | Host Plants | Effect/Mechanism | Heavy Metal(s) | References |
|---|---|---|---|---|
| Phialophora mustea Pr27; Leptodontidium sp. Me07 | Noccaea caerulescens | Improvement in plant mineral nutrition; Uptake of Zn (30%) and Cd (90%). | Zn, Cd | [9] |
| Bacillus amyloliquefaciens RWL-1 | Rice | Enhancement in seedling biomass and antioxidant levels (POD, PPO, and GHS); Increase of seedling biomass. | Cu | [21] |
| FeS53 | Rice | Promotion of plant growth and nutrient absorption; Alleviation of the effect of Cd stress. | Fe | [45] |
| Pseudomonas putida RE02 | Trifolium repens | Promotion of IAA; P solubilization; Increase of plant biomass. | Cd | [52] |
| Cupriavidus sp. HXC-8 | Mimosa pudica | Improvement in plant length, biomass, chlorophyll contents, and GSH; Alleviation of the effect of Cd stress. | Cu | [53] |
| Serratia sp. AI001; Klebsiella sp. AI002 | Solanum nigrum | Improvement in the chlorophyll content, plant length, and biomass; Induction of Cd translocation. | Cd | [54] |
| Mesorhizobium loti HZ76 | Robinia pseudoacacia | N fixation and production of ACC-deaminase; Improvement in shoot biomass; Decrease in the concentrations of heavy metals (Zn, Pb, Cd) in the roots. | Zn, Pb, and Cd | [55] |
| Pseudomonas lurida EOO26 | Helianthus annuus | Increase in the length and dry weight of plant root and shoot. | Cu | [57] |
| Buttiauxella sp. SaSR13 | Sedum alfredii | Production of IAA and decrease in the concentrations of superoxide anion (O2−) in plants. | Cd | [58] |
| Paenibacillus sp. RM | Tridax procumbens | Production of secondary metabolites, IAA, siderophores, ACC-deaminase, and biosurfactants; Solubilization of phosphate; Resistance to Cu (750 mg/L), Zn (500 mg/L), Pb (450 mg/L), As (400 mg/L). | Cu, Zn, Pb, and As | [59] |
| 7 different endophytic bacteria | maize | Increase in total dry biomass of plants and Hg volatilization. | Hg | [60] |
| Priestia megaterium R2.5.2, Micrococcus luteus S3.4.1, P. megaterium R3.4.5, and P. megaterium L3.5.1) | Pteris vittata | Enhancement in biomass; As accumulation. | As | [61] |
| Bacillus thuringiensis GDB-1 | Alnus firma | Improvement in plant growth and ACC-deaminase activity; Production of IAA, siderophores, and P; Accumulation of heavy metals (including As, Cu, Pb, Ni, and Zn). | As, Cu, Pb, Ni, and Zn | [62] |
| Sphingomonas sp. ZYG-4 | Ageratina adenophora | Secretion of IAA; Solubilization of phosphate; Regulation of root ethylene levels; Increase in root biomass; Pb accumulation. | Pb | [64] |
| Pseudomonas fluorescens Sasm05 | Sedum alfredii | Increase in chlorophyll content, plant biomass, and the expression of Zn transporters SaIRT1 and SaNramp1 in the roots. | Zn | [65] |
| Bacillus cereus BL4 | Miscanthus floridulus | Increase in fresh weight, plant height, chlorophyll content, photosynthetic rate, and root activity; Decrease in MDA content; Increase in the bioavailability of Cd in the soil and the activity of soil enzymes (sucrase, urease, alkaline phosphatase, dehydrogenase, FDA hydrolase, and CAT); increase in the richness and diversity of soil bacteria. | Cd | [66] |
| Penicillium funiculosum LHL06 | Glycine max | Enhancement in the activities of antioxidant systems (GSH, CAT, POD, and SOD); Alleviation of oxidative stress. | Ni, Cu, Pb, Cr, and Al | [69] |
| Bacillus altitudinis WR10 | Triticum aestivum | Enhancement in the expression and activity of POD; Down-regulation of GST; Increase in GSH level. | Cu | [70] |
| Enterobacteriaceae genus CBSB1 | Brassica juncea | Increase in aboveground length, fresh weight, and dry weight; Extraction of Cd and Pb. | Cd, Pb | [72] |
| Klebsiella variicola Y38; Serratia surfactantfaciens Y15 | Medicago sativa | Enhancement in plant growth and antioxidant capacity; Alteration of Cd morphology in soil; Cd bioaccumulation. | Cd | [73] |
| Sinorhizobium meliloti CCNWSX0020; Agrobacterium tumefaciens CCNWGS0286 | Medicago lupulina | Increase in root length and dry weight; Cu uptake. | Cu | [74] |
| Bacillus aryabhattai AS03; Rhizobium pusense AS05 | Macrotyloma uniflorum | Increase in stem length, root length, nodule number, and leghemoglobin content; Decrease in the contents of ROS and activities of antioxidant enzymes. | Cr | [75] |
| Enterobacter HU38, Microbacterium arborescens HU33, and Pantoea stewartii AsI11 | Leptochloa fusca | Increase in root length, stem height, chlorophyll content, and total biomass. | Cr | [76] |
| Enterobacter sp. MN17 | Pisum sativum | Enhancement in plant health; Alleviation of the toxic effects of Cd on plants. | Cd | [78] |
| Burkholderia phytofirmans PsJN | Brassica napus | Promotion of plant growth; Reduction in the GSH-Px activity in soil; Stabilization of Cr in the soil. | Cr | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Quan, S.; Li, L.; Lei, G.; Li, S.; Gong, T.; Zhang, Z.; Hu, Y.; Yang, W. Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals. Microorganisms 2024, 12, 2137. https://doi.org/10.3390/microorganisms12112137
Liu L, Quan S, Li L, Lei G, Li S, Gong T, Zhang Z, Hu Y, Yang W. Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals. Microorganisms. 2024; 12(11):2137. https://doi.org/10.3390/microorganisms12112137
Chicago/Turabian StyleLiu, Ling, Shujing Quan, Liangliang Li, Gao Lei, Shanshan Li, Tao Gong, Zhilong Zhang, Yiliang Hu, and Wenling Yang. 2024. "Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals" Microorganisms 12, no. 11: 2137. https://doi.org/10.3390/microorganisms12112137
APA StyleLiu, L., Quan, S., Li, L., Lei, G., Li, S., Gong, T., Zhang, Z., Hu, Y., & Yang, W. (2024). Endophytic Bacteria Improve Bio- and Phytoremediation of Heavy Metals. Microorganisms, 12(11), 2137. https://doi.org/10.3390/microorganisms12112137







