Contribution of Other Respiratory Viruses During Influenza Epidemic Activity in Catalonia, Spain, 2008–2020
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethical Considerations
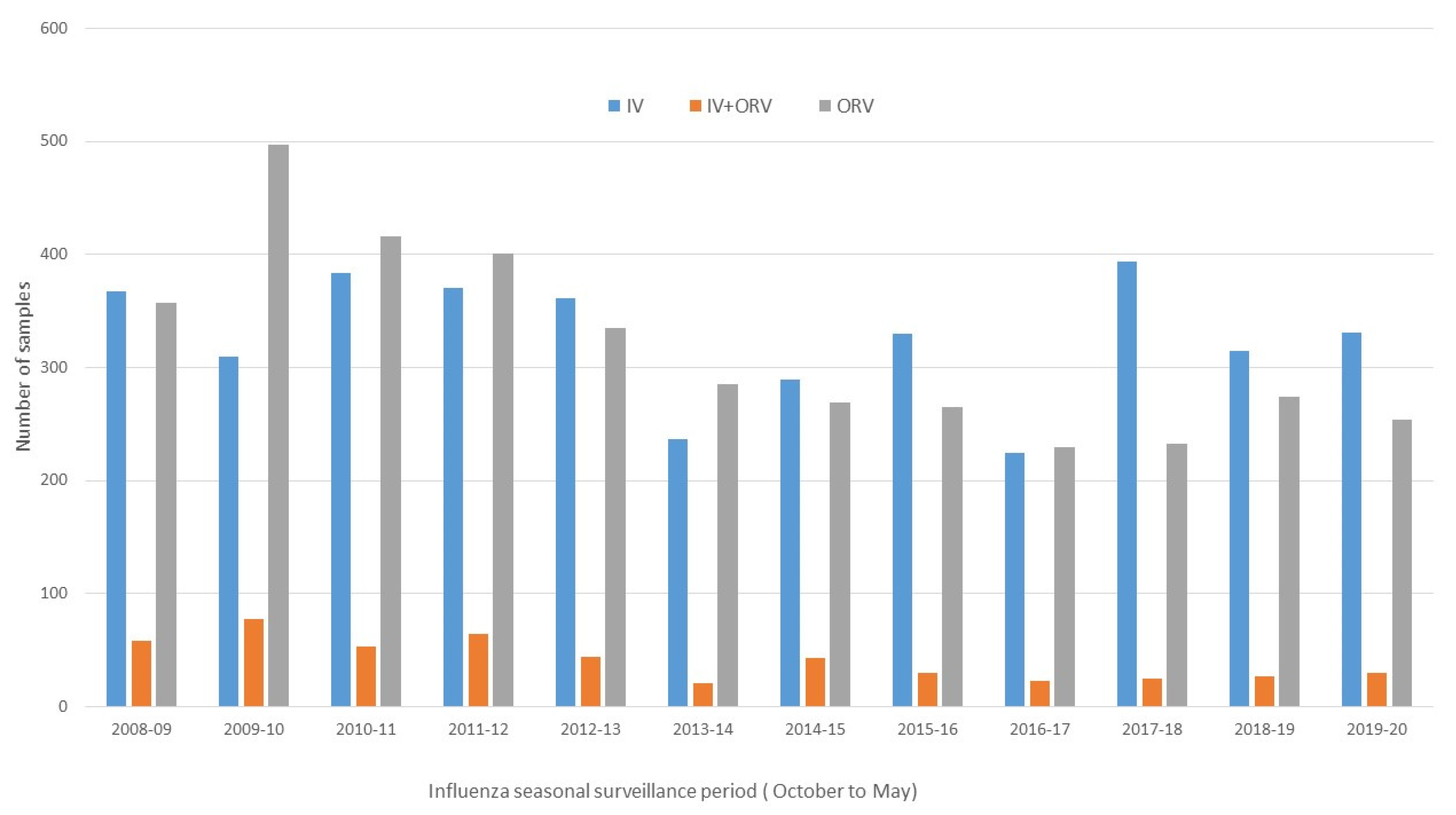
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Influenza (Seasonal), n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 28 December 2018).
- Capitani, E.; Montomoli, E.; Camarri, A.; Bova, G.; Capecchi, P.L.; Mercone, A.; Nante, N.; Manini, I. Epidemiological and virological surveillance of Severe Acute Respiratory Infections in the 2019/2020 season in Siena, Tuscany, Italy. J. Prev. Med. Hyg. 2021, 62, E782–E788. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Meijer, A.; Nunes, M.C.; Henaff, L.; Zounon, M.; Boudewijns, B.; Del Riccio, M.; Paget, J. Probable extinction of influenza B/Yamagata and its public health implications: A systematic literature review and assessment of global surveillance databases. Lancet Microbe 2024, 5, 100851. [Google Scholar] [CrossRef] [PubMed]
- Opatowski, L.; Baguelin, M.; Eggo, R.M. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: A key role for mathematical modelling. PLOS Pathog. 2018, 14, e1006770. [Google Scholar] [CrossRef]
- Bosch, A.A.T.M.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.M.; Bogaert, D. Viral and Bacterial Interactions in the Upper Respiratory Tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Cesario, T.C. Viruses Associated With Pneumonia in Adults. Clin. Infect. Dis. 2012, 55, 107–113. [Google Scholar] [CrossRef]
- Mina, M.J.; Klugman, K.P. The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir. Med. 2014, 2, 750–763. [Google Scholar] [CrossRef]
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141. [Google Scholar] [CrossRef]
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2365. [Google Scholar] [CrossRef]
- Vos, L.M.; Bruning, A.H.L.; Reitsma, J.B.; Schuurman, R.; Riezebos-Brilman, A.; Hoepelman, A.I.M.; Oosterheert, J.J. Rapid Molecular Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review of Diagnostic Accuracy and Clinical Impact Studies. Clin. Infect. Dis. 2019, 69, 1243–1253. [Google Scholar] [CrossRef]
- Avolio, M.; Venturini, S.; De Rosa, R.; Crapis, M.; Basaglia, G. Epidemiology of respiratory virus before and during COVID-19 pandemic. Infez. Med. 2022, 30, 104–108. [Google Scholar] [CrossRef]
- Ortiz-Hernández, A.A.; Nishimura, K.K.; Noyola, D.E.; Moreno-Espinosa, S.; Gamiño, A.; Galindo-Fraga, A.; Vázquez, R.V.; Aquino, M.M.; Ramirez-Venegas, A.; Salgado, R.V.; et al. Differential risk of hospitalization among single virus infections causing influenza-like illnesses. Influenza Other Respi. Viruses 2019, 13, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Schanzer, D.L.; Langley, J.M.; Tam, T.W. Role of influenza and other respiratory viruses in admissions of adults to Canadian hospitals. Influenza Other Respi. Viruses 2008, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Basile, L.; Jané, M. Pla D’informació de les Infeccions Respiratòries Agudes a Catalunya (PIDIRAC)—2019–2020; Sub-Direcció General de Vigilància i Resposta a Emergències de Salut Pública: Barcelona, Spain, 2019. [Google Scholar]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef]
- Decret 2013/2015, de 15 de setembre, pel qual es crea la Xarxa de Vigilància Epidemiològica i es regulen els sistemes de notificació de malalties de declaració obligatòria i els brots epidèmics. DOGC 2015, 6958, 1–19, ISSN 1988-298X.
- Instituto de Salud Carlos III. Informe anual SiVIRA de Vigilancia de gripe, COVID-19 y VRS. Temporada 2021-22. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Informes_Anuales_Vigilancia_IRAs.aspx (accessed on 15 November 2022).
- Weidmann, M.D.; Green, D.A.; Berry, G.J.; Wu, F. Assessing respiratory viral exclusion and affinity interactions through co-infection incidence in a pediatric population during the 2022 resurgence of influenza and RSV. Front. Cell. Infect. Microbiol. 2023, 13, 1208235. [Google Scholar] [CrossRef]
- Luo, M.; Gong, C.; Zhang, Y.; Wang, X.; Liu, Y.; Luo, Q.; Li, M.; Li, A.; Wang, Y.; Dong, M.; et al. Comparison of infections with respiratory syncytial virus between children and adults: A multicenter surveillance from 2015 to 2019 in Beijing, China. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1387–1397. [Google Scholar] [CrossRef]
- Bermúdez-Barrezueta, L.; López-Casillas, P.; Rojo-Rello, S.; Sáez-García, L.; Marugán-Miguelsanz, J.M.; Pino-Vázquez, M.d.l.A. Outcomes of viral coinfections in infants hospitalized for acute bronchiolitis. Virol. J. 2023, 20, 235. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of trained immunity by influenza vaccination—Impact on COVID-19. PLOS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Lim, F.J.; de Klerk, N.; Blyth, C.C.; Fathima, P.; Moore, H.C. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology 2016, 21, 648–655. [Google Scholar] [CrossRef]
- Barrezueta, L.B.; Zamorano, M.G.; López-Casillas, P.; Brezmes-Raposo, M.; Fernández, I.S.; Vázquez, M.d.l.A.P. Influence of the COVID-19 pandemic on the epidemiology of acute bronchiolitis. Enfermedades Infecc. Microbiol. Clin. 2023, 41, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2022, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Babawale, P.I.; Guerrero-Plata, A. Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens 2024, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]

| A | 0–4 years | 5–14 years | 15–64 years | <64 years |
| Positivity | 82.2% | 78.4% | 65.9% | 63.9% |
| At least one IV (IV or IV coinfection) | 42.9% | 63.5% | 50.6% | 43.6% |
| At least one ORV (ORV or coinfection of ORV) | 28.5% | 7.8% | 11.2% | 16.8% |
| Coinfection IV + ORV | 10.7% | 7.2% | 4.0% | 3.4% |
| Coinfection ORVs | 6.9% | 0.9% | 1.2% | 1.4% |
| % Coinfection * | 17.6% | 8.5% | 5.3% | 4.8% |
| B | 0–4 years | 5–14 years | 15–64 years | <64 years |
| At least one IV (IV or IV coinfection) | 52.2% | 81.0% | 76.9% | 68.3% |
| At least one ORV (ORV or coinfection of ORV) | 34.8% | 9.9% | 17.0% | 26.4% |
| Coinfection IV + ORV | 13.0% | 9.2% | 6.1% | 5.4% |
| Coinfection ORVs | 8.5% | 1.1% | 1.9% | 2.2% |
| % Coinfection * | 21.5% | 10.8% | 8.2% | 7.6% |
| 0–4 years | 5–14 years | 15–64 years | <64 years | |
| Positive samples | 875 | 1081 | 1344 | 186 |
| ORVs (with or without coinfection) | 304 (34.7%) | 107 (9.9%) | 228 (17.0%) | 49 (26.3%) |
| OR (95% CI) | 4.85 (3.80–6.18) | Ref. 1 | 1.86 (1.45–2.38) | 3.26 (2.22–4.77) |
| A. Other Respiratory Viruses (ORVs) | Vaccinated | Unvaccinated |
| Coinfection ORVs | 15/126 (11.9%) | 13/747 (17.4%) |
| ORVs without coinfection | 111/126 (88.1%) | 617/747 (82.6%) |
| OR (IC 95%) 0.64 (0.36–1.14); p = 0.13 | ||
| B. Influenza (IV) | Vaccinated | Unvaccinated |
| Coinfection IV + ORVs | 32/262 (12.2%) | 317/3157 (10.0%) |
| Influenza without coinfection | 230/262 (87.8%) | 2840/3157 (90.0%) |
| OR (IC 95%) 1.25 (0.85–1.84); p = 0.26 | ||
| Influenza (N = 3075) | ORV (N = 873) | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Cardiovascular disease | 63 (2.0%) | 23 (2.6%) | 0.77 (0.48–1.25) | 0.30 | 0.64 (0.39–1.05) | 0.08 |
| Chronic respiratory disease | 157 (5.1%) | 54 (6.2%) | 0.82 (0.59–1.12) | 0.21 | 0.86 (0.58–1.28) | 0.46 |
| Chronic liver disease | 12 (0.4%) | 3 (0.3%) | 1.14 (0.32–4.04) | 0.84 | 0.82 (0.21–3.11) | 0.77 |
| Chronic kidney disease | 18 (0.6%) | 4 (0.5%) | 1.28 (0.43–3.79) | 0.66 | 1.69 (0.48–5.98) | 0.42 |
| Metabolic disease | 47 (1.5%) | 25 (2.9%) | 0.53 (0.32–0.86) | 0.01 | 0.44 (0.27–0.73) | 0.01 * |
| Obesity | 32 (1.0%) | 11 (1.3%) | 0.82 (0.41–1.64) | 0.58 | 0.80 (0.33–1.92) | 0.62 |
| Immunodeficiency | 19 (0.6%) | 14 (1.6%) | 0.38 (0.19–0.76) | 0.01 | 0.38 (0.17–0.85) | 0.02 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torner, N.; Soldevila, N.; Basile, L.; Mosquera, M.M.; de Molina, P.; Marcos, M.A.; Martínez, A.; Jané, M.; Domínguez, A.; The Working Group for the Catalan Influenza and Acute Respiratory Infection Sentinel Surveillance Network (PIDIRAC). Contribution of Other Respiratory Viruses During Influenza Epidemic Activity in Catalonia, Spain, 2008–2020. Microorganisms 2024, 12, 2200. https://doi.org/10.3390/microorganisms12112200
Torner N, Soldevila N, Basile L, Mosquera MM, de Molina P, Marcos MA, Martínez A, Jané M, Domínguez A, The Working Group for the Catalan Influenza and Acute Respiratory Infection Sentinel Surveillance Network (PIDIRAC). Contribution of Other Respiratory Viruses During Influenza Epidemic Activity in Catalonia, Spain, 2008–2020. Microorganisms. 2024; 12(11):2200. https://doi.org/10.3390/microorganisms12112200
Chicago/Turabian StyleTorner, Nuria, N. Soldevila, L. Basile, M. M. Mosquera, P. de Molina, M. A. Marcos, A. Martínez, M. Jané, A. Domínguez, and The Working Group for the Catalan Influenza and Acute Respiratory Infection Sentinel Surveillance Network (PIDIRAC). 2024. "Contribution of Other Respiratory Viruses During Influenza Epidemic Activity in Catalonia, Spain, 2008–2020" Microorganisms 12, no. 11: 2200. https://doi.org/10.3390/microorganisms12112200
APA StyleTorner, N., Soldevila, N., Basile, L., Mosquera, M. M., de Molina, P., Marcos, M. A., Martínez, A., Jané, M., Domínguez, A., & The Working Group for the Catalan Influenza and Acute Respiratory Infection Sentinel Surveillance Network (PIDIRAC). (2024). Contribution of Other Respiratory Viruses During Influenza Epidemic Activity in Catalonia, Spain, 2008–2020. Microorganisms, 12(11), 2200. https://doi.org/10.3390/microorganisms12112200








