Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. EPS Purification
2.3. Spectral Analysis
2.4. Detection of the Molecular Weight (Mw)
2.5. Monosaccharide Composition Analysis
2.6. Congo Red Experiment
2.7. Scanning Electronic Microscope (SEM) Analysis
2.8. X-Ray Diffraction (XRD) Analysis
2.9. Particle Size and Zeta Potential
2.10. Differential Scanning Calorimeter (DSC) Analysis
2.11. In Vitro Antioxidant Activities
2.11.1. ABTS+ Scavenging Assay
2.11.2. DPPH Scavenging Assay
2.11.3. Hydroxyl Radical (·OH) Quenching Assay
2.11.4. Reducing Power Assay
2.12. In Vitro Antibiofilm Activities
2.12.1. Biofilm Inhibition Assay
2.12.2. The Impact of EPS on Preformed Bacterial Biofilm
2.13. The Analysis of the Antibiofilm Mechanism of EPS715 on H. alvei
2.13.1. Effects of EPS715 on the Growth of H. alvei
2.13.2. Effects of EPS715 on the Auto-Aggregation and Hydrophilic Ability of H. alvei
2.13.3. Effects of EPS715 on the Swarming and Swimming Ability of H. alvei
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of EPS715
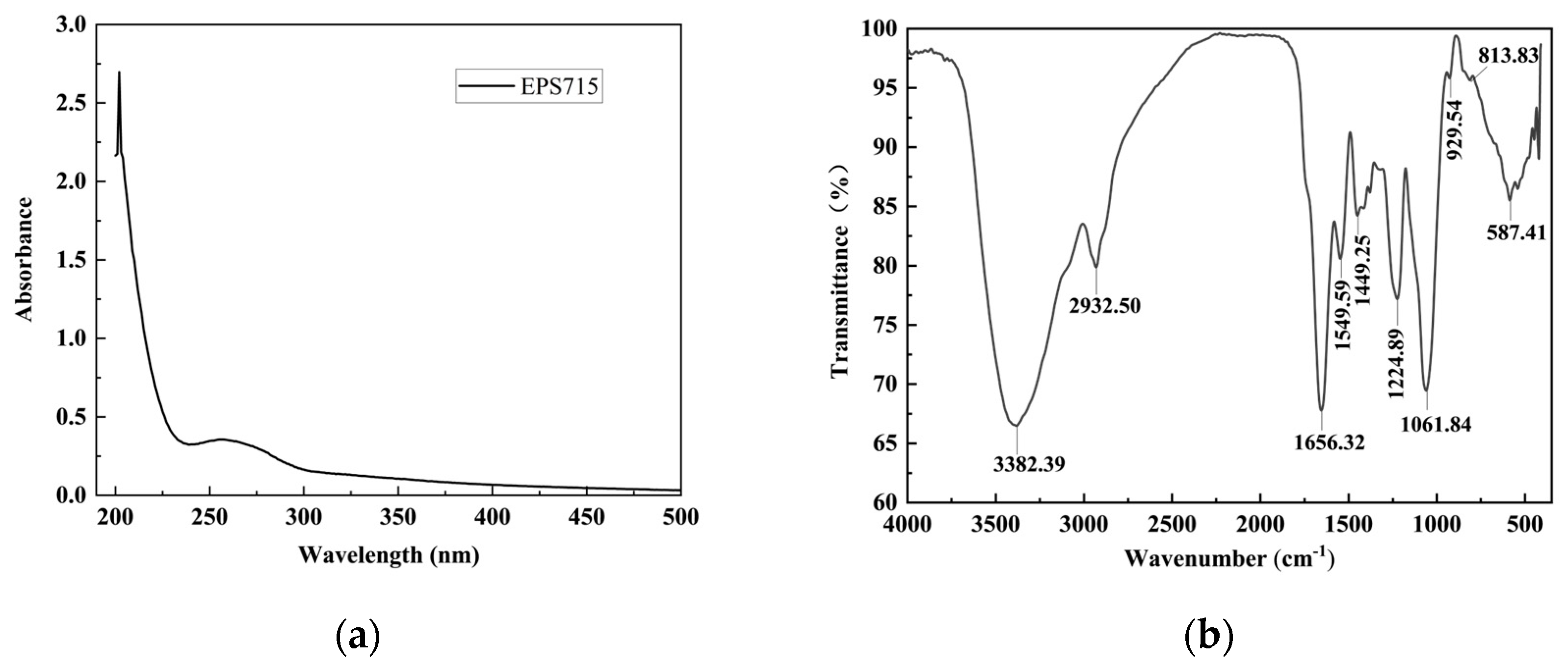
3.1.1. Spectra Analysis
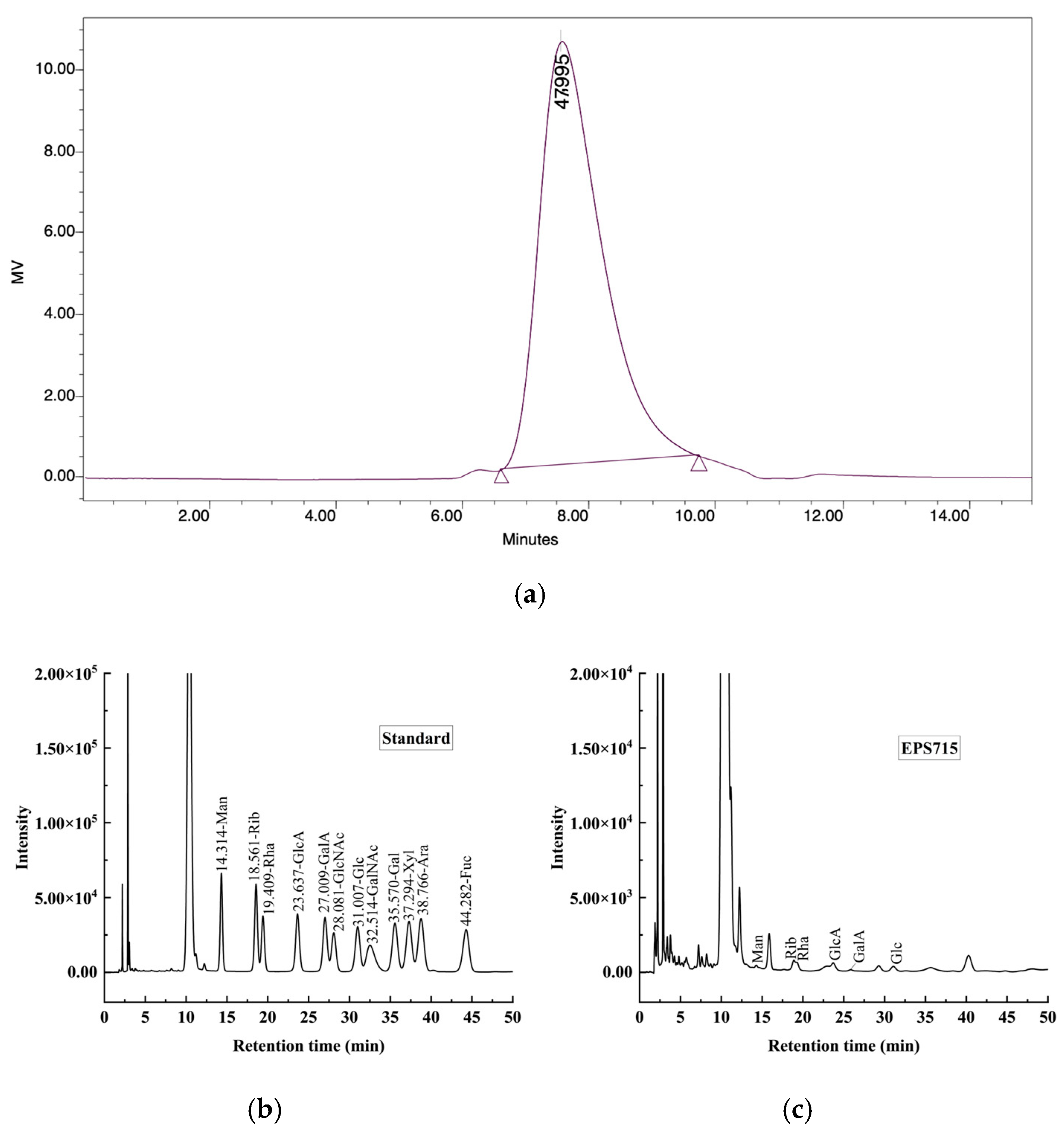
3.1.2. Molecular Weight (Mw) and Monosaccharide Composition Analysis
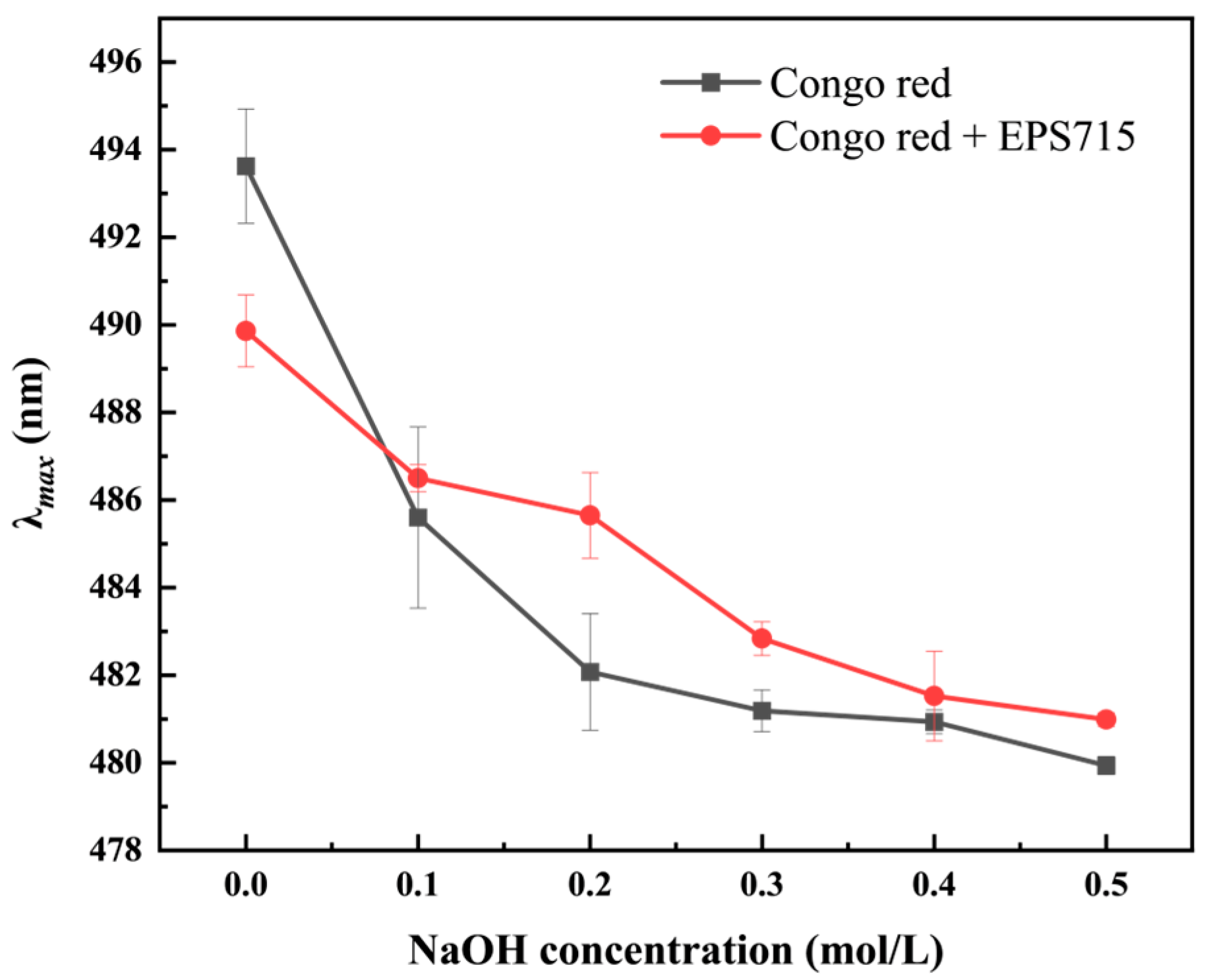
3.1.3. Congo Red Test
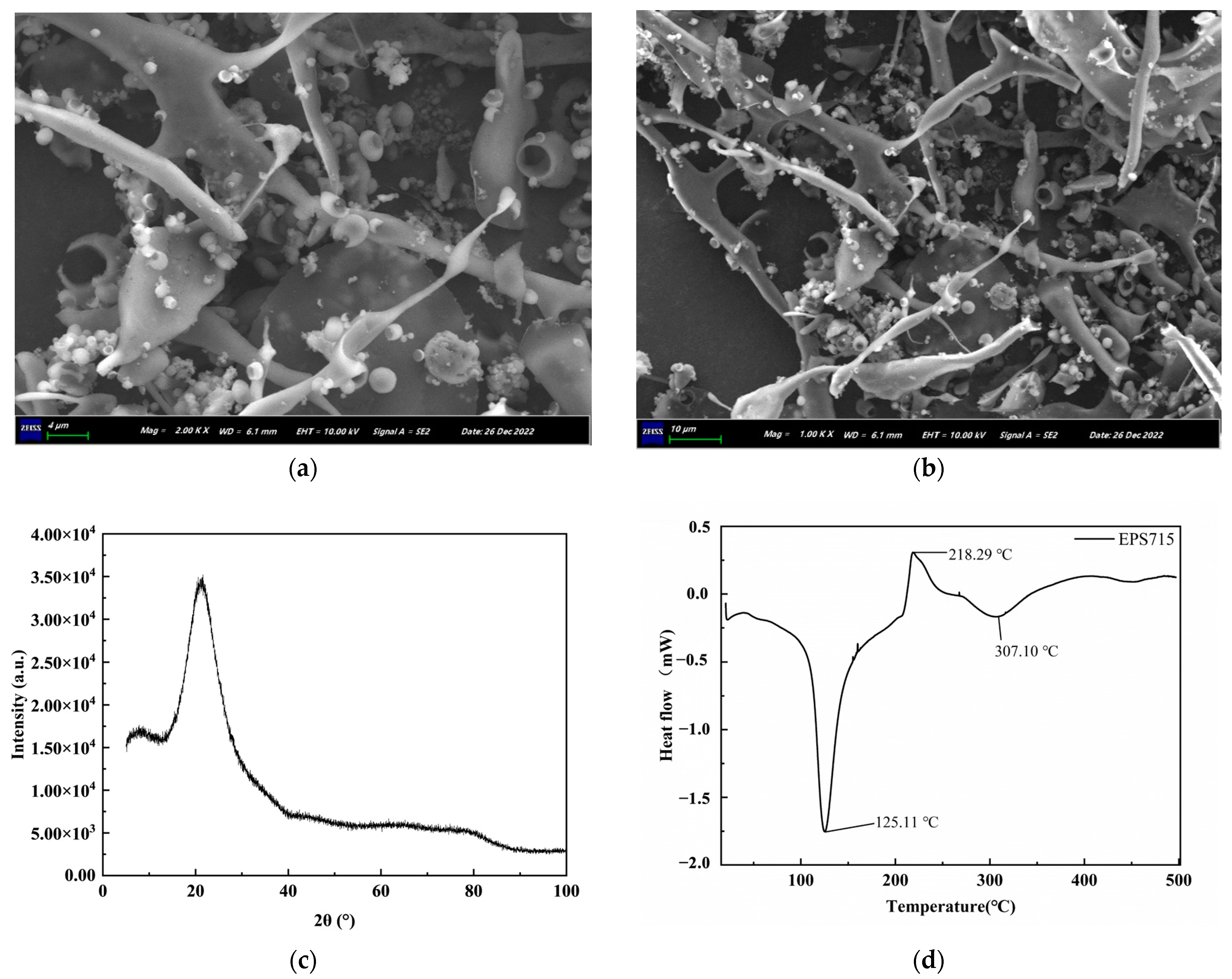
3.1.4. Microstructural and Thermal Characteristic Analysis
3.2. In Vitro Antioxidant Activities of EPS
3.3. In Vitro Antibiofilm Activities of EPS715
3.4. Potential Antibiofilm Mechanism of EPS715 on H. alvei
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teja, C.R.; Karlapudi, A.P.; Vallur, N.; Mamatha, K.; Babu, D.J.; Venkateswarulu, T.C.; Kodali, V.P. Antioxidant Potential and Optimization of Production of Extracellular Polysaccharide by Acinetobacter Indicus M6. J. Genet. Eng. Biotechnol. 2021, 19, 39. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and Characterization of Dextran Produced by Lactobacillus Sakei L3 from Hubei Sausage. Carbohydr. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Sathiyanarayanan, G.; Balaji, P.; Mathivanan, K.; Pugazhendhi, A.; Ma, Y.; Sekar, S.; Thirumurugan, R. Struc tural Characterization, Functional and Biological Activities of an Exopolysaccharide Produced by Probiotic Bacillus Licheniformis AG-06 from Indian Polyherbal Fermented Traditional Medicine. Int. J. Biol. Macromol. 2021, 174, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Geng, L.; Xue, B.; Wang, Z.; Xu, W.; Shu, C.; Zhang, J. Structure Characteristics and Function of a Novel Extracellular Polysaccharide from Bacillus Thuringiensis Strain 4D19. Int. J. Biol. Macromol. 2021, 189, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, D.; Huang, Y. Exopolysaccharides from Lactiplantibacillus Plantarum: Isolation, Purification, Structure–Function Relationship, and Application. Eur. Food Res. Technol. 2023, 249, 1431–1448. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Ibrahim, M.I.A. Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus Plantarum EI6 With in Vitro Wound Healing Activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus Plantarum WLPL04 Ex opolysaccharide Amplifies Its Antioxidant Activities in Vitro and in a Caco-2 Cell Model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopol ysaccharide Produced by Lactobacillus Plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, L.; Yu, T.; Ma, J.; Lu, S.; Ren, J.; Li, X.; Guo, X. Exopolysaccharide Production by Lactobacillus Plantarum T10 Is Responsible for the Probiotic Activity in Enhancing Intestinal Barrier Function in Vitro and in Vivo. Food Funct. 2024, 15, 3583–3599. [Google Scholar] [CrossRef]
- Pradeepa; Shetty, A.D.; Matthews, K.; Hegde, A.R.; Akshatha, B.; Mathias, A.B.; Mutalik, S.; Vidya, S.M. Multidrug Resistant Pathogenic Bacterial Biofilm Inhibition by Lactobacillus Plantarum Exopolysaccharide. Bioact. Carbohydr. Diet. Fibre 2016, 8, 7–14. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2022, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Champion, M.; Portier, E.; Vallée-Réhel, K.; Linossier, I.; Balnois, E.; Vignaud, G.; Moppert, X.; Hellio, C.; Faÿ, F. Anti-Biofilm Activity of a Hyaluronan-like Exopolysaccharide from the Marine Vibrio MO245 against Pathogenic Bacteria. Mar. Drugs 2022, 20, 728. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Haliscelik, O.; Gursoy, M.; Kiran, F.; Loimaranta, V.; Söderling, E.; Gursoy, U.K. Analysis of Chemical Structure and Antibiofilm Properties of Exopolysaccharides from Lactiplantibacillus Plantarum EIR/IF-1 Postbiotics. Microorganisms 2022, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.P.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas Aeruginosa Lectin LecB Binds to the Exopolysaccharide Psl and Stabilizes the Biofilm Matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Amini, E.; Salimi, F.; Imanparast, S.; Mansour, F.N. Isolation and Characterization of Exopolysaccharide Derived from Lactica seibacillus Paracasei AS20(1) with Probiotic Potential and Evaluation of Its Antibacterial Activity. Lett. Appl. Microbiol. 2022, 75, 967–981. [Google Scholar] [CrossRef]
- Hu, J.; Tian, X.; Wei, T.; Wu, H.; Lu, J.; Lyu, M.; Wang, S. Anti-Helicobacter Pylori Activity of a Lactobacillus sp. PW-7 Exopoly saccharide. Foods 2021, 10, 2453. [Google Scholar] [CrossRef]
- Tan, J.-Y.; Yin, W.-F.; Chan, K.-G. Quorum Sensing Activity of Hafnia Alvei Isolated from Packed Food. Sensors 2014, 14, 6788–6796. [Google Scholar] [CrossRef]
- Hou, H.-M.; Zhu, Y.-L.; Wang, J.-Y.; Jiang, F.; Qu, W.-Y.; Zhang, G.-L.; Hao, H.-S. Characteristics of N-Acylhomoserine Lactones Produced by Hafnia Alvei H4 Isolated from Spoiled Instant Sea Cucumber. Sensors 2017, 17, 772. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Hou, H.M.; Zhang, G.L.; Wang, Y.F.; Hao, H.S. AHLs Regulate Biofilm Formation and Swimming Motility of Hafnia Alvei H4. Front. Microbiol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Langsrud, S.; Moen, B.; Møretrø, T.; Løype, M.; Heir, E. Microbial Dynamics in Mixed Culture Biofilms of Bacteria Surviving Sanitation of Conveyor Belts in Salmon-processing Plants. J. Appl. Microbiol. 2016, 120, 366–378. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, Purification and Characterization of Exopolysaccha ride Produced by Leuconostoc Pseudomesenteroides YF32 from Soybean Paste. Int. J. Biol. Macromol. 2018, 114, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Sun, Q.; Zhang, S.-M.; Sun, X.-Y.; Li, C.-Y.; Zheng, M.-X.; Xiang, W.-L.; Tang, J. Characterization and Anti oxidant Activity of Released Exopolysaccharide from Potential Probiotic Leuconostoc Mesenteroides LM187. J. Microbiol. Biotechnol. 2021, 31, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Pang, X.; Wang, P.G.; Chen, M. Isolation and Characterization of an Antioxidant Exopolysaccharide Produced by Bacil lus sp. S-1 from Sichuan Pickles. Carbohyd. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pei, F.; Zhu, J.; Xue, D.; Liu, Y.; Liu, D.; Li, H. Production, Characterization and Antioxidant Activity of Exopolysaccha ride from Sporidiobolus Pararoseus PFY-Z1. World J. Microbiol. Biotechnol. 2023, 39, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-Helix Polysaccharides: Formation Mechanisms and Ana lytical Methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of Highly Branched Dextran Pro duced by Leuconostoc Citreum B-2 from Pineapple Fermented Product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Jia, M.; Chen, J.; Liu, X.; Xie, M.; Nie, S.; Chen, Y.; Xie, J.; Yu, Q. Structural Characteristics and Functional Properties of Soluble Dietary Fiber from Defatted Rice Bran Obtained through Trichoderma Viride Fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.-Y.; Li, W.; Sun, Z. Structure Characterization, Antioxidant Capacity, Rheological Characteristics and Expression of Biosynthetic Genes of Exopolysaccharides Produced by Lactococcus Lactis subsp. Lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Bekar, F.; Kamiloglu, A. Effect of Different Carbon Sources on Exopolysaccharide Production of Lactiplantibacillus Pentosus KS27 and Optimization of Production Conditions Using Box-Behnken Designing. Biomass Convers. Biorefin. 2023, 14, 26139–26148. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kim, J.-S.; Park, H.M.; Kim, S.; Paek, N.-S. Antioxidant Activity and Short-Chain Fatty Acid Production of Lactic Acid Bacteria Isolated from Korean Individuals and Fermented Foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.C.; Sukumaran, V.; Park, S.C. Antioxidant, Antibacterial, and Anti-Adhesive Activities of Biosurfactants Iso lated from Bacillus Strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.; Ishola, R.; Oyewunmi, T. Characterization, Antioxidant and Immunomodulatory Potential on Exopolysac charide Produced by Wild Type and Mutant Weissella Confusa Strains. Biotechnol. Rep. 2018, 19, e00271. [Google Scholar] [CrossRef]
- Zeinivand, M.; Aghaei, S.S.; Zargar, M.; Ghasemzadeh, M.A. Exopolysaccharide-Mediated Silver Nanoparticles Synthesized from Lactobacillus Paracasei with Antimicrobial, Antibiofilm and Antioxidant Activities. Arch. Microbiol. 2023, 205, 210. [Google Scholar] [CrossRef]
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of Exopolysaccharide Produced by Levilactobacillus Brevis HDE-9 and Evaluation of Its Potential Use in Dairy Products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Pei, F.; Cao, X.; Zhang, W.; Du, R.; Ge, J.; Ping, W. Purification of Exopolysaccharides from Lactobacillus Rhamnosus and Changes in Their Characteristics by Regulating Quorum Sensing Genes via Polyphenols. Int. J. Biol. Macromol. 2023, 240, 124414. [Google Scholar] [CrossRef]
- Dhanya, B.E.; Prabhu, A.; Rekha, P.D. Extraction and Characterization of an Exopolysaccharide from a Marine Bacterium. Int. Microbiol. 2022, 25, 285–295. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, K.; Zhang, N.; Chitrakar, B.; Huang, P.; Wang, X.; Yang, B.; Sang, Y. Structural Characterization and Immunomod ulatory Effects of Extracellular Polysaccharide from Lactobacillus Paracasei VL8 Obtained by Gradient Ethanol Precipitation. J. Food Sci. 2022, 87, 2034–2047. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and Characterization of Dextran Produced by Leuconostoc Citreum NM105 from Manchurian Sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Vuyst, L.D.; Degeest, B. Heteropolysaccharides from Lactic Acid Bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, Bioactivities, and Rheological Properties of Exopolysaccharide Produced by Novel Probiotic Lactobacillus Plantarum C70 Isolated from Camel Milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef]
- Das, D.; Baruah, R.; Goyal, A. A Food Additive with Prebiotic Properties of an α-d-Glucan from Lactobacillus Plantarum DM5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant Activities of Different Fractions of Polysaccharide Conjugates from Green Tea (Camellia Sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, M.; Lai, T.; Yang, Z. Characterization and In Vitro Fecal Microbiota Regulatory Activity of a Low-Molecular-Weight Exopolysaccharide Produced by Lactiplantibacillus Plantarum NMGL2. Foods 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and Antioxidant Activity of an Acidic Exopoly saccharide from Lactobacillus Plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and Bioactivities of an Exopolysaccharide Produced by Lacto bacillus Plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wu, Z.; Huang, B.; Sun, L.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Zhou, L.; et al. Extraction, Antioxidant and Antibacterial Activities of Broussonetia Papyrifera Fruits Polysaccharides. Int. J. Biol. Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, P.; Qu, Z.; Bai, D.; Gao, X.; Zhao, C.; Chen, J.; Gao, W. Physicochemical Characterizations of Polysaccha rides from Angelica Sinensis Radix under Different Drying Methods for Various Applications. Int. J. Biol. Macromol. 2019, 121, 381–389. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ping, W.; Ge, J. Characterization of Exopolysaccharides Produced by Weissella Confusa XG-3 and Their Potential Biotechnological Applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, Q.; Dong, M. Structural Characterization and Anti cancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus Helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Liao, Y.; Gao, M.; Wang, Y.; Liu, X.; Zhong, C.; Jia, S. Structural Characterization and Immunomodulatory Activity of Exopoly saccharide from Aureobasidium Pullulans CGMCC 23063. Carbohydr. Polym. 2022, 288, 119366. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Na, R.; Du, R.; Ping, W.; Ge, J.; Zhao, D. Purification, Characterization and Partial Biological Activities of Exopoly saccharide Produced by Saccharomyces Cerevisiae Y3. Int. J. Biol. Macromol. 2022, 206, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Dai, X.; Zhao, B.; Li, Z.; Tao, J.; Wang, T.; Huang, A. Structure-Activity Relationship of Exopolysaccharides Produced by Limosilactobacillus Fermentum A51 and the Mechanism Contributing to the Textural Properties of Yogurt. Food Hydrocoll. 2023, 144, 108993. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Bamigbade, G.; Tarique, M.; Esposito, G.; Obaid, R.; Abu-Jdayil, B.; Ayyash, M. Physicochemical, Rheological, and Bioactive Properties of Exopolysaccharide Produced by a Potential Probiotic Enterococcus Faecalis 84B. Int. J. Biol. Macromol. 2023, 240, 124425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and Bioactivities of the Exopolysaccharide from a Probiotic Strain of Lactobacillus Plantarum WLPL04. J Dairy Sci 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, M.; Velmurugan, T.; Mythili, R.; Kim, W.; Sudha, K.G.; Ali, S.; Kalpana, B.; Ramalingam, S.; Rajeshkumar, M.P. Ex traction and Characterization of Exopolysaccharides from Lactiplantibacillus Plantarum Strain PRK7 and PRK 11, and Evaluation of Their Antioxidant, Emulsion, and Antibiofilm Activities. Int. J. Biol. Macromol. 2023, 242, 124842. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Antibiofilm Activity of Lactobacillus Planta rum 12 Exopolysaccharides against Shigella Flexneri. Appl. Environ. Microb. 2020, 86, e00694-20. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and Antibacterial Activities of Exopolysaccharides from Bifidobacterium Bifidum WBIN03 and Lactobacillus Plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Bayar, S.; Mahdouani, K.; Majdoub, H.; Kouidhi, B. Use of Extracellular Polysaccharides, Secreted by Lactobacillus Plantarum and Bacillus spp., as Reducing Indole Production Agents to Control Biofilm Formation and Efflux Pumps Inhibitor in Escherichia Coli. Microb. Pathog. 2018, 125, 448–453. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Chen, G.; Li, B.; Chen, D.; Li, L.; Xu, Z. Pathogenic Features and Characteristics of Food Borne Pathogens Bio film: Biomass, Viability and Matrix. Microb. Pathog. 2017, 111, 285–291. [Google Scholar] [CrossRef]
- Spanò, A.; Laganà, P.; Visalli, G.; Maugeri, T.L.; Gugliandolo, C. In Vitro Antibiofilm Activity of an Exopolysaccharide from the Marine Thermophilic Bacillus Licheniformis T14. Curr. Microbiol. 2016, 72, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Chen, X.; Yang, R.; Li, W.; Yin, B.; Li, Z.; Dong, M. Structure of a Unique Fucose-Containing Exopolysaccharide from Sayram Ketteki Yoghurt and Its Anti-MRSA Biofilm Effect. Int. J. Biol. Macromol. 2022, 216, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and Anti-Infection of a Marine Bacterial Exopolysaccharide Against Pseudo monas Aeruginosa. Front. Microbiol. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, R.; Zhang, J.; Wu, S. Transcriptional Profiling of Pseudomonas Aeruginosa PAO1 in Response to Anti-biofilm and Anti-infection Agent Exopolysaccharide EPS273. J. Appl. Microbiol. 2021, 130, 265–277. [Google Scholar] [CrossRef]


| EPS Concentration (μg/mL) | Inhibition Rate (%) | ||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. saprophyticus | Acinetobacter spp. | H. alvei | |
| 37.5 | −6.78 ± 21.68% b | 66.51 ± 7.11% ab | 39.16 ± 13.68% a | 18.69 ± 4.14% a | 58.38 ± 2.06% b |
| 62.5 | 32.49 ± 11.82% a | 73.29 ± 3.71% a | 34.38 ± 10.33% a | 29.20 ± 10.96% a | 78.17 ± 1.65% a |
| 125 | 60.08 ± 12.98% a | 41.19 ± 10.99% bc | 8.05 ± 16.51% b | 10.31 ± 14.50% a | 44.01 ± 0.44% c |
| 250 | 56.92 ± 7.42% a | 38.93 ± 3.76% bc | 54.13 ± 0.93% a | 23.33 ± 20.90% a | 30.31 ± 1.19% d |
| 500 | 53.28 ± 15.62% a | 26.74 ± 26.14% c | 46.82 ± 0.90% a | 18.47 ± 7.49% a | 32.06 ± 5.40% d |
| EPS Concentration (μg/mL) | Dispersion Rate (%) | ||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. saprophyticus | Acinetobacter spp. | H. alvei | |
| 37.5 | 75.51 ± 1.95% b | 73.24 ± 4.46% a | 62.35 ± 2.29% a | 4.10 ± 11.48% c | 35.66 ± 11.02% c |
| 62.5 | 83.47 ± 0.84% a | 82.18 ± 4.55% a | 35.14 ± 10.65% bc | 19.30 ± 7.60% bc | 36.38 ± 4.86% c |
| 125 | 50.03 ± 3.34% c | 84.01 ± 3.04% a | 24.97 ± 8.99% c | 36.88 ± 2.33% a | 65.86 ± 1.23% b |
| 250 | 72.80 ± 2.07% b | 81.39 ± 2.62% a | 51.13 ± 2.25% ab | 21.22 ± 5.65% ab | 60.75 ± 4.33% b |
| 500 | 75.20 ± 2.66% b | 64.80 ± 16.21% a | −26.46 ± 8.69% d | 27.61 ± 4.09% ab | 78.92 ± 2.05% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Ma, B.; Wang, X.; Cui, F.; Li, X.; Li, J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms 2024, 12, 2229. https://doi.org/10.3390/microorganisms12112229
Tan X, Ma B, Wang X, Cui F, Li X, Li J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms. 2024; 12(11):2229. https://doi.org/10.3390/microorganisms12112229
Chicago/Turabian StyleTan, Xiqian, Bingyu Ma, Xiaoqing Wang, Fangchao Cui, Xuepeng Li, and Jianrong Li. 2024. "Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei" Microorganisms 12, no. 11: 2229. https://doi.org/10.3390/microorganisms12112229
APA StyleTan, X., Ma, B., Wang, X., Cui, F., Li, X., & Li, J. (2024). Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms, 12(11), 2229. https://doi.org/10.3390/microorganisms12112229






